Introduction
Measles is a febrile exanthematous disease of mandatory surveillance in Mexico in accordance with Mexican legislation and regulations.1,2 The disease is caused by the measles virus, which belongs to the Morbilivirus genus of the Paramixoviridae family.3 Measles receptors are expressed in many cell types, which explains its systemic symptomatic expression. Transmission occurs by propagation of droplets or direct contact with airborne nasopharyngeal secretions when an infected person coughs or sneezes. Up to 90 % of susceptible people in direct contact with the secretions deriving from coughing and sneezing, or with the air or surfaces of the room where the patient is staying, can become infected in that period.4
Initial symptoms include fever, cough, nasal discharge and conjunctivitis, enanthem, maculopapular rash that starts in the head and spreads to the trunk and extremities. It can cause from mild symptoms to serious complications such as diarrhea, pneumonia, fever-associated seizures, corneal scars that result in blindness, encephalitis and death. Encephalitis occurs in one of 100,000 cases and deaths in one to three in 1000; younger than five years and immunocompromised individuals are the most affected. Six to eight years after the condition, one in every 100,000 cases may develop subacute sclerosing pan-encephalitis as a complication due to the persistence of the virus in the cerebrospinal fluid.5
Incubation period is 10 to 12 days; the prodromes begin after exposure. The rash appears 14 days after the initial exposure, within a range of 7 to 21 days. Patients are contagious four days prior to the onset of exanthema and four days after its appearance. Immunocompromised patients are contagious throughout the disease.5 The National Epidemiological Surveillance System defines a “probable case” of measles or rubella as any person of any age with fever and maculopapular rash and one or more of the following signs and symptoms: cough, coryza, conjunctivitis or adenomegalies (retroauricular, occipital or cervical); and a “confirmed case” of measles or rubella as any probable case where measles or rubella virus infection is demonstrated by laboratory techniques recognized by the Institute of Epidemiological Diagnosis and Reference “Dr. Manuel Martínez Báez” (InDRE), or a probable case that does not have a sample or laboratory result and that is epidemiologically associated with another laboratory-confirmed case.6
In Mexico, the Universal Vaccination Program has the triple viral vaccine as a specific protection measure for measles, rubella and mumps. The first dose is applied at 12 months of age. Although, in general, 90 to 95 % of vaccinated individuals respond adequately, a second dose is applied at six years of age in order for those who had an immune response failure to adequately develop antibody protective levels.5
In September 2016, the Pan American Health Organization and the World Health Organization declared the region of the Americas the first in the world to be free of measles after 22 years of coordinated efforts within the member states. Measles became the fifth vaccination-preventable disease to be eliminated, after the eradication of smallpox, polio, rubella and congenital rubella syndrome.7 However, in 2017, an outbreak of measles occurred in Venezuela, which spread to Brazil and Colombia in 2018,8 deriving from migration to these countries. The same year, Argentina, Chile, Ecuador and Peru reported imported cases related to these countries. By the end of 2018, Brazil, Venezuela, the United States, Peru, Mexico, Colombia, Chile, Canada, Ecuador, Argentina, Guatemala, Antigua and Bermuda reported 16,514 confirmed cases.9
By September 29, 2019, the Ministry of Health had a total of 16 confirmed cases reported on the federal government official website,10 four of them classified as imported, two under investigation and the rest associated with importation.11
Laboratory diagnosis
As the national reference laboratory certified by the Pan American Health Organization/World Health Organization, the InDRE belongs to the World Network of Laboratories for the Diagnosis of Measles and Rubella since 1992. That same year, the National Network of Laboratories for Etiological Diagnosis of Febrile Exanthematous Disease started operating in Mexico. Currently, the National Network of Public Health Laboratories (RNLSP – Red Nacional de Laboratorios de Salud Pública) is integrated by 31 state public health laboratories and the Central Epidemiology Laboratory of the Mexican Institute of Social Security. In order to guarantee the quality of epidemiological information and appropriate adoption of control actions, the laboratory system is assessed using international standards.
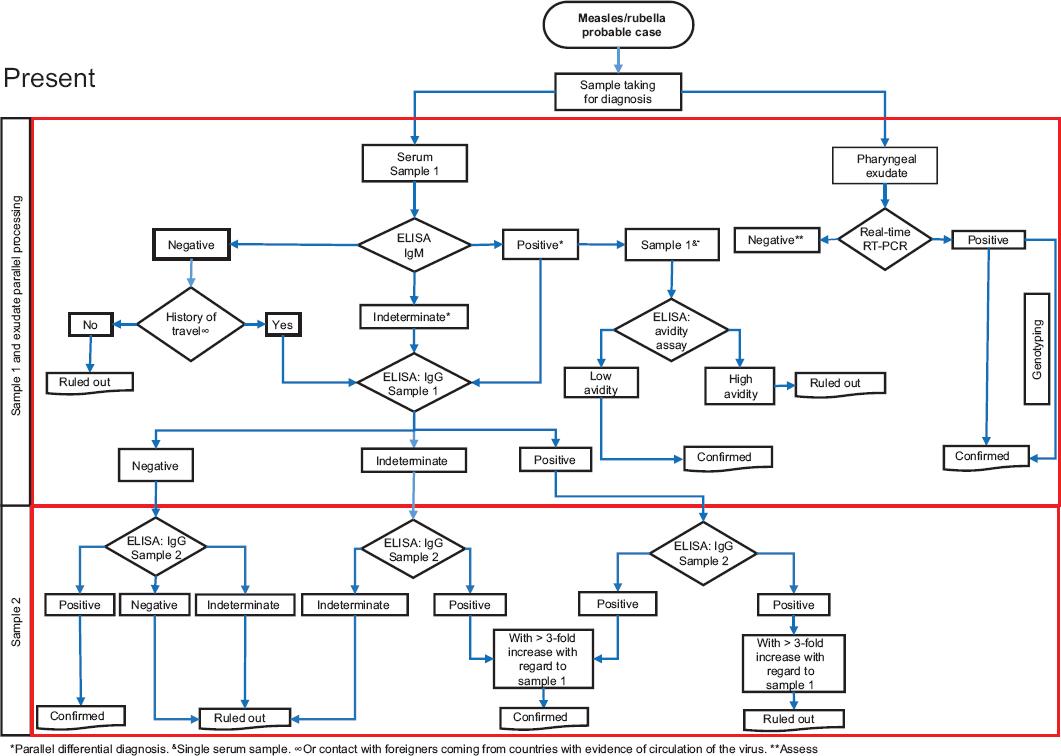
The diagnostic algorithm and procedures issued by the InDRE, through the Laboratory of Febrile Exanthematous Disease, are established in the Guidelines for laboratory monitoring of febrile exanthematous diseases,3 approved by the National Committee for Epidemiological Surveillance and of mandatory observance for the RNLSP (Figure 1).
Sample collection, handling and submission
The samples for the diagnosis of measles, rubella and congenital rubella syndrome are serum and pharyngeal exudate. Both must be mandatorily and simultaneously taken, in order to carry out serological and molecular detection, for the confirmation of cases and genotype identification. The samples must comply with the operational definition of measles/rubella probable case or congenital rubella probable case and with the evolution days to be accepted in the processing laboratory.
Sample collection and handling
Serum
In the period from zero to 35 days of evolution from the date of exanthema onset, 5 mL of blood should be obtained by venous puncture, using a vacuum-extraction plastic tube, without anticoagulant, with or without separating gel. Once the sample is taken, the tube should be labeled with the patient’s name, type of sample and date, and stored at between 2 and 8 °C. It is necessary to separate the serum in a plastic tube with screw closure and identify it with patient and sample data. The volume of serum required is 1 mL. In newborns, 1 mL of blood should be obtained by venous puncture, as the volume required in these cases is 100 to 200 µL. If a second sample is required for follow-up of the case, it should be obtained two weeks after the first one, within 30 days after the onset of exanthema.
Pharyngeal exudate
It should be obtained within the period of 0 to 5 days of evolution from the date of exanthema onset; the posterior wall of the pharynx is rubbed with a sterile dacron or rayon swab in order to detach epithelial cells. The swab is placed in 2 mL of sterile viral transport medium contained in a plastic tube with screw closure, labeled with the patient’s name, date of sampling and type of sample.
Sample submission and transportation
All biological specimens should be transported in a triple packaging system, under refrigeration conditions (2 to 8 °C). The administrative conditions for submitting and receiving samples are described in detail in the Guidelines for laboratory surveillance of febrile exanthematous disease.3 This document describes the conditions whereby a sample could be rejected due to incidents in the process of taking, handling and submission.
Diagnostic algorithm
In the context of measles, rubella and congenital rubella syndrome elimination sustainability, laboratory confirmation with an algorithm standardized for RNLSP is essential.12 Serum samples and pharyngeal exudate are processed in parallel, and must comply with the definition of probable case and days of evolution. All sera are analyzed by enzyme-linked immunosorbent assay (ELISA) for measles/rubella, while all pharyngeal exudates are analyzed by reverse transcription coupled to polymerase chain reaction (RT-PCR), in real time for both diagnoses (Figure 1).
The RNLSP carries out the serological diagnosis for identification of specific IgM and IgG antibodies, both for measles and for rubella, in addition to molecular diagnosis by real-time RT-PCR. The Institute of Epidemiological Diagnosis and Reference carries out the determination of IgG antibodies by avidity tests for measles and rubella (to differentiate primary and secondary immune responses), in addition to virological methods (viral isolation) and genetic characterization of the virus (genotyping), if the sample has an adequate quality.
In countries that have achieved elimination, such as Mexico, if a sample has an IgM-negative result for measles or rubella and the patient has a history of travels to countries with evidence of transmission of the virus, or contact with a person of foreign origin who comes from any of these countries, a second sample should be obtained to look for any increase in IgG antibodies. In samples with an IgM-positive result for measles or rubella, a second sample is also required to look for seroconversion and increase in IgG-specific antibody titers.
Quality standards of the test
Timeliness and quality in the taking and submission of samples, until the reception in the laboratory, are determinant to the reliability of the result. The laboratory guidelines describe the factors that affect the pre-analytical, analytical and post-analytical phase, the timing of the issuance of the result for the epidemiological surveillance system and for interested parties.
Recommendations
It is important for health personnel to know the case operational definitions for febrile exanthematous disease, which will facilitate a proper process of diagnostic confirmation by ensuring adequate collection, handling and submission of samples. The InDRE and the RNLSP have the technical and professional resources to establish a reliable and timely diagnosis and to support the actions of disease prevention and control.











 nueva página del texto (beta)
nueva página del texto (beta)