Introduction
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines chronic obstructive pulmonary disease (COPD) as an air flow persistent limitation, generally progressive, associated with an increase in the inflammatory response of the airway and lung, which is a result of emphysema, small airway inflammation, bronchoconstriction, excess mucus, or a combination of these factors.1 Persistent airflow obstruction is required to diagnose COPD, and the criterion used by GOLD is the ratio between two spirometry parameters after bronchodilator administration: forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) lower than 70 %.1
In Latin America, according to the Latin American Project for Research in Lung Obstruction (PLATINO) study, COPD has a prevalence of 7.8 % in Mexico.2
The main risk factors described for the development of COPD include smoking (smoking index > 10, although only 10 to 15 % of smoker patients develop the disease), exposure to biomass combustion-derived smoke (biomass index > 200 hours/year), age > 40 years, and history of exposure to other dusts, fumes or chemical substances.3
When COPD is considered, the development and worsening of comorbidities typical of the pathophysiological changes it entails, and the socioeconomic impact inherent to the prevalence of the disease as a global public health problem, it is essential to initially establish the epidemiological relationship it may have with infectious diseases such as that caused by human immunodeficiency virus (HIV), with which an association has recently been pointed out.4 HIV represents, together with tuberculosis, the infectious disease with the highest distribution in the world and with the greatest impact on morbidity and mortality rates.5
Approximately 9 to 10 % of individuals with HIV infection have some degree of non-reversible airflow obstruction, according to prevalence rates reported in other parts of the world.6 COPD prevalence epidemiological scope in individuals with HIV infection warrants the search for statistical data specific to the population in Mexico, in order to establish specific preventive measures to limit the higher rate of chronic pulmonary complications resulting from the interaction of the two diseases in question.
The purpose of this investigation was to determine the prevalence of COPD in patients diagnosed with HIV referred to an infectology hospital.
Method
A cross-sectional, observational, prolective study was conducted. Patients included in the sample were individuals with documented HIV infection, regardless of the time since its acquisition, without previous or ongoing antiretroviral treatment, with or without a history of exposure for the development of COPD, who had chronic respiratory symptoms suggestive of said disease, in whom active respiratory infection or clinical data suggestive of an infectious process in recent weeks were ruled out, who had no history of known chronic lung disease, and who were able to perform spirometry with acceptability and repeatability criteria in accordance to the American Thoracic Society (ATS) quality standards. Patients who attended the outpatient services of the Mexican Institute of Social Security La Raza National Medical Center Infectology Hospital between March 1, 2016 and August 31, 2016, were included after signing an informed consent document.
Initially, HIV infection diagnosis was corroborated based on patient records. Patient records were reviewed to identify a history of previous infectious diseases with lung involvement that would have not determined any known chronic lung disease. All patients were interviewed and thorough medical history was taken for directed search for respiratory symptoms or history of lung disease.
An expert pulmonologist performed pre- and post-bronchodilator spirometry on the patients using a portable spirometer (Datospir Micro, Sibelmed®, Barcelona), in accordance with ATS standards. Individuals with a post-bronchodilator FEV1/FVC ratio < 0.70, and who in addition did not meet the reversibility criteria after drug application were classified with COPD diagnosis. In addition, CD4+ cells and HIV-1 RNA viral load determination was requested in the laboratory of the Infectology Hospital. As part of the imaging studies, high-resolution computed tomography was performed on all participants at the Radiology Department of La Raza National Medical Center General Hospital, looking for changes consistent with central acinar, panacinar or paraseptal emphysema, defined as low-attenuation areas of less than -950 Hounsfield units (HU) on tomography; the assessment was validated by an expert pulmonologist.
Descriptive analysis of the sociodemographic characteristics, personal history, respiratory symptoms, tomographic findings and spirometry results of the HIV patients was carried out. Qualitative variables were expressed using counts and percentages, while numerical variables were summarized with medians and 25th and 75th percentiles (P25, P75). Wilcoxons test was used to compare spirometric measurements before and after bronchodilator administration.
The prevalence of non-reversible airflow obstruction was calculated with 95 % confidence intervals (CI), assuming a binomial distribution.
To compare the sociodemographic characteristics, personal history, respiratory symptoms, tomographic findings and spirometry results between the subjects who had non-reversible airflow obstruction and those who did not, Fishers exact test (for qualitative variables) and Mann-Whitneys U-test (for quantitative variables) were used.
Finally, a multiple linear regression model was constructed to assess the correlation between post-bronchodilator FEV1/FVC and age, body mass index, smoking index, biomass exposure, viral load and CD4 count. The analysis was carried out with the Stata program, version 13. A p-value < 0.05 was considered to be statistically significant.
Results
Sixty-six HIV-diagnosed patients, without prior antiretroviral treatment, 64 men and two women, with a median age of 31.5 years, were included. Regarding marital status, 84.8 % were single, 13.6 % were married and 1.5 % were cohabitating; 31.8 % had a college degree; 47 %, completed high school, and 19.7 %, secondary school. Weight, height, and body mass index had medians of 65 kg, 1.7 m, and 23 kg/m2, respectively.
As for comorbidities, 39.4 % of the subjects reported previous high blood pressure diagnosis; 2.1 %, diabetes diagnosis; 16.7 %, positive zoonosis, and 10.6 %, positive Coombs test. Regarding the risk factors for the development of COPD, medians for smoking cessation and exposure to biomass were 0.5 and 0, respectively; i.e., no significant history of exposure was identified in individuals with and without airflow obstruction (Table 1).
Table 1 Characteristics of HIV-diagnosed subjects without previous antiretroviral treatment (n = 66)
| Sociodemographic characteristics | n | % |
|---|---|---|
| Men | 64 | 96.9 |
| Women | 2 | 3.03 |
| Comorbidities | ||
| High blood pressure | 26 | 39.4 |
| Diabetes | 8 | 12.1 |
| Obesity (BMI > 30) | 5 | 7.5 |
| Median | ||
| Age (years) | 31.5 | |
| Smoking index | 0.5 | |
BMI = body mass index, COPD = chronic obstructive pulmonary disease.
As regards characteristics related to HIV infection, a median of 18 years was obtained for the initiation of active sexual life, and regarding the number of sexual partners, the median was 10. Time elapsed since diagnosis was estimated in 0.8 years (Table 2).
Table 2 Subject characteristics related to HIV infection.
| HIV infection | Median | |
|---|---|---|
| HIV evolution time (years) | 0.8 | |
| Median | IQR | |
| CD4 count (cells/μL) | 203.5 | 111-358 |
| Viral load (copies/μL) | 65 369 | 3165-160 000 |
IQR = interquartile range, SD = standard deviation.
The most common respiratory symptoms referred by subjects with HIV were chronic cough (> 2 weeks of evolution) by 10.6 %, dyspnea according to the Modified Medical Research Council (mMRC) scale by 9.1 %; expectoration by 1.3 % and wheezing by 1.5 %. These symptoms occurred both in subjects with airflow obstruction and in those with normal spirometry, without predominance in either group.
As one of the secondary endpoints, high-resolution computed tomography was performed; however, only 28.8 % of the subjects could be assessed using this imaging test. No changes suggestive of emphysema or structural alterations associated with COPD were found, and neither were other data consistent with interstitial or alveolar involvement.
Spirometry
No significant decreases in spirometry values were observed in the post-bronchodilator versus pre-bronchodilator measurement, without the individuals who showed airflow obstruction being considered separately (Table 3).
Table 3 Spirometry results in subjects diagnosed with HIV without prior antiretroviral treatment
| Spirometry measurement | Pre-bronchodilator | Post-bronchodilator | p |
|---|---|---|---|
| FEV1/FVC | 82.5 (74.8) | 82.9 (76.8) | 0.053 |
| FEV1 | 109 (86.1) | 108.5 (88.1) | 0.812 |
| Forced vital capacity (FVC) | 108 (87.1) | 106.5 (89.1) | 0.526 |
| FEF25-75 | 105.5 (76.1) | 104 (77.1) | 0.391 |
| PEF | 119 (82.1) | 115 (82.1) | 0.138 |
Data presented as the median (interquartile range). p-value using Wilcoxons test. *p < 0.05. FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity,
PEF = peak expiratory flow, FEF25-75 = forced expiratory flow measured between 25% and 75% of the forced expiratory maneuver.
When the spirometry results were assessed, COPD prevalence was observed in five cases, i.e., in 7.6 % (95 % CI = 2.5-16.8). Regarding the five patients who had airflow obstruction (FEV1/FVC ratio < 70), four had a FEV1 higher than 80 %, i.e., they were classified with GOLD grade 1 obstruction (mild obstruction); the other patient had FEV1 values between 50 and 80 % of predicted value, and thus he was classified with GOLD grade 2, i.e., with moderate obstruction. Similarly, in three individuals (1.98 %) a decrease in mean expiratory flow could be corroborated, which is defined by a measured flow between 25 and 75 % of the forced expiration maneuver (FEF25-75) lower than 60 % of predicted value.
When a separate analysis of groups with and without airflow obstruction was carried out, no differences were found regarding Coombs, zoonosis, diabetes, hypertension, smoking index, exposure to biomass, onset of active sexual life, number of sexual partners, time of HIV infection evolution or presence of opportunistic infections. However, the group with non-reversible airflow obstruction had a lower CD4 count (27.3 versus 225.9) and higher viral load (165,000 versus 57,722) than the group without obstruction (Fig. 1).
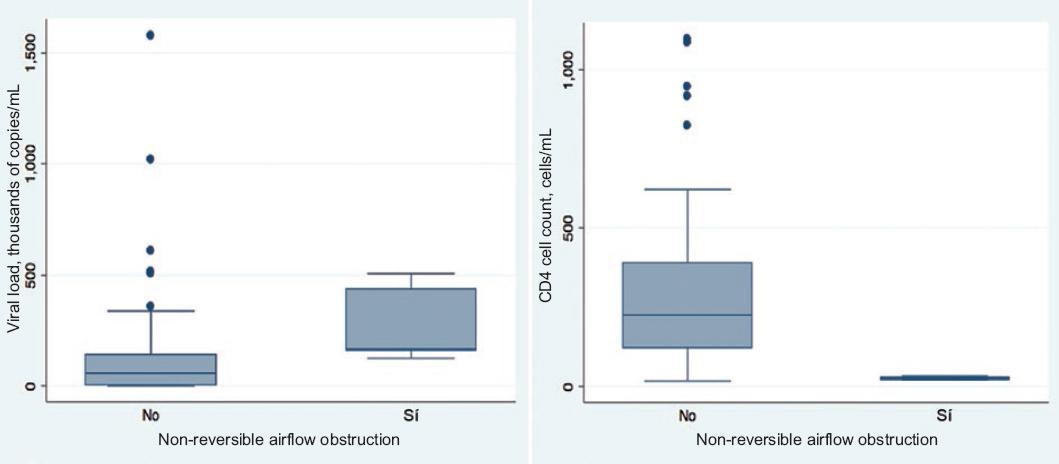
Figure 1 Viral load and CD4+ cell count in subjects diagnosed with HIV without prior antiretroviral treatment, according to the presence of non-reversible airflow obstruction.
Similarly, no differences were found in respiratory symptoms (cough, dyspnea, expectoration or wheezing) or in tomographic findings between subjects with and without non-reversible airflow obstruction. Patients with obstruction had a median for the FEV1/FVC ratio = 69.3 (P25, P75 = 68.3, 69.9) in the pre-bronchodilator measurement and 69.3 (P25, P75 = 69, 69.8) in the post-bronchodilator maneuver (Table 4). On the other hand, subjects without obstruction had a median of 83.2 (P25, P75 = 77.7, 88.1) and 83.3 (P25, P75 = 78.6, 88.2), respectively. As for FEV1/FVC for patients with obstruction, medians were 78 (P25, P75 = 76, 82) and 79 (P25, P75 = 77, 80) in the pre- and post-bronchodilator measurements; in the individuals without obstruction, medians of 111 (P25, P75 = 98, 135) and 113 (P25, P75 = 96, 136) were recorded pre- and post-bronchodilator, respectively. That is, the subjects with obstruction had lower medians in all pre- and post-bronchodilator spirometry measurements; however, lower spirometry values were not related to an increase in respiratory symptoms, presence of areas with decreased attenuation or structural alterations on high-resolution chest tomography.
Table 4 Spirometry results in subjects diagnosed with HIV without prior antiretroviral treatment, according to the presence of non-reversible airflow obstruction
| Spirometry measurement | Non-reversible airflow obstruction (n = 5)* | Without non-reversible airflow obstruction (n = 61)* | p |
|---|---|---|---|
| Pre-bronchodilator | |||
| FEV1/FVC | 69.3 (68.3,69.9) | 83.2 (77.7, 88.1) | < 0.001 |
| FEV1 | 78 (76, 82) | 111 (98, 135) | 0.007 |
| Forced vital capacity | 86 (86, 87) | 109 (91, 124) | 0.022 |
| FEF25-75 | 68 (59, 69) | 107 (79, 141) | 0.006 |
| PEF | 76 (74, 82) | 121 (84, 151) | 0.008 |
| Post-bronchodilator | |||
| FEV1/FVC | 69.3 (69, 69.8) | 83.3 (78.6, 88.2) | < 0.001 |
| FEV1 | 79 (77, 80) | 113 (96, 136) | 0.005 |
| Forced vital capacity | 84 (84, 88) | 108 (94, 124) | 0.015 |
| FEF25-75 | 67 (62, 69) | 108 (78, 138) | 0.005 |
| PEF | 78 (75, 82) | 121 (85, 149) | 0.011 |
*Data presented as medians and percentiles (P25, P75). p-value using Fishers exact test or Mann-Whitneys U-test. p < 0.05.
Finally, a multiple linear regression model was constructed to assess the correlation between post-bronchodilator FEV1/FVC and age, body mass index, smoking index, exposure to biomass, viral load and CD4 count. A correlation was observed between viral load and FEV1/FVC measurement: the lower the viral load, the higher the FEV1/FVC ratio. No correlation was observed between age, body mass index, smoking index or CD4 cell count (Table 5).
Table 5 Correlation between selected variables and post-bronchodilator FEV1/FVC spirometry measurement
| Characteristic | Coefficient | Standard error | p |
|---|---|---|---|
| Age, years | −0.2457 | 0.088 | 0.007* |
| Body mass index | −0.4758 | 0.2569 | 0.069 |
| Smoking index | 0.1061 | 0.2837 | 0.710 |
| Exposure to biomass | −0.3364 | 0.1084 | 0.003* |
| CD4 count (cells/μL) | 0.0027 | 0.0037 | 0.466 |
| Viral load (copies/μL) | −0.000008 | 0.000004 | 0.044* |
The coefficient and standard error values of the multiple linear regression model are shown.
*p < 0.005
Discussion
In our study, a prevalence of spirometry-measured non-reversible airflow obstruction consistent with chronic obstructive pulmonary disease diagnosis of 7.6 % was identified. These findings contrast with the report by Kunisaki et al.7 of an international study that included 1026 patients from 20 countries: the combined prevalence for Mexico and South America of HIV patients without previous exposure to antiretroviral therapy was 2.7 and 3.3 %, according to the GOLD criterion, with a lower limit of normality; estimated global prevalence was 5.5 %. This study only included individuals with a CD4 count > 500 cells/μL, whereas, in our research, a specific CD4 count or viral load was not considered as an inclusion criterion.
We were able to document that individuals diagnosed with COPD had a lower CD4 count (27.3 versus 225.9 cells/μL) and higher viral load (165,000 versus 57,722) in comparison with subjects without airflow obstruction. The observation regarding the CD4 count is consistent with Crothers et al. observations in a cohort study8 where a prevalence of COPD of 10 % was also estimated in subjects with HIV, regardless of previous exposure to antiretroviral therapy.
Regarding viral load, in an analysis of a larger sample from the Veterans Ageing Cohort Study carried out by Crothers,9 a significantly higher incidence of COPD was found in patients with higher viral load. However, after constructing a multiple linear regression model, we did not find a significant correlation for CD4 count or viral load with regard to the FEV1/CVF ratio in our group.
As for the reported respiratory symptoms, 10.6 % of the subjects had chronic cough (> 2 weeks of evolution), 9.1 % had dyspnea according to the mMRC scale; 3 %, hyaline-looking expectoration, and only 1.5 %, wheezing. Prevalences are very low when considering the findings of Díaz et al.10 in a cohort of 327 HIV patients without previous pulmonary complications: up to 42 % reported having dyspnea and 40 % had cough; in that study, up to 52.2 % of HIV patients were active smokers, with a mean of 12.2 packs/year, which might explain the high proportion of respiratory symptoms. In our sample, none of the subjects had a significant smoking or biomass index.
High-resolution tomography was carried out in 19 patients (28.8 %); in none of them were areas with decreased attenuation less than -950 HU observed, which are consistent with emphysema. It should be noted that Gelman et al.11 carried out chest CT scans at inspiration and expiration on 48 HIV-positive and 11 HIV-negative subjects, by means of which they found that up to 62.5 % of HIV-positive subjects had focal air trapping in comparison with 27.3 % of HIV-negative subjects; considering that the smoking index medians, expressed as packs/year, were 14.36 versus 18.59, respectively, the prevalence of emphysematoid changes was higher in the group of subjects with HIV. The significant tobacco consumption index in said sample might explain the discrepancy with regard to our findings: none of the subjects in whom a tomography was performed had the described changes.
On the other hand, no differences were found between the groups with and without non-reversible airflow obstruction with regard to a history of Coombs, zoonosis, chronic degenerative diseases, smoking index, exposure to biomass, onset of active sexual life, number of sexual partners, HIV infection evolution time and opportunistic infections. This can be explained because, particularly, the frequency of exposure history was very low in the subjects included in the sample.
Among the strengths of our study we can mention the following:
− Although the sample was calculated in a single reference center, we did not identify a previous estimation of the prevalence of chronic obstructive pulmonary disease in HIV patients in the country. Our research was the first to assess the lung function in patients with no previous exposure to antiretroviral therapy, regardless of CD4 count or viral load at the time of inclusion.
− Drummond et al.12 reported the use of antiretroviral treatment as an independent factor associated with a higher prevalence of obstructive pulmonary disease, and for this reason this confounding variable was eliminated by including only naive patients in our sample.
− In addition to the basic parameters for interpreting spirometry, evaluation of mean expiratory flows was included to assess incipient changes in the small caliber airway. With this, a decrease below 60 % of predicted value was detected in 1.98 % of patients, which could suggest a significant change in the obstructive type that is not yet reflected in FEV1/FVC ratio or FEV1. This partially coincides with the observations reported by Onyedum,13 who found that FEF25-75 values were significantly lower in HIV-positive men and women in comparison with HIV-negative controls; however, the proportion of HIV patients who show an alteration in this spirometry parameter below a specific predicted value has not been established. A FEV1/FVC ratio < 0.7 was used as a parameter to establish the diagnosis, which allowed the criteria to be homogenized with regard to other published studies.
Our investigation has certain limitations, the first of which being that median age of the sample was only 31.5 years, which might underestimate the prevalence of COPD, which tends to occur more frequently in subjects older than 40 years, with an increase in incidence being recorded as age increases. On the other hand, the research was carried out in a reference center for patients with HIV, which could overestimate the prevalence of chronic lung disease, tentative CD4 lower count and higher viral load in subjects referred for treatment at the time of inclusion.
Multiple risk factors for the development of COPD have been described, among which, in order of importance, exposure to tobacco smoke, fumes derived from biomass combustion, industrial gases, etc., stand out. However, the role of HIV infection as an independent factor for the development of COPD has recently been described, in which common pathophysiological pathways that compromise protease/anti-protease balance, metalloproteinases activation, increased oxidative stress and free radical formation are implicated.
The results of our study are correlated with those of Ronit et al.,14 who documented HIV as a risk factor for a decrease in FEV1 and FVC unrelated to smoking or low socioeconomic status. Considering our findings, several questions arise, especially regarding the potential differences in the rate of decline in the lung function, general behavior of the disease, and exacerbation characteristics in subjects with COPD related to HIV infection and that are classically associated with tobacco and combustion-derived fumes. Some questions that require further research are: which one of them has better prognosis? Which one is associated with better treatment response? Do the deleterious effects on the lung parenchyma and airway increase as CD4 count decreases and viral load increases?
Furthermore, our research suggests the need for an assessment with routine respiratory function tests in patients with HIV at the time of diagnosis and periodically during their follow-up since, in the subjects of our sample, a decrease in spirometry parameters was recorded, even without significant respiratory symptoms. Therefore, early identification and management of incipient structural alterations that cause a decrease in air flow might decrease disease progression and, therefore, intervene on pathophysiological pathways that perpetuate damage in small and large caliber airways.











 nueva página del texto (beta)
nueva página del texto (beta)


