Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.18 no.2 Ciudad de México ago. 2008
Removal of epiphytes of the kelp Macrocystis pyrifera (L.) Agardh using different biocides
Remoción de epifitas de Macrocystis pyrifera (L.) Agardh con diferentes biocidas
M. del Pilar Sánchez–Saavedra1,*, Domenico Voltolina3, Jorge Simental1, 2 and Miriam Jazmín Carbajal–Miranda1
1 Centro de Investigación Científica y de Educación Superior de Ensenada (CICESE), Kilómetro 107 Carretera Tijuana a Ensenada, Ensenada, Baja California, 22860, México.
2 Centro de Estudios Superiores del Estado de Sonora (CESUES), Laboratorio de Tecnologías de Cultivo de Organismos Acuáticos, Periférico Sur y Carretera a Huatabampo, Navojoa, Sonora, 85870, México.
3 Centro de Investigaciones Biológicas del Noroeste, Laboratorio de Estudios Ambientales UAS–CIBNOR, P.O. Box 1132, Mazatlán, Sinaloa, 82000, México.
*Corresponding author:
M.P. Sánchez-Saavedra.
P.O. Box 434844,
San Diego, CA 92143, USA.
Phone: +52 (646) 1750500 ext. 24460 and 24425; Fax +52 (646) 1750572.
E–mail: psanchez@cicese.mx
Recibido: 14 de febrero de 2007.
Aceptado: 5 de junio de 2008.
ABSTRACT
The aim of this work was to evaluate the effectiveness of some biocides for the removal of epiphytes from the blades of the kelp Macrocystis pyrifera. The lowest initial removal was with 1% KMnO4 and with the water purifier Fit®, but both gave better results in the long term. The treatments with distilled and tap water, which avoid the use of biocides, gave a better than 75% reduction of the epiphytes and had a long–lasting effect. In addition, the photosynthetic activity of the controls was similar to that of these two treatments. This confirms that a simple immersion in freshwater may achieve a good initial removal of epiphytic diatoms and prevent their subsequent growth.
Keywords: Epiphytes removal, Macrocystis pyrifera, Navicula incerta.
RESUMEN
En este trabajo se determinó la mejor eficacia de diferentes biocidas para la eliminación de epifitas de las frondas de Macrocystis pyrifera. La menor remoción inicial fue con 1% KMnO4 y con el purificador de agua Fit®, pero ambos tuvieron el mejor efecto a largo plazo. Los tratamientos con agua destilada y agua de uso doméstico, que evitan el uso de biocidas, dieron una reducción inicial superior al 75% y su efecto fue a largo plazo. La actividad fotosintética de la macroalga después de estos tratamientos resultó similar a la del tratamiento control. Estos resultados confirman que una simple inmersión en agua dulce puede lograr una buena remoción de diatomeas epifitas y prevenir su crecimiento después del tratamiento.
Palabras clave: Remoción de epifitas, Macrocystis pyrifera, Navicula incerta.
INTRODUCTION
The giant kelp Macrocystis pyrifera ranges from Alaska to Baja California, Mexico, and grows from outside the surf area to depths of 50 meters. Its young blades have been harvested and processed for alginate production for the food industry as well as for medical applications since 1910, with an estimated worldwide generated income of close to 250 million US $ (Vásquez, 1999).
In more recent times it has also been cultured and used as food for several grazers such as abalone, sea urchins, and sea cucumbers (Hahn, 1989; Fallu, 1991) or as a supplement for animal diets, mainly in the aquaculture industry (Cruz–Suárez et al., 2000; Plana et al., 2007).
However, in our studies on its dietary value for abalone it became evident that, because of the high specific richness of its epiphytic diatoms, such as Cocconeis cf. britannica Naegeli ex Kützing, C. speciosa Gregory, Gonphonemopsis pseudoexigua (Simon–Sen) Medlin, Climacosphenia moniligera Ehrenberg, and Navicula spp. (Siqueiros–Beltrones et al., 2002), it was necessary to separate the relative contribution of kelp and epiphytes to abalone diets (Siqueiros–Beltrones et al., 2005).
The information on the effectiveness of the techniques used for the removal of epiphytic diatoms from macroalgae blades is scarce and mentions the use of scrubs with a brush in freshwater or of germanium dioxide (GeO2) which prevents diatom growth (Lewin, 1966; Shea & Thierry, 2007), although none of these treatments is completely effective and long–lasting (Gledhill et al., 1998). In addition, scrubbing tends to damage the macroalgae tissues (Lafferty, 2001) and, although the effective dosage of GeO2 (0.1 to 0.5 mg/L) is within the range described as nephrotoxic to rats (Furst, 1987), there are no specific criteria for the aquatic environment or for invertebrate diets.
In this paper, we examine the effectiveness of some treatments that were tested to achieve a lasting removal of epiphytic diatoms from the kelp blades that were being evaluated as diets for juvenile red abalone Haliotis rufescens Swainson.
MATERIALS AND METHODS
Sixty six disks with a diameter of 2.1 cm and a total surface area (considering both sides) of 6.92 cm2 were cut with a cork–borer from the apical portion of young M. pyrifera blades freshly harvested from a natural bed off the coast of northern Baja California (31° 51' 30" N, 116° 38' 38" W).
Since the discs had few epiphytes, they were left standing for two hours in individual Petri dishes with a suspension (5 × 105 cell/mL) of a 4–days old culture of Navicula incerta Grunow, 1880, grown in non–axenic batch cultures of progressively increasing volumes (10, 150, 900 mL, and 10 L) in f/2 medium (Guillard, 1975) with double silicate concentration, at a constant temperature of 16 °C and with 100 µE/m2/s continuous photoflux.
This diatom was chosen as the dominant epiphyte, because several Navicula species have been described as common on the M. pyrifera blades used as food when abalone juveniles are weaned from a microalgae to a macroalgae diet (Siqueiros–Beltrones et al., 2002, 2005), and because it was one of the common diatoms in the gut content of juvenile H. rufescens (Siqueiros–Beltrones & Voltolina, 2000).
After this enrichment, all disks were individually rinsed with 1–µm filtered seawater and placed in clean Petri dishes with fresh, sterile seawater. Six received no treatment and served as controls. The remaining 60 were treated for 10 minutes, in groups of six disks for each treatment, with seven treatments common in aquaculture, used at the concentrations suggested by Lázaro–Chávez (1985), as well as with three biocides (Biopur® = 0.35% Ag2SO4; Microdin® = 0.35% Ag2SO4; Fit® = CH3(CH2)11OSO3Na and Na3PO4·12H2O), in the concentrations used for vegetable and fruit disinfection for human consumption (Biopur and Microdin: 1 drop/L; Fit: 4.5 g/L: Table 1). After treatment, the disks were rinsed with sterile seawater to remove any residue of the cleaning agents.
Both sides of three disks of each treatment were individually scraped under a microscope with a soft brush to remove all epiphytic diatoms. These were counted with a haemocytometer after ultrasonic treatment to achieve their thorough dispersion for ease and precision of counting (Voltolina, 1991). The results were used to evaluate the initial efficiency of removal as the mean percentage of epiphytic diatoms (original community and N. incerta) found on the three disks of each treatment, in comparison to the mean value of the three control disks.
Physiological damage was evaluated comparing the photosynthetic activity and the chlorophyll content of the disks treated to that of the controls. The maximum photosynthetic O2 evolution (Pmax) of each disk was measured under a light gradient of 0 to 1000 mE/m2/s in a 7 ml acrylic chamber, using a Clark–type Yellow Spring Instruments 5221 O2 electrode (Mercado et al., 2004). Chlorophyll was determined as in Strickland & Parsons (1972).
The long–term effectiveness of each treatment was evaluated as positive or negative diatom growth on the remaining disks, incubated at 18 °C for 4 days in f/2 medium, using the equation:
Gi = log2 (Nti / Nto),
where Nti = number of live diatoms on disk i after four days; Nto = mean number of live cells on the three disks used for the evaluation of initial efficiency after treatment and Gi = total number of positive or negative duplications of the cell number on disk i (one negative duplication = 50% reduction of the cell number in comparison to Nto; Gómez–Villa et al., 2002).
The removal efficiency, the photosynthetic activity and the chlorophyll contents were compared by one–way analysis of variance (ANOVA) or Kruskall–Wallis tests, depending on the results of the Lilliefors' and Bartlett's tests of normality and equal variances, separating the significant differences with Tukey's or Dunn's tests. All statistical analysis were performed with α = 0.05 (Zar, 1996).
RESULTS AND DISCUSSION
The best initial results were with NaClO, iodine, acetic acid (AA = C2H4O2), Biopur® and Microdin® and the lower removals were with KMnO4 and Fit ®. However, these two treatments gave the lowest epiphyte concentration after four days, whereas that on the AA–treated disks had rebounded to more than 60% of the initial value, from 0.11 to 2.06 × 103 cells/cm2, for a total of 4.26 cell duplications during the four days of incubation after treatment. Fit ® gave the best final result, with the highest mean value (–2.89) of negative cell duplications (Table 1).
There was also a greater than 75% initial removal of the epiphytes present on the disks with the two types of freshwater, which also gave negligible growth after treatment (Table 1). Although significantly lower than most treatments, this seems of particular interest because it avoids the use of biocides. In addition, these treatments did not affect the consistency or texture of the blades because the cell membranes of this species are permeable to solutes but not to water (Bold & Wynne, 1985).
CuSO4 and iodine stained the disks, indicating their presence in the blade tissues. In spite of this, all disks were readily consumed by abalone and for this reason the use of these biocides should be avoided if kelp is intended as food for grazers.
According to Gledhill et al. (1998), the total elimination of epiphytes from the blade surface of Fucus vesiculosus may be achieved using a combination of chemical (ethanol, ascorbic acid, and sodium hypochlorite) and physical treatments (brushing or scrubbing).
However, although there were no significant differences between the photosynthetic activity of the controls and those of any other treatment, the mean chlorophyll content of the disks treated with acetic acid, copper sulphate and Biopur® were significantly lower than the controls (Table 2), and in addition our previous experience with M. pyrifera showed that scrubbing caused cell damage and excessive exopolysaccharide production (Simental, 2005).
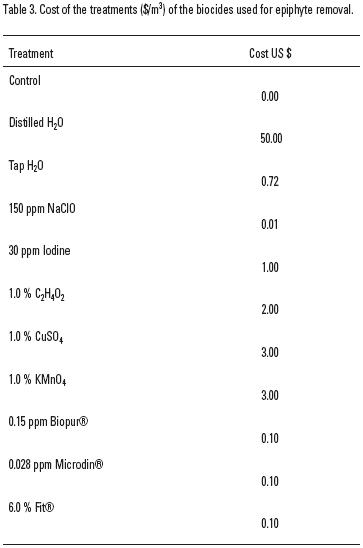
In contrast, a simple immersion in freshwater removes most epiphytic diatoms, prevents their subsequent growth and does not cause any significant textural or physiological damage to the blades. Our results show that this cheap and environmentally friendly treatment (Table 3), which is commonly used in abalone hatcheries to eliminate potential pathogenic organisms (Lafferty, 2001), is also effective for the removal of epiphytes from the kelp blades. This should be taken into consideration, because this treatment may affect the dietary quality of macroalgae, because it removes epiphytic diatoms, which have a high nutritional value (Simental et al., 2004).
ACKNOWLEDGMENTS
The first author acknowledges a CONACyT Ph.D. scholarship. Research supported by CICESE Project 7073 and CONACyT Projects 45844 and 239.
REFERENCES
Bold, H. C. & M. J. Wynne. 1985. Introduction to Phycology. 2nd. Edition. Prentice Hall. Englewood Cliffs. New Jersey. Unites States of America. 720 p. [ Links ]
Cruz–Suárez, L. E., D. Rique–Marie, M. Tapia–Salazar & C. GuaJardo–Ba. 2000. Uso de harina de kelp (Macrocystis pyrifera) en alimentos para camarón. In: Cruz–Suárez, L. E., D. Ricque–Marie, M. Tapia–Salazar, M. A. Olvera–Novoa. & R. Civera–Cerecedo (Eds.). Avances en Nutrición Acuícola V. Memorias del V Simposium Internacional de Nutrición Acuícola. Centro de Investigaciones Avanzadas–IPN. Mérida, Yucatán México. pp.19–22. [ Links ]
Fallu, R. 1991. Abalone Farming. Fishing News Books. Osney. 195 p. [ Links ]
Furst, A. 1987. Biological testing of Germanium. Toxicology and Industrial Health 3: 167–204. [ Links ]
Gledhill, M., M. T. Brown, M. Nimmo, R. Moate & S. J. Hill. 1998. Comparison of techniques for the removal of particulate material from seaweed tissue. Marine Environmental Research 45: 295–307. [ Links ]
Gómez–Villa, H., D. Voltolina, M. Nieves, P. Piña & J. López–Ruiz. 2002. Reduction of copper toxicity for two microalgae using artificial zeolites. Journal of the World Aquaculture Society 33: 214–219. [ Links ]
Guillard, R. L. L. 1975. Culture of phytoplankton for feeding marine invertebrates. In: Smith, M. L. & M. H. Chanley (Eds.). Culture of Marine Invertebrate Animals. Plenum Press, New York. pp. 29–60. [ Links ]
Hahn, K. O. 1989. Handbook of Culture of Abalone and Other Marine Gastropods. CRC Press Inc., Boca Raton, Florida. 348 p. [ Links ]
Lafferty, K. D. 2001. Restoration of the white abalone in Southern California: population, assessment, broodstock collection, and development of husbandry technology. Western Ecological Research Center, USGS. Final Report. 14 p. [ Links ]
Lázaro–Chávez, M. 1985. Sustancias, desinfectantes y drogas de utilidad en las piscifactorías: manual de usos. AGT Editor S.A., Mexico. 90 p. [ Links ]
Lewin, J. 1966. Silicon metabolism in diatoms. V. Germanium dioxide, a specific inhibidor of diatom growth. Phycologia 6: 1–12. [ Links ]
Mercado, J. M., M. P. Sánchez–Saavedra, J. G. Correa–Reyes, L. Lubián, O. Montero & F. L. Figueroa. 2004. Blue light effect on light absorption characteristics and photosynthesis of five benthic diatom species. Aquatic Botany 78: 265–277. [ Links ]
Plana, J., A. Mansilla, M. Palacios. & N. P. Navarro. 2007. Estudio poblacional de Macrocystis pyrifera (L.) C. Agardh (Laminariales: Phaeophyta) en ambientes protegido y expuesto al oleaje en Tierra del Fuego. Gayana 71(1): 66–75. [ Links ]
Shea, R. & Ch. Thierry. 2007. Effects of germanium dioxide, an inhibitor of diatom growth, on the microscopio laboratory cultivation stage of the kelp, Laminaria saccharina. Journal of Applied Phycology 19(1): 27–32. [ Links ]
Simental, J. A. 2005. Enriquecimiento de láminas de Macrocystis pyrifera con películas de Navicula incerta y su utilización como alimento para abulón (Haliotis spp.). Tesis Doctoral en Ciencias. Departamento de Acuicultura. Centro de Investigación Científica y de Educación Superior de Ensenada. Ensenada, Baja California, México. 112 p. [ Links ]
Simental, J. A., M. P. Sánchez–Saavedra & N. Flores–Acevedo. 2004. Growth and survival of juvenile red abalone (Haliotis rufescens) fed with macroalgae enriched with a benthic diatom film. Journal of Shellfish Research 23: 995–999. [ Links ]
Siqueiros–Beltrones, D. A. & D. Voltolina. 2000. Grazing selectivity of red abalone Haliotis rufescens postlarvae on benthic diatoms films under culture conditions. Journal of the World Aquaculture Society. 31(2): 239–245. [ Links ]
Siqueiros–Beltrones, D. A., E. Serviere–Zaragoza & U. Argumedo. 2002. Epiphytic diatoms of Macrocystis pyrifera (L.) C. Ag. from the Baja California Península. Oceánides 17(1): 31–39. [ Links ]
Siqueiros–Beltrones, D. A., S. Guzmán del Próo & E. Serviere–Zaragoza. 2005. Main diatom taxa in the natural diet of juvenile Haliotis fulgens and H. corrugata (Mollusca: Grastropoda) in Bahía Tortugas and Bahía Asunción, B.C.S., Mexico. Pacific Science 59 (4): 581–592. [ Links ]
Strickland, J. D. H. & T. R. Parsons. 1972. A Practical Handbook of Seawater Analysis. Second Edition. Bulletin of the Fisheries Research Board of Canada. 167. 311 p. [ Links ]
Vásquez, J. A. 1999. The effect of harvesting of brown seaweeds: a social, ecological and economical importance resource. Word Aquaculture Magazine 31(1): 19–22. [ Links ]
Voltolina, D. 1991. A comparison of methods for benthic diatom culture dispersion. Cryptogamie–Algologie 12: 183–187. [ Links ]
Zar, J. H. 1996. Biostatistical analysis. Third Edition. Prentice–Hall, Englewood Cliffs, N.J. 662 p. [ Links ]














