Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Geofísica internacional
On-line version ISSN 2954-436XPrint version ISSN 0016-7169
Geofís. Intl vol.49 n.1 Ciudad de México Jan./Mar. 2010
Articles
Rare–earth element distribution in water from El Chichón Volcano Crater Lake, Chiapas Mexico
O. Morton–Bermea1*, M. Aurora Armienta1, S. Ramos2
1 Instituto de Geofísica, Universidad Nacional Autónoma de México, Ciudad Universitaria, Del. Coyoacán, 04510, Mexico City, Mexico. * Corresponding Autor: omorton@geofisica.unam.mx
2 Universidad de Ciencias y Artes de Chiapas, Tuxtla, Gtz. Chiapas, Mexico
Received: October 2, 2008
Accepted: September 1, 2009
Resumen
Esta investigación puede considerarse como un estudio piloto en el volcán El Chichón para determinar si la distribución de los patrones normados de tierras raras (TR) pueden utilizarse como trazadores rutinarios en el sistema geotérmico del volcán. Dieciocho muestras de agua del lago alrededor del cráter fueron tomadas durante tres campañas de campo de marzo de 2006 a mayo de 2008. Los datos químicos han documentado cambios en la evolución del sistema hidrotermal, sin embargo, estas muestras no evidencian variaciones significativas en la geoquímica de tierras raras que corresponde a la estabilidad de la actividad volcánica observada durante el periodo de estudio. Las concentraciones de TR son altas y alcanzan valores entre 5.58 ppb (Ce) y 0.01 ppb (Lu). Los patrones normados de TR de muestras de agua de lago del Volcán El Chichón presentan enriquecimiento de TR pesadas con respecto a muestras pomez de la erupción de 1982 (representativas de la roca encajonante). La pequeña anomalía de Eu observada en muestras recientes puede haberse originado por el aumento de agentes acomplejantes tales como sulfatas solubles, cloruros y fluoruros. Cambios significantes en el comportamiento de las TR pueden estar asociados con cambios mayores de la actividad magmática, que no se han presentado durante el tiempo de estudio.
Palabras clave: Tierras raras, geoquímica, Volcán El Chichón, ICP–MS, México.
Abstract
This research can be regarded as a pilot study at El Chichón Volcano to assess whether REE distributions can be used as routinely geochemical tracers in the Crater Lake geothermal system. Eighteen lake water samples were collected during 3 field campaigns (Group 1, Group 2, and Group 3) from March 2006 to May 2008 at the same sites around the lake. The chemical data has documented the temporal evolution of the hydrothermal system as changes in major element chemistry. However, these samples do not evidence significant variations in the REE geochemistry corresponding to the observed volcanic stability during this period. The concentrations of the REE ranged between 5.58 ppb (Ce) and 0.01 ppb (Lu). Lake water from El Chichón Volcano show HREE enriched patterns with respect to the 1982 pumice samples (as representative of the host rock). The small Eu anomaly observed in recent samples may be due to the increase of complexing agents such as soluble sulfates, chlorides and fluorides.
Key words: Rare earth elements, geochemistry, El Chichón Volcano, ICP–MS, Mexico.
Introduction
Rare Earth Elements (REEs) are a suite of 14 metals from atomic number 57 (La) to 71 (Lu). Due to their similar configuration of valence electrons and ionic ratios, they are expected to show comparable chemical and physical behavior. In general, REEs are present in the natural environment as trivalent cations, however, some of them have an anomalous behavior, as Cerium that occurs in the +IV oxidation state under oxidizing conditions, and Eu that may occur in the +II state, due to the relative stability of this chemical form. The REEs behavior has allowed their use in geological environments as a powerful tool to identify geochemical processes (Henderson, 1984; Brookins, 1989).
During the last decade, the study of the geochemical behavior of REE have attracted much attention in hidrogeochemical research (Lewis et al., 1997; van Middlesworth and Wood, 1998; Johannesson et al., 1996a, 1996b, 2000), because it provides information concerning the thermodynamic conditions controlling water–rock interaction processes. The REEs are relatively immobile during water–rock reactions, but they can be mobilized during some hydrothermal and metasomatic processes (Hopf, 1993; Worral and Pearson, 2001). Their concentration in waters is a function of many parameters. Most relevant are: REE concentration in the host rock, REE distribution in mineral phases and chemistry of fluids like pH and presence and concentrations of complexing ligands (Johannesson et al. 1996a; Johannesson et al. 1996b; Johannesson and Zhou 1999; Johannesson et al. 2000). Those influences determine REE patterns in fluids respect to the host rock to be depleted in HREEs or LREEs and to exhibit Eu or Ce anomalies. Johannesson et al. (1999) demonstrated that REEs may be particularly useful for investigating groundwater–rock interactions, groundwater recharge regions and groundwater mixing, because these elements are thought to be derived chiefly from the aquifer rock matrix. Banks et al. (1999) used REEs in groundwaters to identify reservoir lithology at depth in a heavily glaciated landscape. In this study they showed that water samples from different aquifers have distinct REE signatures. REE distribution in groundwater, being directly related to the REE distribution in the host rock, may be of importance from the point of view of both, mineral prospecting, and geochemical and geological mapping. Because El Chichón volcano has erupted very homogenus andesitic magmas during the Holocene (Espíndola et al., 2000) and ever during the Pleistocene (Layer et al., 2009) we used the composition of the 1982 pumice sample as a host rock.
The geochemistry of rare earth elements (REE) in acid waters (natural acid springs and mine water samples) was studied by Verplanck et al. (2004), to determine the dominant control on REE in acid waters either source–related or post–dissolution process–related. This knowledge may be used to determinate contributions of metals from ores to the streams. Since REE patterns of acid waters seem to reflect those of the host rocks, a contrast in the REE composition between the host rock and the acid mine water is needed to use REE patterns of acid waters as a source signature.
In the last years, several processes that are responsible for controlling dissolved REE concentrations, specially the negative Eu anomaly have been discussed (Haas et al., 1995; Wood, 1990a, 1990b; Brookins, 1989). Lewis et al. (1997) reported that REE speciation in hydrothermal solutions is controlled by temperature, pH, salinity, and relative abundances of suitable ligands. Based on the relation among those processes and volcanic dissolved gases, REE behavior in geothermal and volcanic systems and its relation with volcanic activity has been investigated (Lewis et al. 1997, Gammouns et al 2005, Wood 2006; Varekamp et al. 2009).
The chondrite–normalized REE behavior in hydrothermal waters of theYellowstone National Park showed LREE–enrichment and negative Eu anomalies similar to the rhyolite rocks in the area, indicating that little or no fractionation had occurred across the REE series during water–rock interaction, although there was a general tendency for slight enrichment in the LREE in the fluid and a marked Eu anomaly (Lewis et al., 1997). This behavior was consistent with the abundances of potential complexing agents such as sulphate or chloride at low pH. Gammons et al. (2005) reported a slight negative Eu anomaly in the REE trends of samples from the Lower Rio Agrio (northern Patagonia, Argentina). Based on the magnitude of these anomalies they concluded that its appearance could be explained in part by analytical error. Wood (2006) evaluated REE behavior in acidic geothermal waters from the Taupo Volcanic Zone, New Zealand. Results showed that acid–sulfate waters (pH ranging between 1.5 and 2.8) have a distinctive "gull–wing" chondrite–normalized pattern with a negative Eu anomaly and heavy and light REE "wings". The heavy REE wings are more or less parallel to the patterns of the host rocks, but the light REE wings exhibit depletion of the lightest REE (La–Nd) compared to host rocks. In this case, the negative Eu anomaly in the waters appears to simply reflect a negative Eu anomaly in the host rocks. On the other hand, waters from hyperacidic volcanic crater lakes do not exhibit these "gull–wing" REE patterns. The higher acidity of the crater–lake waters may result in host–rock REE fractionation, at least during pre–eruptive periods. Based on these results it is concluded that below a certain critical pH (below 0.5–2), waters acquire REE from rock or magma with little fractionation across the entire series whereas waters with pH in the range 2–4 tend to fractionate the light REE.
Varekamp et al. (2009) established that the chemistry of acid waters from Copahue volcano, Argentina, results from changes in water rock interaction. Most fluids have LREE enrichments relative to the rock matrix, but during periods of new magma intrusion the LREE enrichment decreases as does the magnitude of the negative Eu anomaly in the fluids. This behavior is ascribed to dissolution of plagioclase, olivine and volcanic glass that occurs during intrusion of new magma into the hydrothermal system. At El Chichón during our observation period, pH ranged from 2.35 to 2.70, and sulfate, chloride, and fluoride concentrations showed important variations. Samples collected in 2004 with a pH around 2 had a parallel pattern of REE to that of host rocks.
El Chichón is the northernmost volcano of a small volcanic system known as the Chiapanecan Volcanic Arc (Damon and Montesinos, 1978). El Chichón volcano is a complex structure composed of craters and domes with an approximate volume of 26 km3. El Chichón Crater Lake was formed after the 1982 eruptions of E1 Chichón Volcano, Mexico within a a 1–km wide crater with a maximum rim elevation of 1100 masl, having steep inner walls with an average height of 160 m (Casadevall, et al., 1984; Macias et al., 2008). The lake covers the central area within the crater; therefore, changes in the chemistry of lake waters can be expected to reflect variations in the activity of the volcano. El Chichón Lake water pH increased from 1983 (0.56) to ~2.5 since 1986 (Armienta et al., 2000). Its chemical composition has shown strong variations along the years as a result of differences on environmental and volcanic factors. The fluid from the crater–lake was acid, calcium chloride type in 1983, and has varied in the relative proportions of cations and anions (sulfate and chloride) since then (Armienta and De la Cruz 1994; Taran et al. 1998; Armienta et al., 2000; Tassi et al. 2003; Taran et al. 2008; Rouwet et al. 2008). The significant hydro–geochemical fluctuations (Armienta and De la Cruz, 1994; Taran et al., 1998; Tassi et al., 2003) observed after the Chichón volcanic disaster (Tilling, 2009) have provided a detailed chemical characterization of fluid manifestations of the volcano. Taran et al., (1998) ascribed the changes in the crater–lake chemistry to the activity of near–neutral geyser–like springs in the crater (Soap Pool). These Soap Pool springs represented the interaction between condensed magmatic vapor with volcanic rocks. Armienta et al. (2000) presented a chemical characterization of the El Chichón lake in relation with other Mexican crater–lakes. These authors recorded a concentration decrease with time (from 1983 to 2000) of volcanic–related species like sulfate, chloride, boron and fluoride at El Chichón suggesting a concomitant lowering of the magmatic contribution to the crater–lake. Tassi et al. (2003) interpreted that the chemical and physical changes recorded in 1998–2000 were possibly due to variations in the permeability of the conduit system feeding the fluid discharges at the surface. They also reported that the magmatic–hydrothermal system of El Chichón is probably related to interaction processes between a deep magmatic source and a surficial cold aquifer. Taran et al. (2008) used major and trace element composition of water to develop a geochemical model for the volcano–hydrothermal system of El Chichón with recommendations for monitoring chemical changes in hot springs as precursors of volcanic unrest. Rouwet et al. (2008) and Taran et al. (2008) related the hydrochemical dynamics of the El Chichón volcanic system to the geyser–like springs in the crater and stated that a future dome growth, not observed yet, may be anticipated by changes in the lake and springs chemistry. They also reported the absence of a relevant volcanic activity during the studied period.
The objective of the present study is to investigate the REE concentrations in the acid waters of El Chichón Volcano Lake (Mexico) and their correlation with changes in the concentration of main ions to evaluate their use as routinely tracers of the magmatic activity, which may contribute to the information available for the assessment of the volcano hazards.
Methodology
For this work, 18 lake water samples were collected during three field campaigns (Group 1, Group 2 and Group 3) from March 2006 to May 2008 at the same sites around the lake. Sampling sites and respective coordinates are indicated in Fig. 1. Rare earth element (REE) concentrations were determined by Inductively Coupled Plasma–Mass Spectrometry (ICP–MS) using a PQ3 VGElemental at the Instituto de Geofísica, UNAM. The instrument was optimized using a standard tuning solution of 10 mu/l Be, Co, In, Tb, Bi and Ba. The isobaric oxide interferences were reduced below 3% BaO/Ba. Mass interferences were considered negligible. The instrument detection limits were calculated as the concentration equivalent to three times the standard deviation of five replicated analysis of a blank solution (measured on nonconsecutive days). They varied across the entire series from 0.001 ppb (Tm) to 0.03 ppb (Sm). In addition, two 1982 pumice samples (CHI92123 and CHI9366) were analyzed by an acid digestion method with a mixture of HClO4 and HF, followed by ICP–MS analysis for REEs. The data are show in Table 2. These two samples have REE concentrations within the average composition of rocks erupted during the Holocene (Luhr et al., 1984; Espíndola et al., 2000) and Pleistocene (Layer et al., 2009), for which they can be used as representative of the El Chichón host rock.
The precision and accuracy of the analytical method was assessed by comparison of measured and reference values with an international standard reference material (JA–2). The precision was calculated in terms of standard deviation among 10 replicates and for all reported values it was better than 1.3 % RSD. The estimation of the accuracy was assessed in terms of % deviation between reported and experimental results. The results show a good agreement with the reported values and varied between 0.05– 0.51 %.
Chemical analyses of main ions were performed at the Laboratorio de Química Analítica, Instituto de Geofísica, UNAM. Sodium and potassium were measured by atomic emission spectroscopy using a Perkin Elmer 2380. Magnesium and calcium concentrations were obtained by complexometric titration with EDTA. Sulfates were determined by turbidimetry. Chloride was potentiometrically determined with an ion–selective electrode, adding a 5 M solution of NaNO3 as ionic strength adjuster. Fluoride concentrations were determined also with an ion selective electrode, adding a TISAB solution for decomplexing and adjusting the ionic strength. The pH values were measured in the laboratory.
Results and discussion
Chemical analyses results of samples collected from the El Chichón Lake are shown in Tables 1 and 2. The pH of water during the studied period did not show a significant change, it ranged from 2.35 to 2.64. The chemical composition of the main ions has shown variations along the studied period. Sulphate, chloride and fluoride as well as Ca2+, Na+ and Mg2+ content increased from October 2006 to April 2008 (Group 1 to Group 3). Sulphate and fluoride levéis varied slightly from 345.1 to 470.5 mg/L, and from 0.12 a 0.23 mg/L respectively. However, chloride concentration increased noticeable from 8.1 to 240.8 mg/ L. These data are in accordance to the variability of major ions composition reported (Tassi et al., 2003; Taran et al., 2008), related to a very dynamic geothermal activity inside the crater, and not linked to seasonal variations as previously stated by Rowet et al. (2008). Figs. 2, 3 and 4 show a concentration change of main ions ratios with time (SO4–2, Cl–, Na+, Ca2+, Mg2+)This variation disregards the possibility that rain dilution effects might have produced the concentration changes and might be attributed to mixing between different groundwater reservoirs.
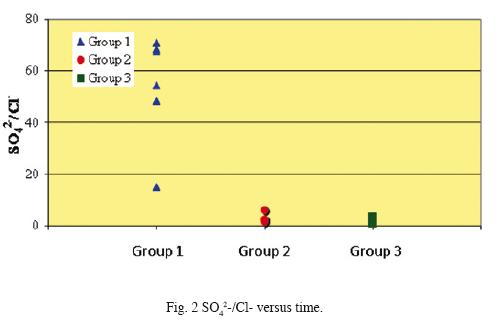
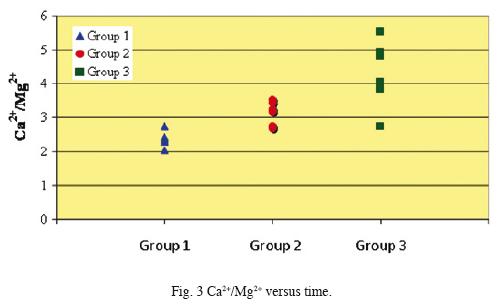
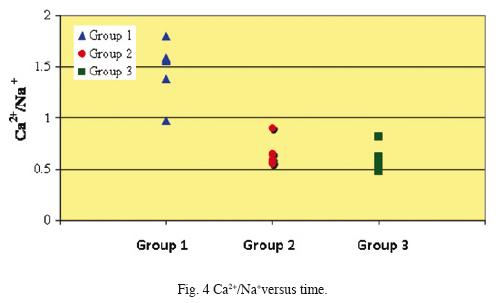
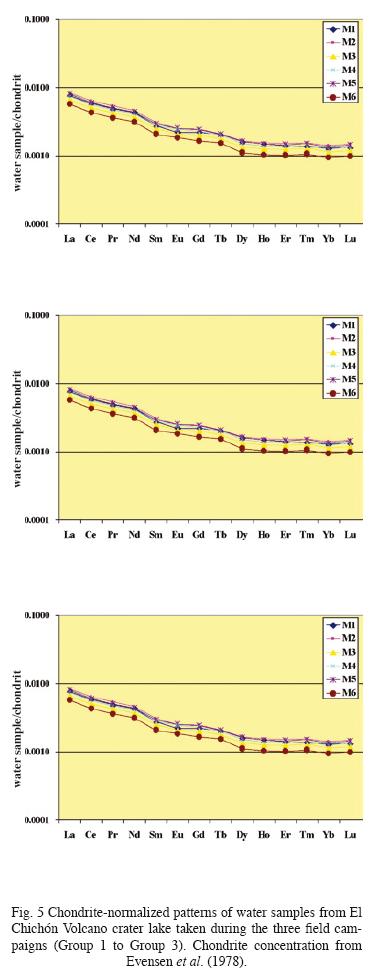
Concentrations of REE in the analyzed water samples ranged from 55.8 ppb (Ce) to 0.01 ppb (Lu). These concentrations fall within the ranges reported for lake waters, acidic groundwaters, and volcanically acidified watershed springs (Wood, 2006; Gammons et al., 2005; Johannesson and Zhou, 1999; Worrall and Pearson 2001.) Fig. 5 shows chondrite–normalized REE of the analyzed fluids samples (Group 1 to Group 3). Groups 1 and 2 show similar REE distribution patterns although Group 2 samples are enriched in all REE. Besides, chondrite–normalized REE from Group 3 show similar REE abundances as Groups 1 and 2, however, these samples present a widest range in concentrations.
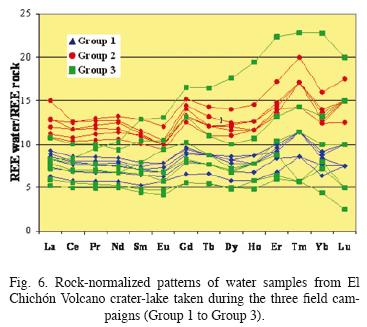
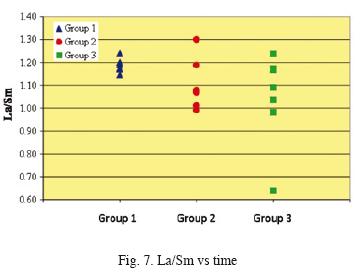
A more detailed view of water/rock interactions is shown in a rock–normalized REE diagram (Fig. 6), where horizontal fluids–REE patterns would represent that crater–lake waters acquire REE from rock without REE fractionation across the entire series. Group 1 patterns are very fíat. Group 2 samples are enriched in HREE with respect to Group 1. The same conclusions may be pursued from Fig. 7 showing that the slope of La/Sm versus time becomes more variable with time.
The similarity of REE patterns between filtered and unfiltered aliquots (Fig. 4) indicates that most of the REE load is probably present in true solution or as fine colloids. Lewis et al. (1997) sustain that REE exist in solution primarily as complexes with halides, sulphate, phosphate, hydroxide, carbonate, or as the free ion, being REE speciation controlled by temperature, pH, salinity and relative abundance of suitable ligands.
The only previously reported REE contents in waters of El Chichón Volcano were presented by Taran et al. (2008) from samples taken in 1998. Those concentrations were determined also in our laboratory. The results of both studies give almost coincident values and show a stable REE behavior since 1998. Taran et al. (2008) also established that concentration of REE in the water corresponded to the complete dissolution of about 1 g of the El Chichón trachyandesite reported by Luhr et al. (1984).
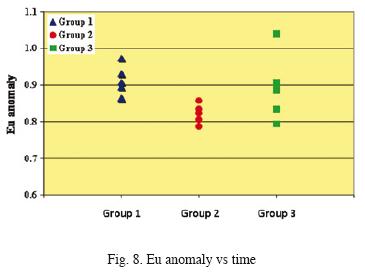
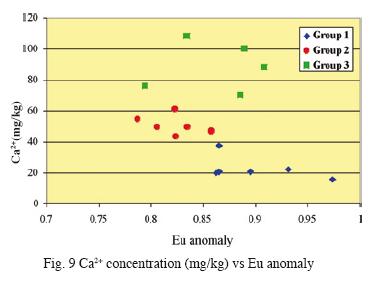
Fig. 8 shows the nature of Eu anomaly as a function of time. Samples of Group 3 present the highest and most dispersed values of the Eu anomaly. Information related with water/rock interaction is given by Fig. 9 and 10. It can be observed a lack of relation between Eu anomaly and Ca+2 concentration. The increase in HREE concentration is directly linked with that of Mg2+.
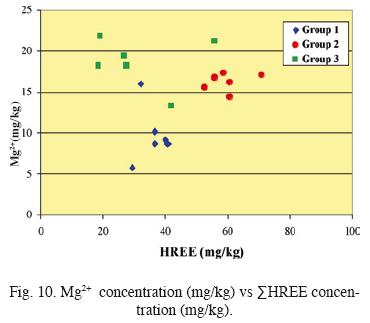
This change in the behavior cannot be correlated with an evident increase in the volcanic activity or with a change on the pH. The assessment of REE signature in El Chichón volcano lake waters, suggests that REE distribution is controlled mainly by the mineralogy of their associated host rock. Luhr et al., (1984) stated that the plagioclase contained in El Chichón trachyandesite has strong LREE enrichment and a positive Eu anomaly. However this enrichment and anomaly were not observed in out study. Our results indicate that probably plagioclase dissolution is not determinant in REE behavior but is linked with an increase in the concentration of complexing agents such as sulphate, chloride or fluoride.
The REE patterns of samples of El Chichón volcano lake determined in this study (Fig 5) do not have a "gull–wing" chondrite–normalized REE pattern (depletion of the lightest REE compared to host rocks) observed by Wood (2006) in geothermal waters from the Taupo Volcanic Zone (New Zealand) with similar pH values, in the range 2–4. On the contrary, the REE behavior of the analyzed samples is similar to hyperacidic lake waters (pH in the range 1.5–2.0), with little REE fractionation across the entire series as reported by Wood (2006). Results of the present study are not comparable either with the acid water of Copahue Volcano, Argentina (Varekamp et al, 2009) because our samples have an enrichment in light REE with respect to Copahue rocks.
Comparison of the REE behavior among diverse geothermal sy stems indicates that the unique characteristics of each of these low–temperature systems (such as the nature of the source rocks, pH and concentration of ligands) are important in determining fluid REE contents and patterns.
Evolution of main ions chemistry indicates that the observed geochemical changes since the lake formation are associated to different kinds of minor activity mainly related with thermal manifestations. This activity has not yet produced changes in the parameters that control the REE behavior as those reported at other geothermal systems. Data collected during a continuous monitoring system will allow us to relate a change in the REE patterns with other premonitory warnings such as an increase of seismicity or deformation, to make a better assessment of the usefulness of REE at El Chichón.
Conclusions
The chemical data has documented an evolution of the hydrothermal system in major element chemistry during the studied period. Unlike the study of Wood (2006), who concluded that crater lake waters over a critical pH (in the range 2–4) tend to fractionate light REEs, our results indicate that lake waters from the El Chichón Volcano have a HREE enrichment with respect to the host rock, probably related with the dissolution of mineral phases rich in these elements. Additionally, the REE trend of recent samples showed a slight Eu anomaly that may be related to the concentration increase of complexing agents such as sulphate, chloride, and fluoride.
The results of this study did not show significant changes in the REE geochemistry during this period and correspond to the observed volcanic stability. However, although it is well known that to achieve a good understanding of the hydrothermal geochemistry of the REE in volcanic systems more research is required, this data can provide valuable information concerning these systems and may be used as an additional tool for active–volcano monitoring.
Acknowledgements
The authors are grateful to Elizabeth Hernández, A. Aguayo, N. Ceniceros and O. Cruz for their skillful chemical determinations. We thank Irma Fabiola Mendiola (Laboratorio de Sedimentología Volcánica, UNAM) and Dr. Jose Luis Macías for providing the rock samples. We also sincerely thank Dr. J.C. Varekamp and two anonymous reviewers that greatly improved this paper. We thank Ángel Gómez (CENAPRED) for providing the lake photography.
Bibliography
Armienta, M. A. and S. De la Cruz–Reyna, 1994. Some hydrogeochemical fluctuations observed in México related to volcanic activity. Appl. Geochem. 10, 215–227. [ Links ]
Armienta, M. A., S. De la Cruz–Reyna and J. L. Macías, 2000. Chemical characteristics of the crater lakes of Popocatetetl, El Chichón, and Nevado de Toluca volcanoes, Mexico. J. Volcanol. Geotherm. Res. 97, 105–125. [ Links ]
Banks, D., G. Hall, C. Reimann and U. Siewers, 1999. Distribution of rare earth elements in crystalline bedrock groundwaters: Oslo and Bergen regions, Norway. Appl. Geochem. 14, 27–39. [ Links ]
Brookins, D. G., 1989. Aqueous geochemistry of rare earth elements, in Lipin, B.R. and McKay, G.A., eds., Geochemistry and mineralogy of rare earth elements: Washington, D. C., Mineralogical Society of America. 201–225. [ Links ]
Casadevall, T. J., S. De la Cruz–Reyna, W. Rose, S. Bagley, D. L. Finnigan and W. Zoller, 1984. Crater Lake and Post– Eruption Hidrotermal activity. J. Volcanol. Geotherm. Res. 23, 169–191. [ Links ]
Damon, P. and E. Montesinos, 1978. Late Cenozoic volcanism and metallogenesis over an active Benioff Zone in Chiapas, Mexico. Arizona Geological Society Digest 11, 155–168. [ Links ]
Espíndola, J. M., J. L. Macías, R. I. Tilling and M. F. Sheridan, 2000. Volcanic history of El Chichon Volcano (Chiapas, Mexico) during the Holocene, and its impact on human activity. Bull. Volcanol. 62. 90–104. [ Links ]
Evensen, N. M. P. J. Hamilton, R. K. O'Nions, 1978. Rare–earth abundances in chondritic meteorites. Geochim. Cosmochim. Acta. 42, 1199–1212. [ Links ]
Gammons, Ch. H., S. A. Wood, F. Pedrozo, J. C. Varekamp, B. J. Nelson, Ch. L. Shope and G. Baffico, 2005. Hydrogeochemistry and rare earth element behavior in a volcanically acidified watershed in Patagonia, Argentina. Chem. Geol. 222, 249– 267. [ Links ]
Haas, J. R., E. L. Shock and D. C. Sassani, 1995. Rare earth elements in hydrothermal systems: Estimates of standard partial molal thermodynamic properties of aqueous complexes of the rare earth elements at high pressures and temperatures. Geochim. Cosmochim. Acta. 59, 4329–350. [ Links ]
Henderson, P., ed., 1984. Rare Earth Element Geochemistry. Elsevier, Amsterdam, 510 pp. [ Links ]
Hopf, S., 1993. Behaviour of rare earth elements in geothermal systems of New Zealand. J. Geochem. Explor. 47, 333–357. [ Links ]
Johannesson, K. H., K. J. Stetzenbach, V. F. Hodge, W. B. Lyons, 1996a. Rare earth element complexation behavior in circumneutral pH groundwaters: Assesing the role of carbonate and phosphate ions. Earth Planet. Sci. Lett. 139, 305–319. [ Links ]
Johannesson, K. H., W. B. Lyons, M. A. Yelken, H. E. Gaudette and K. J. Stetzenbach, 1996b. Geochemistry of rare earth elements in hypersaline and dilute acidic natural terrestial waters: complexation behavior and middle rare–earth element enrichments. Chem. Geol. 133, 125–144. [ Links ]
Johannesson, K. H. and X. Zhou, 1999. Origin of middle rare earth element enrichments in acid waters of a Canadian High Arctic lake. Geochim. Cosmochim. Acta. 63, 153–165. [ Links ]
Johannesson, K. H., X. Zhou, C. Guo, K. J. Stetzenbach and V. F. Hodge, 2000. Origin of rare earth element signatures in groundwaters of circumneutral pH from southern Nevada and eastern California, USA. Chem. Geol. 164, 239–257. [ Links ]
Layer, P. W., A. García–Palomo, D. Jones, J. L. Macías, J. L. Arce and J. C. Mora, 2009. El Chichón volcanic complex, Chiapas, México: Stages of evolution based on field mapping and 40Ar/39Ar geochronology. Geofísica Inetrnacional.48, 33–54. [ Links ]
Lewis, A. J., M. A. Palmer, N. C. Sturchio and A. J. Kemp, 1997. The rare earth element geochemistry of acid–sulphate and acid–sulphate–chloride geothermal systems from Yellowstone National Park, Wyoming, USA. Geochim. Cosmochim. Acta. 61, 695–706. [ Links ]
Luhr, J. F., I. S. E. Carmichael and J. C. Varekamp, 1984. The 1982 eruptions of El Chichón volcano, Chiapas, Mexico: mineralogy and petrology of the anhydrite–bearing pumices. J. Volcanol. Geotherm. Res. 23, 69–108. [ Links ]
Macías, J. L., L. Capra, J. L. Arce, J. M. Espíndola, A. García–Palomo and M. F. Sheridan, 2008. Hazard map of El Chichón volcano, Chiapas, México: Constraints posed by eruptive history and computer simulations. J. Volcanol. Geoth. Res 175, 444–458. [ Links ]
Rouwet, D., Y. A. Taran, S. Inguaggiato, N. Varley and J. A. Santiago Santiago, 2008.Hydrochemical dynamics of the "lake–spring" system in the crater of El Chichon volcano (Chiapas, Mexico). J. Volcanol. Geotherm. Res. 178, 237–248. [ Links ]
Taran, Y., T. P. Fischer, B. Pokrovsky, Y. Sano, M. A. Armienta and J. L. Macias, 1998. Geochemistry of the volcano–hydrothermal system of El Chichón Volcano, Chiapas, Mexico. Bull. Volcanol. 59, 436–449. [ Links ]
Taran, Y., D. Rouwet, S. Inguaggiato, A. Aiuppa, 2008. Major and trace element geochemistry of neutral and acidic thermal springs at El Chichón volcano, Mexico. Implications for monitoring of the volcanic activity. J. Volcanol. Geotherm. Res. 178, 224–236. [ Links ]
Tassi, F., O. Vaselli, B. Capaccioni, J. L. Macías, A. Nencetti, G. Montegrossi and G. Magro, 2003. Chemical composition of fumarolic gases and spring discharges from El Chichón volcano, Mexico: causes and implications of the changes detected over the period 1998–2000. J. Volcanol. Geotherm. Res. 123, 105–121. [ Links ]
Tilling, R. I., 2009. El Chichón's "surprise" eruption in 1982: Lessons for reducing volcano risk Volcano Hazards. Geofísica Internacional 48, 3–19. [ Links ]
Van Middlesworth, P. and S. A. Wood, 1998. The aqueous geochemistry of the rare earth elements and yttrium. Part 7. REE, Th and U contents in thermal springs associated with the Idaho batholit. Appl. Geochem. 13, 861–884. [ Links ]
Verplanck, P. L., D. K. Nordstrom, H. E. Taylor, B. A. Kimball, 2004. Rare earth element partitioning between hydrous ferric oxides and acid mine water during iron oxidation. Appl. Geochem. 19, 1339–1354. [ Links ]
Varekamp, J. C., A. P. Ouimette, S. W. Herman, K. S. Flynn, A. Bermudez, D. Delpino, 2009. Naturally acid waters from Copahue volcano, Argentina. Appl. Geochem. 24, 208–220. [ Links ]
Wood, S. A., 1990a. The aqueous geochemistry of the rare–earth elements and yttrium 1. Review of the available low–temperature data for inorganic complexes and the inorganic REE speciation of natural waters. Chem. Geol. 82, 159–186. [ Links ]
Wood, S. A., 1990b The aqueous geochemistry of the rare–earthelements and yttrium 2. Theoretical predictions of speciation in hydrothermal solutions to 350°C at saturation water vapor pressure. Chem. Geol. 88, 99–125. [ Links ]
Wood, S. A., 2006. Rare earth element systematics of acidic geothermal waters from the Taupo Volcanic Zone, New Zealand. J. Geochem. Explor. 89, 424–42. [ Links ]
Worral, F. and D. G. Pearson, 2001. Water–rock interaction in an acid mine discharge as indicated by rare earth element patterns. Geochim. Cosmochim. Acta. 65, 3027–3040. [ Links ]














