Introduction
Morphea, also known as localized scleroderma, is a chronic inflammatory disease of the dermis and subcutaneous cellular tissue in which a sclerotic process with diffuse thickening and induration of the skin is generated, as well as atrophy at various levels.1-3 Usually, its evolution is benign; however, 10 % of patients suffer severe sclerosis and atrophy, which can cause deformity, contractures or growth disorders.2,3
Morphea is considered an entity that is different to systemic sclerosis and does not evolve into it, although both share physiopathogenic factors. Often, patients with morphea can have extracutaneous involvement, mainly ocular, articular and central nervous system compromise.2
Diagnosis and management of patients with morphea is not always easy and can be a challenge for the dermatologist. In this article, the basic concepts of the disease are reviewed, with special focus on the diagnosis, classification and treatment.
Basic concepts
Morphea is relatively uncommon, with an annual incidence of 2.7 per 100,000 population.4 It predominates in women, with a ratio of 2.6:1;5,6 and although it affects all ethnicities, it predominates in the Caucasian population. In 75 to 90 % of patients it occurs between the ages of 20 and 50 years.1 It is possible that due to the delay in diagnosis, the true prevalence of morphea is underestimated.3
Morphea etiology and pathogenesis are not yet fully understood. Autoimmune, environmental factors and various events that trigger the production of cytokines are thought to be involved, including infections, radiation or trauma.3,7 Borrelia burgdorferi infection might have a pathogenic role; however, a causal association has not been confirmed.7 There is an unproven hypothesis that morphea may be due to post-cygotic mosaicism by the blaschkoid or dermatomic distribution of many lesions8 (Fig. 1).

Figure 1 Patient with generalized morphea, with multiple indurated and hyperpigmented plaques with blaschkoid distribution.
The skin sclerosis process comprises three main events, which can be therapeutic targets:
– A primary microvascular lesion, with SVCAM-1 and sE-selectin elevation, which are indicators of endothelial activation; as well as expression of adhesion molecules, thickening of the basement membrane and intimal hyperplasia.9
– Fibroblast function control by perivascular CD4+ T cells that produce interleukin-4 and transforming growth factor b, which are cytokines that direct differentiation towards the Th2 phenotype, recruit eosinophils (which can be found at morphea early stages) and modify the synthesis of collagen by fibroblasts.10
– Pathological production of collagen (I, II, III) by fibroblasts, as well as other extracellular matrix proteins.3
Classification and clinical presentation
Morphea includes a variety of conditions where the skin or subcutaneous cell tissue are affected by sclerosis. Classification can guide the treatment and prognosis; however, it is not simple, because the different types are not always clear and overlap.11 The current accepted classification is detailed in Table 1.12
Table 1 Current classification of morphea according to the European Society of Pediatric Rheumatology, 200469
| Subtype | Modality | Clinical characteristics |
|---|---|---|
| Circumscribed or plaque morphea | Superficial | Single or multiple round- or oval-shaped lesions limited to the epidermis and dermis. |
| Deep | Single or multiple round- or oval-shaped lesions that involve subcutaneous, fascia or muscle. | |
| Linear morphea | Trunk/extremities | Linear lesions involving the dermis and subcutaneous or deep tissue. |
| Head | Linear lesions in the head, may involve underlying bone; either "en coup de sabre" type or progressive hemifacial atrophy. | |
| Generalized morphea | 4 or more plaques, in 2 or more anatomical sites. | |
| Disabling pan-sclerotic morphea of childhood | Circumferential lesion of most of the body surface area (sparing finger and toe tips) that affects the skin, subcutaneous cell tissue, muscle or bone. No visceral involvement. | |
| Mixed morphea | Combination of any of the subtypes. |
Morphea can affect any part of the body and is characterized by one or several well-defined plaques of varying sizes of thickened, bright, atrophic skin; either hyper or hypopigmented. The lesions begin insidiously as erythematous-edematous or violet plaques that spread centrifugally; they are usually asymptomatic and go unnoticed by the patient. As they evolve, they start to become sclerotic, indurated tissue, acquire a bright white color and, in the periphery, violet rings are appreciated, which disappear when progression of the lesion stops and give rise to residual pigmentary changes (Fig. 2). They evolve chronically until atrophy reaches various levels: epidermis, dermis or subcutaneous cellular tissue. Sometimes, different clinical forms are combined in one single patient. Activity of the lesions lasts from three to five years, with a tendency to remission; however, there may be recurrences. In children, more severe, mutilating and disabling forms occur, such as disabling pan-sclerotic morphea of childhood.3,13,14

Figure 2 A) Erythematous edematous plaque in the paramedial line of the face that corresponds to an initial linear morphea lesion. B) The same lesion several months later, where sclerosis, atrophy and loss of annexes is evident.
Linear morphea is the most common variant during childhood or adolescence; it begins at this stage in 40 to 70 % of patients.11 It mainly affects the extremities, the head in the frontal area (morphea en coup de sabre) (Fig. 2) and the chest. It is accompanied by loss of adnexal structures, pruritus and xerosis. Nearly 50 %1 of patients with this type of morphea also have plaque lesions (mixed morphea), which is common in children and rare in adults.5,6,15 Up to 30 % of patients with linear morphea can experience extracutaneous involvement with ophthalmological or neurological disorders such as migraines, headaches, seizures or uveitis; or musculoskeletal disorders in patients with limb involvement.15,16
Circumscribed or plaque morphea is the most common type in adults and the most superficial form due to the limitation of the fibrotic process to the dermis. It is characterized by circumscribed or oval, single or multiple, 1- to 30-cm sclerotic plaques (Fig. 3).3,13

Figure 3 Plaque morphea in the trunk. Epidermal and dermal superficial atrophy with visible vessels.
Generalized morphea is a severe form of the disease, defined as four or more plaques larger than 3 cm that converge and involve more than two anatomical sites (Fig. 1). It occurs in 7 to 9 % of patients with morphea and is characterized by rapid plaque spread.13 It is usually not associated with extracutaneous manifestations.11
Pan-sclerotic morphea (also called childhood disabling morphea) is an aggressive and mutilating variant that most often affects children. Presentation is rapidly progressive and deteriorates deep structures such as muscles, tendons and bone by generating joint contractures, painful ulcerations and calcifications that lead to significant disability (Fig. 4).14,17
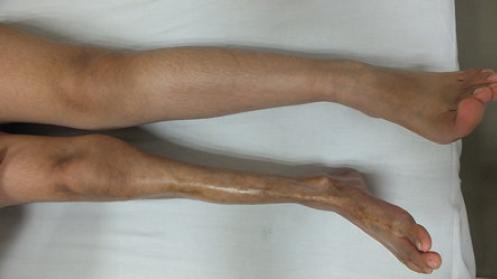
Figure 4 Pan-sclerotic morphea of childhood in left lower extremity. Note the circumferential and deep involvement of sclerosis and atrophy.
Some less common variants of morphea are described in Table 2.3,5,14,16,18,19
Table 2 Morphea less common variants1,11,19
| Morphea guttata | Sclero-atrophic whitish small lesions that lack the firm characteristics of morphea, with dilated follicular orifices. |
| Nodular (keloid) morphea | Nodular lesions resembling keloids. |
| Atrophoderma | Multiple subtly depressed, oval-shaped and hyperpigmented plaques appear on the trunk and upper limbs, without the presence of sclerosis. |
| Parry-Romberg syndrome or progressive facial hemiatrophy | Uniform atrophy of the dermis, subcutaneous and muscular cellular tissue and, in some cases, bone, in a hemifacial or trigeminal distribution. It is associated with neurological symptoms in up to 30% of cases. |
| Eosinophilic fasciitis or Shulman syndrome | Of sudden onset, with bilateral painful induration exclusively of the extremities. It is accompanied by peripheral eosinophilia and increased erythrocyte sedimentation. It is rare in childhood. |
| Lichen sclerosus et atrophicus | White or pale purple-colored atrophic plaques with a shiny appearance. It frequently affects the anogenital area, but can occur in any area of the body. Associated with circumscribed and generalized morphea. |
Diagnosis
The diagnosis of morphea is mainly clinical, through history and careful physical examination of the skin. The interogation of the patient about evolution of the lesions or comparison with previous photographs is quite helpful, as well as identifying the different morphology patterns: plaque or circumscribed, linear, generalized, superficial or deep.19-22
Laboratory tests
Laboratory tests are not considered necessary for the diagnosis or follow-up of patients with morphea. Erythrocyte sedimentation rate and blood count are within normal ranges in most cases, but eosinophilia may appear, especially at early stages of the disease. The presence of antinuclear antibodies or anti-double-stranded anti-DNA and anti-histone antibodies occurs in approximately 30% of patients, mainly in those with linear or generalized morphea.5,6,13,15,23
Due to the frequency of extracutaneous alterations, especially in patients with linear morphea, it is important to deliberately look for them through patient interrogation and consultation with the ophthalmology, neurology or orthopedics departments, in addition to relevant paraclinical tests. Some authors recommend performing brain magnetic resonance imaging and routine ophthalmological examination in patients with linear morphea in the head.16
Histopathology
Morphea histology of depends on two factors: the stage of the disease and the depth to which it extends. At early stages, perivascular lymphocytic inflammatory infiltrate, endothelial edema and, sometimes, eosinophils, plasma cells and mast cells are found, as well as skin appendageal atrophy and vessels with thickened walls and narrow lumen (Fig. 5).24 At later stages, the inflammatory infiltrate decreases or disappears, and loss of appendages, flattened dermo-epidermal junction and collagen bundles in the reticular dermis are observed, with a packaged appearance and alignment parallel to the dermo-epidermal junction.24 Deep morphea primarily affects the subcutaneous cellular tissue, and produces extensive sclerosis and hyalinization, which extends to the underlying fascia.25
Evaluation of morphea activity and severity
Assessing the activity of the disease is extremely important for making therapeutic decisions; however, it is not always easy, since currently there are no objective, clear or objective standardized outcome measures.26 The factors and characteristics referred to in Table 3 may be helpful in determining the activity of a morphea plaque.1,3,27
Table 3 Useful characteristics and factors to assess morphea activity and severity27,30
| Appearance of new lesions or expansion of existing lesions in the last 3 months. |
| Erythema or moderate or severe edema in the lesions. |
| Erythema/violet borders of lesions. |
| Induration or progressive increase or sclerosis in the lesion. |
| Hair-loss associated with the lesions. |
| Documentation by the physician of disease activity or progression to deep tissues, through activity scales, photographs, magnetic resonance imaging or ultrasound. |
| Skin biopsy demonstrating disease activity. |
Some scales have been developed to assess disease activity, such as the Localized Scleroderma Severity Index (LoSSI,) and the Localized Scleroderma Damage Index (LoSDI); together, they make up the Localized Scleroderma Cutaneous Assessment Tool (LoSCAT), which has been validated (Table 4).28,29 The DIET scale is another tool that has been proposed to assess morphea plaque; however, it has not been validated (Table 5).27
Table 4 Localized Scleroderma Cutaneous Assessment Tool (LoSCAT)
| mLoSSI (Modified Localized Scleroderma Skin Activity Index) | LoSDI (Localized Scleroderma Skin Damage Index) | ||||
|---|---|---|---|---|---|
| New lesion or growth | Erythema | Thickening | Dermal atrophy | Subcutaneous atrophy | Depigmentation |
| (within previous month) | 0 = No | 0 = No | 0 = No | 0 = No | 0 = No |
| 0 = No | 1 = Pink | 1 = Slight | 1 = Shiny surface | 1 = Flat | 1 = Mild |
| 3 = Yes | 2 = Red | 2 = Moderate | 2 = Visible blood vessels | 2 = Concave | 2 = Moderate |
| 3 = Dark red/Violet | 3 = Severe | 3 = “Cliff-drop” sign | 3 = Severe atrophy | 3 = Severe | |
| 0-3 | 0-3 | 0-3 | 0-3 | 0-3 | 0-3 |
A score from 0 to 3 should be assigned for each one of the 6 items. Highest score: 18.
Table 5 DIET scale (depigmentation, induration, erythema and telangiectasia)
| Depigmentation (hypo or hyper) | 0-3 |
| Induration | 0-3 |
| Erythema | 0-3 |
| Telangiectasia | 0-3 |
A score of 0 to 3 should be subjectively assigned to each one of the 4 items. Highest score: 12.
There is significant correlation between the different morphea activity scales (LoSSI, LoSCAT, DIET) and even with a visual analog or subjective scale of lesion activity from the physician´s and patient´s point of view; 27 therefore, it is acceptable to use the more practical to assess patients.
Other tools proposed to assess morphea activity include the durometer (which evaluates skin thickness), a computed scale that calculates the exact area of induration, Doppler ultrasound and infrared thermography.30
Treatment
Morphea treatment can be a challenge, since there is no medication considered to be the gold standard. In addition, the choice of treatment will depend on how active the disease is, its extent, localization, depth and progression; i.e., it will be individualized to each patient (Fig. 1).26 Treatment should be started early, before the appearance of complications in patients with risk associated with the morphea characteristics.31 It is recommended for treatment to continue until the disease activity disappears, even if atrophy and depigmentation do not completely improve.1,11,19,22,32-36
Topical treatment
Topical treatment is limited to the superficial and circumscribed varieties of morphea, without any movement or growth restriction.26,37 Therapeutic options, the level of evidence and the strength of recommendation are listed in Table 6:
Table 6 Topical treatments for morphea
| Treatment | Level of evidence* | Grade of recommendation* |
|---|---|---|
| 5% imiquimod40 | 1b | B |
| Tacrolimus39 | 1b | B |
| Pirfenidone43 | 4 | C |
| Calcipotriol42 | 4 | C |
| Calcipotriol/betamethasone dipropionate41 | 4 | C |
| Calcipotriol/PUVA cream70 | 5 | D |
PUVA = psoralen+phototherapy with ultraviolet light A.
*Highest level of evidence according to the Centre for Evidence-Based Medicine, Oxford (Appendix 1).
– Tacrolimus (0.1 %): calcineurin inhibitor, with good effectiveness and safety.38,39
– Imiquimod: immunomodulator that regulates interferon (IFN) a and g and inhibits the production of collagen by fibroblasts by negatively regulating transforming growth factor b. It is particularly effective in indurated lesions.40
– Calcipotriol: vitamin D-derived, inhibits fibroblast proliferation in vitro; it is clinically effective alone and combined with betamethasone dipropionate.41,42
Other anecdotally used medications and in small series of patients with apparent benefit are pirfenidone and subcutaneous immunoglobulin.25,43 Intralesional IFN g has been shown not to be effective with good evidence (level 1b).44
Topical steroids, used by more than 90% of doctors, have not been formally studied.36
Systemic treatment
It should be used in linear morphea in the head and extremities with potential functional or cosmetic disability, in generalized or rapidly progressive lesions and in pan-sclerotic morphea.1,3,11,19,21,22,26,32-35,37,45,46 Therapeutic options, the level of evidence and the strength of recommendation are listed in Table 7; the most efficacious are the following:
Table 7 Systemic treatments for morphea
| Treatment | Level of evidence* | Grade of recommendation* |
|---|---|---|
| Methotrexate47 | 1b | A |
| LD UVA157 | 1b | A |
| MD UVA157 | 1b | A |
| HD UVA157 | 2b | B |
| LD UVA1/calcipotriol | 2b | B |
| NB UVB57 | 2b | B |
| Fractioned CO2 laser versus LD UVA171 | 2b | B |
| Oral PUVA72 | 4 | C |
| Bath/topical PUVA58 | 4 | C |
| Abatacept73 | 4 | C |
| Cyclosporine74 | 4 | C |
| Mycophenolate mofetil75,76 | 4 | C |
| Tocilizumab77 | 4 | C |
| PUVA/retinoic acid | 4 | C |
| D-penicillamine78 | 4 | C |
| Imatimib79,80 | 5 | D |
| Methotrexate+excimer laser81 | 5 | D |
| Leflunomide/infliximab82 | 5 | D |
| Etretinate83 | 5 | D |
| EC photopheresis+BB UVA84 | 5 | D |
CO2 = carbon dioxide, UVA1 = phototherapy with ultraviolet A1 light (LD = low dose, less than 30 J/cm2, MD = medium dose, 50-70 J/cm2, HD = high dose, 130 J/cm2), NB UVB = phototherapy with narrow band ultraviolet B light, PUVA = psoralen+phototherapy with ultraviolet A light, EC = extracorporeal, BB UVA = phototherapy with broadband ultraviolet A light.
*According to the Centre for Evidence-Based Medicine, Oxford (Appendix 1).
– Methotrexate: tetrahydrofolate reductase inhibitor. It is the drug with most evidence regarding its effectiveness in morphea and is currently considered the first line treatment, with or without systemic corticosteroids for a short period of time (prednisone 0.5-1 mg/kg/day) or in pulses (intravenous methylprednisolone 1-2 mg/kg/day for three days each month for three months). Methotrexate should be continued for at least 12 to 18 months and be discontinued until six months have elapsed without disease activity.47-51
– Phototherapy: with ultraviolet (UV) light A1, UV B or with psoralen (PUVA) the course of disease is altered, with resolution or marked improvement in patients. It induces the expression of metalloproteinase 1, a collagenase that reduces procollagen and collagen in the skin and is effective in all morphea subtypes. UVA1 at different doses (low, medium and high) and broadband UVA have been found to be effective, although the benefit with regard to the risk of oral, bath or topical PUVA is less clear.52-59 Narrowband UVB has also been shown to be effective and to have a very good safety profile.57
There is evidence that prednisone as monotherapy (level 2b)47 and oral calcitriol 1,25 dihydroxyvitamin D3 (level 1b)60,61 are not effective treatments.
Physical therapy
Physiotherapy is mandatory in patients in whom morphea causes mobility alterations or di
sability it is considered part of the treatment in patients with linear morphea in extremities or extensive lesions, to preserve the degree of mobility and minimize contractures, although its effectiveness has never been studied.35
Surgical or cosmetic treatment
Once the disease is inactive without treatment for at least one or two years and children have reached full growth, residual damage can be treated in various forms such as plastic surgery, lipoimplants, filling material or fatty tissue-free flaps.62-65 The goal of surgical treatment is specifically volume restoration, since asymmetry is one of patients’ main concerns.66 Orthopedic surgery may be indicated if patients develop joint and bone deformity as sequelae of linear or deep morphea, including the release of joint contractures and limb lengthening procedures.67,68
Prognosis
The prognosis of circumscribed or superficial morphea is good, although the disease shows a tendency towards progression. Generally, the disease is inactivated in between three and five years, but approximately 30 % of patients will experience relapses,38 especially if morphea started in childhood and if treatment was not adequate. The linear and deep forms can leave important sequelae with functional and esthetic disability.
Conclusions
Morphea is an inflammatory disease of common onset in childhood and predominance in females, where localized sclerosis of the skin and underlying tissue occurs. Its pathophysiology is not fully understood; however, it is known that there is initial microvascular damage and abnormal T-lymphocyte response, which generates abnormal collagen production and fibrosis. In some cases, its classification is difficult, but lesion location and the degree of potential disability that the disease can generate in the patient should always be considered in order to choose the treatment, which should be individualized and promptly started. Topical treatment is preferred for circumscribed or localized forms; tacrolimus and imiquimod are the most recommended options. Systemic therapy is the treatment of choice for linear or severe forms; methotrexate and phototherapy with UVA1 are considered the first-line options. Approximately 30 % of patients with linear morphea may have extracutaneous, neurological, articular or ophthalmological lesions, which should be deliberately searched and discarded.











 nueva página del texto (beta)
nueva página del texto (beta)



