Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de biodiversidad
versión On-line ISSN 2007-8706versión impresa ISSN 1870-3453
Rev. Mex. Biodiv. vol.84 no.4 México dic. 2013
https://doi.org/10.7550/rmb.33904
Ecología
Tama-risk? Avian responses to the invasion of saltcedars (Tamarix ramosissima) in Sonora, Mexico
Respuestas de las aves ante la invasión del pino salado (Tamarix ramosissima) en Sonora, México
Ian MacGregor-Fors1, Rubén Ortega-Álvarez2, Alfredo Barrera-Guzmán3, Lucero Sevillano4 and Ek del-Val4*
1 Red de Ambiente y Sustentabilidad, Instituto de Ecología, A.C. Carretera antigua a Coatepec 351, El Haya, 91070 Xalapa, Veracruz, México.
2 Iniciativa para la Conservación de las Aves de América del Norte-México, Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Liga Periférico-Insurgentes Sur, Núm. 4903, Col. Parques del Pedregal, 14010 México, D. F., México.
3 Museo de Zoología Alfonso Herrera, Facultad de Ciencias, Universidad Nacional Autónoma de México, Circuito exterior s/n, 04510 México, D. F., México.
4 Centro de Investigaciones en Ecosistemas, Universidad Nacional Autónoma de México, Campus Morelia. Antigua Carretera a Pátzcuaro 8701, Ex Hacienda de San José de la Huerta, 58190 Morelia, Michoacán, México. *ekdelval@cieco.unam.mx
Recibido: 09 octubre 2012
Aceptado: 21 junio 2013
Abstract
Although exotic plant invasions are one of the most important components of global change, previous studies have found some of the alien species to provide resources and/or conditions to native biota. One example of this is the saltcedar (Tamarix ramosissima). This exotic invasive tree has been related to several dramatic environmental changes in North America. However, previous studies suggest that they offer resources and conditions for native biota, such as the threatened southwestern willow flycatcher (Empidonax traillii). In this study, we surveyed avian communities and bird nests at sites severely invaded by saltcedars, moderately invaded sites, and non-invaded sites in northwestern Mexico. Our results show that although bird species richness and abundances do not differ among the studied conditions, species composition did. Also, bird nest density differed among the studied conditions, with non-invaded sites having the highest functional diversity of nesting birds. We suggest that future studies should gather natural history and ecological information that allows managing this invasive species correctly both in the USA and Mexico.
Key words: alien species, bird communities, biodiversity, exotic invaders, northwestern Mexico, species turnover.
Resumen
Las especies invasoras han sido reconocidas como uno de los principales componentes del cambio global; sin embargo, algunos estudios han encontrado que también pueden proveer servicios o condiciones benéficas para la biota nativa. Un ejemplo de esta situación lo representa el árbol exótico conocido como pino salado (Tamarix ramosissima). Este árbol exótico ha estado involucrado con algunos de los cambios dramáticos en las condiciones ambientales de los ecosistemas ribereños de Norteamérica. Sin embargo, algunos estudios han sugerido que también ofrece recursos para la biota nativa, en particular para el mosquero saucero (Empidonax traillii). En este trabajo estudiamos a las comunidades de aves y la abundancia de nidos en sitios con diferentes niveles de invasión del pino salado (Tamarix ramosissima) en el norte de Sonora, México. Nuestros resultados muestran que a pesar de que la riqueza y la abundancia de aves no estuvo significativamente influenciada por el nivel de invasión del pino salado, la composición de especies si lo estuvo. Por otro lado, la densidad de nidos de aves fue diferente dependiendo de la condición de invasión del sitio, siendo los sitios sin T. ramosissima los que mostraron una mayor diversidad funcional de nidos. Sugerimos que estudios posteriores recaben información ecológica y de historia natural que permita establecer planes de manejo para controlar esta especie invasora tanto en México como en los EUA.
Palabras clave: especies exóticas, comunidad de aves, biodiversidad, invasoras exóticas, noroeste de México, recambio de especies.
Introduction
The invasion of exotic species is one of the most important components of global change, posing severe threats to biodiversity, ecosystem functioning, resource availability, economy, and even human health (Czech et al., 2000; IUCN, 2000; Ricciardi et al., 2000). The establishment of exotic species grows annually along with their negative effects throughout the globe (Vitousek et al., 1997). Specifically, the invasion of exotic plants has been widely studied. Results from previous investigations show both detrimental effects and positive ones, such as providing some ecosystems services (Pejchar and Mooney, 2009); however, such positive effects are specific and often overridden by their costs (Vitousek et al. 1997).
Saltcedars (Tamarix spp.) are Eurasian trees that were introduced in the 1800's to semiarid riparian systems in southern USA and northern Mexico with 2 main purposes: as wind barriers among crop plots, and as shade providers for cattle (Brock, 1994). Saltcedar populations increased dramatically in the 1960's (Robinson, 1965). Their establishment has been related to many environmental alterations, such as river morphology modifications, increasing soil salinity, displacing native vegetation, and modifying fire regimes (Dudley et al., 2000; Zavaleta, 2000; Lewis et al., 2003). Since saltcedars covered extensive areas in the USA, an intensive biological control program was developed several years ago (USDA, 2005). As many as 80 000 ha in Colorado, Utah, and Nevada are now completely cleared from this exotic species thanks to the use of the saltcedar leaf beetle as a biological control agent (Diorhabda elongata; De Loach et al. 2009). The saltcedar leaf beetle was not as successful in Texas due to climatic mismatches; however, another closely related species (D. sublinneata) was used. Since 2009, this leaf beetle has expanded its populations and decimated saltcedars southward down to Mexico (Zamorano, 2012).
Apart from saltcedar invasion, the environmental status of rivers in northern Mexico is worrisome. Among the main alterations, changes in water flow, damming, and underground water pumping head the list (Zavaleta, 2000). Altogether, the environmental status of rivers per se and the presence of aggressive exotic plant species (e.g., saltcedars, giant reeds-Arundo donax) have generated a complicated scenario for the wildlife associated to riparian systems, representing a severe threat for them. Some authors claim that among other wildlife groups (e.g., insects, reptiles, amphibians; King, 2005), birds use saltcedars as surrogate habitat, even for nesting (Hunter et al., 1985; Suckling et al., 1992; Guertin, 2003; King, 2005; Sogge et al., 2008). One example of this is the threatened southwestern willow flycatcher (Empidonax traillii). In fact, Paradzick (2005) found that southwestern willow flycatchers tend to select similar habitat traits in cottonwood-willow and saltcedar habitat patches. However, other studies have found negative effects of saltcedars on birds, diminishing species richness and abundance in severely invaded areas (Anderson et al., 1983; Anderson and Ohmart, 1984; King, 2005), with species-dependent effects (van Riper III et al., 2008).
Even though saltcedars have invaded many areas of northern Mexico, few studies have documented their effects on the native biodiversity of this country (Scott et al., 2009), and little is known about the threats that saltcedars pose to Mexican bird communities. Previous studies carried out in the USA show that saltcedar invaded areas can support up to 49 bird species; however, its quality as habitat varies across sites (Sogge et al., 2008). In this study, we assessed shifts in bird communities associated to the recent local invasion of saltcedars in northwestern Mexico (as suggested by Hunter et al., 1985, 1988). For this, we surveyed bird communities at severely invaded sites, moderately invaded sites, and non-invaded sites, contrasting diversity and composition metrics (i.e., species richness, relative abundance, species turnover). We also assessed the role that saltcedars play as nesting sites for birds.
Materials and methods
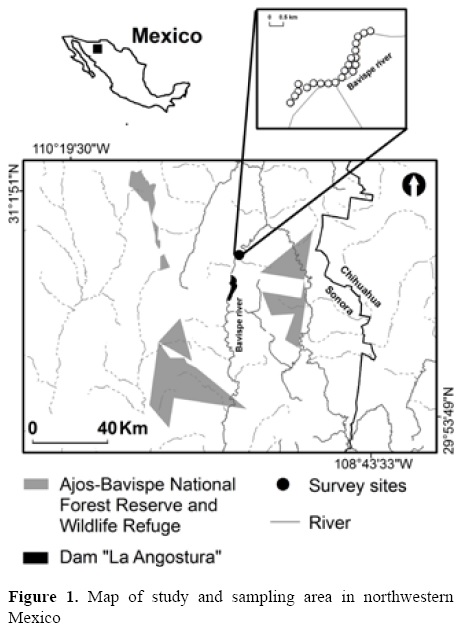
Study area. This study was carried out in the surroundings of the Ajos-Bavispe National Forest Reserve in Sonora (30°40'32.4" N, 109°21'10.9" W), located in northwestern Mexico, along the banks of the Bavispe river (Fig. 1). The vegetation in the area is mainly comprised by a riparian forest, including original tree species, such as Acer grandidentatum, Alnus oblongifolia, Populus fremontii, Platanus wrightii, Juglans major, and Fraxinus velutina; as well as shrubs and lianas, such as Rhus trilobata, Rosa woodsi, Ceanothus fenleri, Vitis arizonica and Rhus radicans (Molina-Freaner and Van de Vender, 2010). The conservation status of riparian vegetation in the Sonoran Desert is of special concern since there have been many hydrological exploitation projects that diverted water flows from most of the region's major rivers (including the Bavispe river) by constructing dams and associated irrigation canals. Inundating vegetation in reservoirs behind dams and changes in river flows are among the most severe pressures to threatened plants and nesting birds in the Mexico-USA borderlands (Zamorano, 2012). Groundwater pumping for agricultural and domestic purposes is also severe in the region and has affected nearly all river valleys leading to ground subsidence, salinization, and threatening riparian forests in the Sonoran desert (Nabham and Holdsworth, 1998).
Bird and nest surveys. We surveyed diurnal breeding birds from 07:00-11:00 in June 2011 using 5 min point counts (25 m radius) located at least 250 m apart from each other to assure survey independence (Ralph et al., 1996). We used limited-radius point counts for assuring that all birds recorded were actively using the surveyed area and not nearby conditions with different habitat attributes, and to maintain an identical sampled area per point count. All birds seen or heard using the sampled area (e.g., perching, foraging, nesting) were recorded and included in our analyses.
We established survey sites in 3 habitat conditions related to saltcedar invasion: 1) severely invaded sites (> 90% saltcedar cover), 2) moderately invaded sites (presence of saltcedars embedded in native plant communities), and 3) non-invaded sites (absence of saltcedars). Due to the nature and distribution of these conditions we could not proceed with a balanced design. Thus, we performed 8 point-counts in severely invaded sites, 3 in moderately invaded sites, and 11 in non-invaded sites.
For nest surveys, we located a total of 32 sampling plots near to the point counts. Of them, 12 were located in severely invaded sites, 5 in moderately invaded sites, and 15 in non-invaded sites. We defined 25 m radius survey areas at each nest sampling plot where we searched for nests in all vegetation strata with equal sampling efforts (20 min, 2 observers). Within each plot, we recorded all nests found and identified the species that built them whenever possible.
Habitat characterization. To characterize vegetation traits in our survey sites, we recorded 13 variables within the 25 m radius where birds were surveyed: 1) tree cover, 2) tree species richness, 3) tree maximum height, 4) tree minimum height, 5) dominant tree species, 6) shrub cover, 7) shrub species richness, 8) shrub maximum height, 9) shrub minimum height, 10) herb cover, 11) herb species richness, 12) herb maximum height, and 13) herb minimum height.
Analyses. To contrast bird species richness values, we compared the species richness statistical expectation for each condition using Estimates (Colwell, 2008). This expectation is generated by the repeated re-sampling of all pooled samples, allowing the statistical comparison of different habitats/treatments (Gotelli and Colwell, 2001). To determine if species richness values were statistically different among the studied conditions, we compared their 84% confidence interval (CI). If their CIs did not overlap, we considered the data to be statistically different with an alpha= 0.05 (following Payton et al., 2003; MacGregor-Fors and Payton, 2013). To assess if bird abundances differed among habitats, we used a Kruskal-Wallis test. To assess bird community composition shifts, we used a Bray-Curtis multivariate cluster analysis (single linkage), based on the abundance-based version of Bray and Curtis's (1957) species turnover index using BioDiversity Pro (McAleece, 1997). This analysis outputs a dendrogram with the single linkage similarity among the compared conditions. To assess if habitat traits were related to bird community richness and abundance, we performed regression trees using R (R Development Core Team, 2010). Regression trees allow the interpretation of datasets where complex nonlinear relationships occur between the set of response and predictor variables (Deíath and Fabricius, 2000). This analysis uses binary recursive partitioning to identify threshold values of a set of predictor variables, which can be a mix of continuous and categorical variables that are related to the response variable. Thus, regression trees identify successive critical values of predictor variables splitting the response variable in a dichotomous and hierarchical manner (Palomino and Carrascal, 2007). These types of trees are analogous to multiple regression models, specifically those using forward selection of predictor variables (Crawley, 2007). Finally, we report the number of nests found in severely, moderately, and non-invaded sites, using a Fisher's exact test to assess differences in the frequency of nests we could identify. We used this test as it is most suitable for small sample sizes.
Results
Due to the nature of our sampling design, with incomparable numbers, we could not contrast bird species richness expectations using one single comparable accumulated abundance cut-off, as suggested by Moreno (2001) and Magurran (2004). Thus, we used 2 abundance cut-off values, given by the least total abundance in the less abundant conditions. Bird species richness values did not show significant differences among the studied conditions using both cut-off values: 1) 11 individuals (total abundance recorded at moderately invaded sites): severely invaded= 8.4 ± 1.4, moderately invaded= 8.0 ± 3.3, non-invaded= 8.4 ± 1.6 computed species, and 2) 31 individuals (total abundance recorded at severely invaded sites): severely invaded= 16 ± 3.3, non-invaded= 17.3 ± 3.0. Similarly, relative abundances did not differ significantly among the studied conditions (individuals / point count: H3 23= 5.7, p= 0.12). The Bray-Curtis multivariate cluster analysis revealed that the studied bird communities were highly different among conditions, regardless of their closeness, showing low average similarity among them (~33% similarity). Specifically, the most similar paired comparison was 'severely invaded-non-invaded' (38.1%), while the most dissimilar was 'moderately invaded-non-invaded' (25.4% similarity). Only 3 of the 13 measured explanatory variables were considered by the regression tree analyses for both bird species richness and abundance: 1) tree cover, 2) shrub cover, and 3) tree maximum height. In both cases (i.e., bird richness, abundance), tree cover was the variable that explained most of the variation and was positively related.
We found a total of 43 bird nests in the sampled plots, of which 49% corresponded to 3 generalist bird species: 1) Sinaloa wren (Thryothorus sinaloa), 2) common ground-dove (Columbina passerina), and 3) gila woodpecker (Melanerpes uropygialis). When we calculated the relative number of recorded nests per condition (total number of nests / plot), moderately invaded areas ranked highest (2 nests / plot), followed by severely invades sites (1.4 nests / plot), and non-invaded sites (1.06 nests / plot). Although we could not determine the exact species related to all recorded nests because they were not active and were presumably from a prior breeding season (32% unidentified nests), results from the set of nests that could be identified indicate a higher number of species nesting in non-invaded sites (6 species, including unidentified hummingbirds, verdins-Auriparus flaviceps, vermilion flycatchers-Pyrocephalus rubinus, and house finches-Haemorhous mexicanus). On the other hand, severely invaded sites had nests of 5 identified species, and moderately invaded sites only had nests of 3 bird species (Table 1). The Fishe's exact test revealed significant differences in the number of species nesting among the 3 studied saltcedar invasion conditions (p= 0.004).
Discussion
Ecosystem alterations, including the impacts of invasive exotic species, jeopardize the demographic status of native species worldwide. In fact, the negative impacts of invasive exotic species can go beyond the loss of certain species, impacting several ecological aspects including negative effects on native species' populations, communities, biotic interactions, and even ecosystem processes (Vila et al., 2011). Moreover, the presence and effects of invasive species often imply substantial economic losses, severe sanitary problems, and therefore represent a direct threat to human health (Simberloff, 1996; Pimentel et al., 2001). In Mexico, there is a dearth of knowledge about the biology and ecology of most invasive exotic species. For most invasive species, we ignore the consequences of their presence on biodiversity, ecosystem processes, and their effect on the human communities that directly interact with them. We do know that there are at least 800 exotic invasive species in the country and most of them, are plants (83%; Aguirre-Muñoz et al., 2009). In this sense, the study of the ecology and impacts of invasive species, and particularly of the aggressive Tamarix ramosissima in northern Mexico, are crucial to understand them and therefore create a solid background that justifies the implementation of control strategies.
Previous studies show that the response of bird communities to the invasion of saltcedars can vary. Although the dominance of saltcedars often reduce bird species richness and abundance (Anderson et al., 1983; Anderson and Ohmart, 1984), the presence of this exotic invasive plant has also resulted in the increase of both bird species richness and abundance under certain conditions (Brock, 1994; King, 2005; van Riper III et al., 2008). In this study, bird species richness and abundance did not show significant differences in the studied conditions, not matching any of the previous studies we know of. This result could be due to several non-exclusive factors, such as: 1) the time-scale of our study, 2) the recent invasion of saltcedars in our study area (1970s-1980s) resulting in a time-lag between the invasion process and the response of bird communities, 3) the importance of tree cover as an explanatory variable of bird species richness and abundance, regardless of the species it is comprised by, and 4) a possible landscape configuration that results in sink-and-source dynamics, with invaded areas having low habitat quality, and thus, high avian mortality, and non-invaded areas generating enough individuals to support similar numbers in all conditions (Hunter et al., 1988). Recording a similar number of individuals of a similar number of species in the 3 studied conditions suggests that saltcedar stands offer suitable conditions for a set of bird species (as suggested by van Riper III et al., 2008). However, our bird composition analysis revealed high dissimilarity among the recorded bird communities (~67% dissimilarity), showing that the studied conditions offer resources for different birds, with saltcedars incorporating novel resources into the landscape (van Riper III et al., 2008).
Our surveys show that birds are using saltcedars for nesting when areas are severely invaded, but not in moderately invaded areas, where birds preferred to nest on native plant species (e.g., mesquites, cottonwoods, acacias). This result indicates that plant composition including native species is crucial for nesting birds, as birds prefer them over exotic species. We recorded several common ground-dove nests in severely invaded sites and none in the other studied conditions. Common gound-doves are a common generalist species capable of breeding in diverse habitats throughout the region, preferring disturbed conditions (Hensley, 1959; Short, 1974; Howell and Webb, 1995). We also recorded 2 Zenaida dove nests and 2 Sinaloa wren nests in severely invaded sites. As common gound-doves, Zenaida doves are also a generalists species associated with open and disturbed areas; while Sinaloa wrens where the most abundant nester in the study area, with the least number of nests recorded in severely invaded sites. Interestingly, only 47% of the recorded nests in severely invaded sites were constructed on saltcedars. We found a low number of nests (n= 10) at moderately invaded sites; however, at this condition none of the nests were constructed on saltcedars. Not surprisingly, we recorded a higher number of nesting species pertaining to different functional groups (i.e., omnivore, insectivore, granivore, nectarivore) at non-invaded sites. Additionally, we recorded an important number of Sinaloa wren nests (n= 7) at non-invaded sites. Among the other species recorded nesting at non-invaded sites, the vermilion flycatcher was also recorded nesting in severely invaded sites. This is completely expectable, as it is a common insectivore species associated to disturbed areas and known to nest near water sources (Howell and Webb, 1995). Aside of this species, we recorded 1 gila woodpecker nest, 1 verdin nest, and 1 house finch nest at non-invaded sites. Although all these species are common and nest widely in the study area, we did not record them nesting in severely invaded sites.
In summary, our results show, for the first time in saltcedar invaded areas in Mexico, that although bird diversity values (i.e., species richness, abundance) did not differ among severely invaded, moderately invaded, and non-invaded sites, species composition varied greatly with the invasion and degree of dominance of this exotic invasive plant species, suggesting that invaded and non-invaded sites offer a different array of resources for birds. Most interestingly, we found that bird only nest in saltcedars when the exotic invasive tree is dominant, while non-invaded sites had a higher amount of nests pertaining to a wider array of birds, both taxonomically and functionally. It is noteworthy to underline that we did not record any threatened bird species in areas invaded by saltcedars, including the southwestern willow flycatcher, which is of conservation concern in the USA.
Although our results are quite robust and suggest a concrete response of birds to saltcedar invasion in northwestern Mexico, we suggest that future studies gather multi-taxonomic data in order to generate information that could aid to develop management plans, precise policy making, and further actions to diminish, or void, the ecosystem effects that saltcedar have on recently invaded systems. We strongly suggest to take into consideration the role that saltcedars are playing in Mexican ecosystems, particularly in agroecosystems across Chihuahua and Sonora in order to avoid social discontent since people have actively planted them in some areas to increase shade for cattle and as wind-barriers among agricultural plots.
Acknowledgements
We are most grateful with the personnel of the Ajos-Bavispe National Preserve, specially the director Juan Mario Cirett, and our field collaborators Roberto Martínez, Guadalupe González y Manuel Munguía. The project was funded by the Conabio-JE004.
Literature cited
Anderson, B. W., R. D. Ohmart and J. Rice. 1983. Avian and vegetation community structure and their seasonal relationships in the Lower Colorado River Valley. Condor 85:392-405. [ Links ]
Anderson, B. W. and R. D. Ohmart. 1984. Vegetation management study for the enhancement of wildlife along the lower Colorado river. Comprehensive final report to USA Bureau of reclamation. Boulder City. 97 p. [ Links ]
Aguirre-Muñoz, A. R. and R. Mendoza Alfaro. 2009. Especies exóticas invasoras: impactos sobre las poblaciones de flora y fauna, los procesos ecológicos y la economía. In Capital natural de México, Vol. II: estado de conservación y tendencias de cambio, R. Dirzo, R. González and I. J. March (eds.). Conabio, México, D. F. p. 277-318. [ Links ]
Best, L. B. and D. F. Stauffer. 1980. Factors affecting nesting success in riparian bird communities. Condor 82:149-158. [ Links ]
Brock, J. H. 1994. Tamarix spp. (Salt Cedar), an invasive exotic woody plant in arid and semiarid riparian habitats of western USA. In Ecology and management of invasive riverside plants, L. C. Waal, L. E. Child, P. M. Wade and J. H. Brock (eds.). John Wiley & Sons, Cambdridge. p. 27-44. [ Links ]
Colwell, R. K. 2008. EstimateS: Statistical estimation of species richness and shared species from samples, version 8.2 http://purl.oclc.org/estimates; last access: 7.X.2012. [ Links ]
Crawley, M. J. 2007. The R Book. Wiley and Sons, Chichester, England. 1076 p. [ Links ]
Czech, B., P. R. Krausman and P. K. Devers. 2000. Economic associations among causes of species endangerment in the United States. Bioscience 50:593-601. [ Links ]
De'ath, G. and K. E. Fabricius. 2000. Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology 81:3178-3192. [ Links ]
De Loach, C. J., P. A. Lewis, J. C. Herr, R. I. Carruthers, J. L. Tracy and J. Johnston. 2003. Host specificity of the leaf beetle, Diorhabda elongate deserticola (Coleoptera: Chrysomelidae) from Asia, a biological control agent for saltcedars (Tamarix: Tamaricaceae) in the Western United States. Biological Control 27:117-147. [ Links ]
De Loach, C. J., A. E. Knuston, P. J. Moran, J. H. Everitt, G. J. Michels, M. A. Muegge, C. W. Randal, T. G. Fain, M. P. Donet and C. M. Ritzi. 2009. Progress on biological control of saltcedar in the Western U.S.: Emphasis-Texas 20042009. USDA-ARS, Texas. 67 p. [ Links ]
Dudley, T. L., C. J. De Loach, J. Lovich and R. I. Carruthers. 2000. Saltcedar invasion of western riparian areas: impacts and new prospects for control. Transactions 65th North American Wildlife and Natural Resources Conference. 24-28 March 2000, Chicago. p. 345-381. [ Links ]
Ellis, L. M. 1995. Bird use of saltcedar and cottonwood vegetation in the middle Rio Grande Valley, New Mexico. Journal of Arid Environments 30:339-349. [ Links ]
Gotelli, N. J. and R. K. Colwell. 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4:379-391. [ Links ]
Hausner, V. H., N. G. Yoccoz, K. Strann and R. A. Ims. 2002. Changes in bird communities by planting non-native spruce in coastal birch forests of northern Norway. Ecoscience 9:470-481. [ Links ]
Hensley, M. 1959. Notes on the nesting of selected species of birds of the Sonoran desert. Wilson Bulletin 71:86-92. [ Links ]
Hinojosa-Huerta, O. M., H. Iturribarría-Rojas, Y. Carrillo-Guerrero, M. de la Garza-Treviño and E. Zamora-Hernández. 2004. Bird conservation plan for the Colorado river delta. Pronatura Noroeste, Sonora. 70 p. [ Links ]
Howell, S. N. G. and S. Webb. 1995. A guide to the birds of Mexico and northern Central America. Oxford University Press, Oxford. 1010 p. [ Links ]
Hunter, W. C., R. Ohmart and B. Anderson. 1988. Use of exotic saltcedar (Tamarix chinensis) by birds in arid riparian systems. Condor 90:113-123. [ Links ]
Hunter, W.C., B. W. Anderson and R. D. Ohmart. 1985. Summer avian community composition of Tamarix habitats in three southwestern desert riparian systems. In Riparian ecosystems and their management: reconciling conflicting uses, R. R. Johnson, C. D. Ziebell, D. R. Patron, P. F. Folliort and R. H. Hamre (eds.). Gen. Tech. Rep. RM-120, U.S. Department of Agriculture, Fort Collins. p. 128-143. [ Links ]
IUCN (International Union for Conservation of Nature). 2000. Guidelines for the prevention of biodiversity loss caused by alien invasive species. Fifth Meeting of the Conference of the Parties to the Convention on Biological Diversity, 15-26 May 2000. Nairobi, Kenya. [ Links ]
King, M. A. 2005. New habitats for old: Tamarisk-dominated riparian communities and marshes in the Grand Canyon. UC Davis, CA. https://www.geology.ucdavis.edu/~shlemonc/html/trips/Grand%20Canyon%20web/html/reports/PDFs/King.pdf; last access: 7.X.2012. [ Links ]
Lewis, P. A., C. J. De Loach, A. E. Knutson, J. L. Tracy and T. Robbins. 2003. Biology of Diorhabda elongata deserticola (Coleoptera: Chrysomelidae), an Asian leaf beetle for biological control of saltcedars (Tamarix sp.) in the United States. Biological Control 27:101-116. [ Links ]
MacGregor-Fors, I. and M. E. Payton. 2013. Contrasting diversity values: Statistical inferences based on overlapping confidence intervals. PLoS One 8:e56794. [ Links ]
Magurran, A. E. 2004. Measuring biological diversity. Blackwell Publishing, Oxford. 254 p. [ Links ]
McAleece, N. 1997. BioDiversity Professional. http://www.sams.ac.uk/peter-lamont/biodiversity-pro/?searchterm=biodiversity%20pro; last access: 7.X.2012. [ Links ]
Molina-Freaner, F. E. and T. R. Van de Vender. 2010. Diversidad biológica de Sonora. UNAM-Conabio, México, D. F. 496 p. [ Links ]
Moreno, C. E. 2001. Métodos para medir la biodiversidad. M&T-Manuales y Tesis SEA, Zaragoza, España. 84 p. [ Links ]
Nabhan, G. P. and A. R. Holdsworth. 1998. State of the Sonoran Desert Biome: uniqueness, biodiversity, threats and the adequacy of protection in the Sonoran Bioregion. Wildlands project. Tucson. 81 p. [ Links ]
Palomino, D. and L. M. Carrascal. 2007. Threshold distances to nearby cities and roads influence the bird community of a mosaic landscape. Biological Conservation 140:100-109. [ Links ]
Paradzick, C. E. 2005. Southwestern willow flycatcher habitat selection along the Gila and Lower San Pedro rivers, Arizona: vegetation and hydrogeomorphic considerations. Msc thesis, Arizona State University. Tempe, Arizona, 172 p. [ Links ]
Payton, M. E., M. H. Greenstone and N. Schenker. 2003. Overlapping confidence intervals or standard error intervals: What do they mean in terms of statistical significance? Journal of Insect Science 3:34 [ Links ]
Pejchar, L. and H. A. Mooney. 2009. Invasive species, ecosystem services and human well-being. Trends in Ecology and Evolution 24:497-504. [ Links ]
Pimentel, D., S. McNair, J. Janecka, J. Wightman, C. Sommonds, C. O'Connell, E. Wong, L. Russel, J. Zern, T. Aquino and T. Tsomondo. 2001. Economic and environmental threats of alien plant, animal and microbe invasions. Agriculture, Ecosystems and Environment 84:1-20. [ Links ]
R Development Core Team. 2010. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org; last access: 7.X.2012. [ Links ]
Ralph, C. J., G. R. Geupel, P. Pyle, T. E. Martin, D. F. DeSante and B. Milá. 1996. Manual de métodos de campo para el monitoreo de aves terrestres. U.S. Department of Agriculture, Albany, California. 44 p. [ Links ]
Ricciardi, A., W. W. M. Steiner, R. M. Mack and D. Simberloff. 2000. Toward a global information system for invasive species. BioScience 50:239-44. [ Links ]
Robinson, T. W. 1965. Introduction, spread and areal extent of saltcedar (Tamarix) in the western states. USA Geological Survey Professional Paper 491-A. U.S. Government Printing Office, Washington, D.C. [ Links ]
Scott, M. L., P. L. Nagler, E. P. Glenn, C. Valdes-Casillas, J. A. Erker, E. W. Reynolds, P. B. Shafroth, E. Gómez-Limón and C. L. Jones. 2009. Assessing the extent and diversity of riparian ecosystems in Sonora, Mexico. Biodiversity and Conservation 18:247-269. [ Links ]
Short, L. L. 1974. Nesting of Southern Sonoran birds during the summer rainy season. Condor 76:21-32. [ Links ]
Simberloff, D. 1996. Impacts of introduced species in the United States. Consequences 2:13-22. [ Links ]
Sogge, M. K., S. J. Sferra and E. H. Paxton. 2008. Tamarix as habitat for birds: implications for riparian restoration in the Southwestern United States. Restoration Ecology 16:146-154. [ Links ]
Stromber, J. C., M. K. Chew, P. L. Nagler and E. P. Glenn. 2009. Changing perceptions of change: the role of scientists in Tamarix river management. Restoration Ecology 17:177-186. [ Links ]
Strong, T. R. and C. E. Bock. 1990. Bird species distribution patterns in riparian habitats in Southeastern Arizona. Condor 92:866-885. [ Links ]
Suckling, K., D. Hoganand and R. D. Silver. 1992. Petition to list the southwest willow flycatcher Empidonax traillii extimus as a federally endangered species. Letter to the Secretary of the Interior. January 25, 1992. Luna, New Mexico. [ Links ]
USDA (United States Department of Agriculture). 2005. Program for biological control of saltcedar (Tamarix spp.) in thirteen states. http://www.aphis.usda.gov/plant_health/ea/downloads/salteafonsi.pdf; last access: 7.X.2012. [ Links ]
Van Horne, B. 1983. Density as a misleading indicator of habitat quality. Journal of Wildlife Management 47:893- 901. [ Links ]
Van Riper III, C., K. L. Paxton, C. O'Brien, P. B. Shafroth and L. J. McGrath. 2008. Rethinking Avian response to Tamarix on the Lower Colorado River: a threshold hypothesis. Restoration Ecology 16:155-167. [ Links ]
Vila, M., J. L. Espinar, M. Hejda, P. E. Hulme, V. Jarosik, J. L. Maron, J. Perlg, U. Schaffner, Y. Sun and P. Pysek. 2011. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecology Letters 14:702-708. [ Links ]
Vitousek, P. M., C. M. D'Antonio, L. L. Loope, M. Rejmánek, R. Westbrooks. 1997. Introduced species: a significant component of human-caused global change. New Zealand Journal of Ecology 21:1-16. [ Links ]
Zamorano, P. 2012. Monitoring of the distribution of the beetle (Diorhabda sublineata) released as biological control of saltcedar (Tamarix spp.) on the banks of the Rio Grande and Rio Conchos. Weeds Across borders Meeting 2012. Cancún, México. http://www.weedcenter.org/wab/2012/sp/docs/Session%207/6%20Zamorano-25abr12.pdf; last access: 7.X.2012. [ Links ]
Zavaleta, E. 2000. The economic value of controlling and invasive shrub. Ambio 29:462-467. [ Links ]














