Study contribution
Through non-convensional foods is possible the modulation of the quantity and quality of intramuscular fat. The inclusion of avocado meal in the diet of pigs, using economic technologies available to pig producers, will help improve growth and meat quality, without affecting pig health indicators. With the study of transcriptomes, it was possible to explain the changes in the expression of genes, involved in various biological processes and to highlight their importance when modifying the diet of pigs using non-conventional feeds. With these methodologies we will be able to address other types of non-conventional foods, study their effects on growth, meat quality and animal health.
Introduction
Modulation of the quantity and quality of intramuscular fat (IMF) is possible, through modification of the lipid profiles in the diet.1,2 Several studies in Iberian pigs have reported that meat lipids increase the monounsaturated and polyunsaturated profiles and offer a healthy food to the consumer.2 Additionally, it has been reported that the composition of fat is moldable, through the implementation of diets rich in unsaturated fatty acids, and that according to the ingredients added to the feed, the expression of genes differs in each body tissue of the pig.3,4 Modification of feed ingredients in commercial pigs has shown to reduce their lipogenic potential, back fat and IMF. The expression of genes on lipid metabolism provides new insights into IM deposition and changes in the lipid profile.1,5,6 Therefore, researchers are interested on the explanation of the effect of the diet on the molecular regulation of lipogenesis.7 It has been shown that the diet modifies the proportions of lipids in meat, providing benefits to the consumer’s health; however, increasing the polyunsaturated acids in the diet, meat is more prone to oxidative degradation of lipids. The use in the diet of natural antioxidants such as avocado (Persea americana Mill) could help to slow this process, improving meat quality.8,9 The avocado is a fruit with important nutritional characteristics, providing fat-soluble vitamins (Vitamin E), antioxidants, phenolic compounds, zero cholesterol and a low content of saturated fatty acids, but a high content of unsaturated fatty acids.10 In the fatty acids of the AM there is a nutritional strengthening to the availability of MUFA and PUFA in a high proportion, which makes it an interesting source to incorporate them into animal feed at a lower cost than commercial oils.11 Feeding whole avocado in the meal of pigs could increase polyunsaturated fatty acids in animals and the expression of genes involved in lipid metabolism. Therefore, the objectives of this research were 1) to use transcriptome analysis of L. dorsi and liver to identify the genes involved in the specific biological process, to design strategies for the use of avocado in the differentiated value of pig meat. 2) To determine the effect of the inclusion of avocado meal, in the diet of Landrace-Yorkshire pigs, finished 56 days before slaughter on growth, carcass, muscle fatty acid composition and blood metabolites.
Materials and methods
Ethical statement
This research complied with the requirements of the Research and Postgraduate Secretary of the Autonomous University of Nayarit, with registration SIP15-65.
Animals and diets
Twenty-four castrated Landrace-Yorkshire pigs with an initial weight of 55 ± 3 kg were used, housing one pig per pen with individual access to food and water. Pigs were fed ad libitum, according to the recommendations of the official Mexican animal welfare norm (NOM-062-ZOO-1999). After five days of adaptation to the diets, plus 56 days of experimental study, the pigs were sacrificed by a method approved by national regulation contained in NOM-033-SAG/ZOO-2014 (Lightheadedness followed by cutting of caval veins and brachiocephalic trunk), at an average live weight of 109 ± 4 kg. Eight pigs were assigned to each diet. The diets had 0, 5 and 10 % avocado meal (AM) (Tables 1, 2). The AM was obtained as described9 and the proximal chemical characteristics and fatty acid profile have been reported.11 Hass avocados discarded for human consumption due to their small size and/or physical damage were used in the diets. The fruits were stored at room temperature until they reached maturity. To obtain a homogeneous mixture of ripe and whole avocados (pulp, seed, and shell), the fruit was ground in a mobile hammer mill without a sieve, driven by a 5 HP gasoline engine. The fresh paste was stored at room temperature without additives, in plastic containers, and then, it was left at room temperature for four days until was dried. Then, the dry paste was ground again to incorporate it into the pig diets.
Table 1 Ingredients (%) of experimental diets (on dry matter basis)
| Diets1 | |||
| Ingredients | AM0 | AM5 | AM10 |
| Corn | 81.205 | 75.780 | 70.490 |
| Avocado meal | 0 | 5 | 10 |
| Soybean meal | 15.3 | 15.65 | 15.95 |
| L-Lysine | 0.125 | 0.12 | 0.11 |
| Calcium carbonate | 0.82 | 0.82 | 0.82 |
| Calcium phosphate | 0.65 | 0.73 | 0.73 |
| NaCl | 0.10 | 0.10 | 0.10 |
| Vitamins and minerals premix | 0.30 | 0.30 | 0.30 |
| Zeolite | 1.50 | 1.50 | 1.50 |
| Total | 100.00 | 100.00 | 100.00 |
1Avocado meal (AM) levels of 0, 5 and 10 percentage.
Table 2 Chemical composition and fatty acid analysis (%) of experimental diets (on dry matter basis)
| Diets1 | |||
| Ingredients (%) | AM0 | AM5 | AM10 |
| Metabolizable energy (Mcal/kg) | 3.85 | 3.96 | 4.08 |
| Crude protein (%) | 14.00 | 14.01 | 14.00 |
| Lysine (%) | 0.75 | 0.75 | 0.75 |
| Methionine (%) | 0.24 | 0.23 | 0.23 |
| Threonine (%) | 0.50 | 0.50 | 0.50 |
| Ca (%) | 0.50 | 0.51 | 0.51 |
| Total phosphate (%) | 0.45 | 0.45 | 0.44 |
| Na (%) | 0.06 | 0.06 | 0.06 |
| Cl (%) | 0.11 | 0.10 | 0.10 |
| C14:0 | 2.10 | 4.95 | 4.10 |
| C14:1c9 | 0.37 | 0.24 | 0.27 |
| C15:0 | 0.35 | 2.02 | 0.32 |
| C15:01 | 1.30 | 1.51 | 0.12 |
| C16:00 | 16.00 | 19.56 | 14.58 |
| C16:1c9 | 1.15 | 3.73 | 1.71 |
| C17:00 | 0.21 | 1.08 | 0.48 |
| C17:1c10 | 1.16 | 0.89 | 0.15 |
| C18:00 | 2.40 | 1.00 | 1.23 |
| C18:1c9 | 21.91 | 27.13 | 31.18 |
| C18:2n-6 cis | 50.19 | 32.53 | 42.06 |
| C18:2n-6 trans | 0.41 | 0.48 | 0.45 |
| C18:3 n-3 | 0.48 | 3.15 | 1.29 |
| C20:00 | 1.97 | 1.74 | 2.05 |
1Avocado meal (AM) levels of 0, 5 and 10 %.
Analysis of transcriptome on in the L. dorsi muscle and liver
Three pig RNA samples were considered per experimental diet for L. dorsi muscle and liver, (n = 18 total samples) and 75 mg of each tissue per pig was taken for RNA extraction. RNA extraction was performed using the nucleic acid extraction kit Direct-zol™ RNA MiniPrep (Zymo Research, USA); whereas, the RNA concentration and purity were quantified by spectrophotometry with a Nanodrop. Massive sequencing was carried out in the UUSMB-UNAM (University Mass Sequencing and Bioinformatics Unit) sequencing service, with Illumina methodology, on a Nextseq 500 device (www.illumina.com/company/legal.html), pared (pair end) of 76 bp, from 18 pork samples, with average quality above Q28 for all samples in all cycles. The sequences obtained were mapped against the Sscrofa11.1 pig reference genome using the Smalt 0.7.6 program (https://bioweb.pasteur.fr/packages/pack@smalt@0.7.6). The per gene counts were carried out using the Bamtools (https://bio.tools/bamtools), coverage bed program (27 280 records), a script in Perl to filter exclusively coding genes for proteins (16 841 records) with final a reading of 15 760 records. With these records, differential expression analysis of L. dorsi muscle and liver, for AM5 vs AM0 and AM10 vs AM0 was done. IDEAmex software (http://www.uusmb.unam.mx/ideamex/),12 was used to apply the EdgeR and DESeq2 methods, and obtain differentially gene expression (DGE), with a reliability statistic of P ≤ 0.01 and a log2 fold change ≥ 1.5.
Growth and carcass traits
At the end of the experimental period, average daily gain (kg), average daily feed intake (kg), feed/gain ratio, slaughter weight (kg), hind carcass weight (kg) without viscera and skin, carcass (%), ham (kg) and back fat (mm) were registered.
Intramuscular fatty acids profile of L. dorsi muscle
After the slaughter of pigs, 100-g L. dorsi muscle samples were collected, and the lipid composition was determined according to the method 920.39.13 To extract L. dorsi muscle lipids, 500 mg of fat was converted into methyl esters fatty acids using chromatographic analysis.
Blood metabolites in finishing pigs
At slaughter, blood samples were obtained from pigs for biochemical assays, which were carried out using Byosistem A160 spectrophotometry equipment. Glucose (mg/dL). Lipid profile: Total cholesterol (mg/dL), triglycerides (mg/dL), HDL (high density cholesterol) (mg/dL), LDL (low density cholesterol) (mg/dL), VLDL (very low density cholesterol) (mg/dL) and HDL/LDL ratio. Liver profile: Urea (mg/dL), Uric acid (mg/dL), R A/G (Albumin/Globulin Ratio), TGO (oxalacetic glutamic transaminase) (mg/dL), TGP (pyruvic glutamic transaminase) (mg/dL) and GGT (gamma-glutamil transferase) (mg/dL) were also measured.
Statistical analysis
The effect of diet on growth, carcass, L. dorsi muscle, fatty acid composition and blood metabolites was determined using one-way analysis of variance under a completely random experimental design, and mean comparisons by Tukey test. Additionally, linear and quadratic regression analyses of the levels of inclusion of AM and measured variables were carried out. Gene annotation: biological function using the NCBI database and Ensembl pig genome databases (http://uswest.ensembl.org/Sus_scrofa/Info/Index), ShinyGO v0.61: Gene Ontology Enrichment Analysis (http://bioinformatics.sdstate.edu/go/) and PHANTER GO (http://geneontology.org/) were searched.
Results
Transcriptome analysis of the L. dorsi muscle and liver
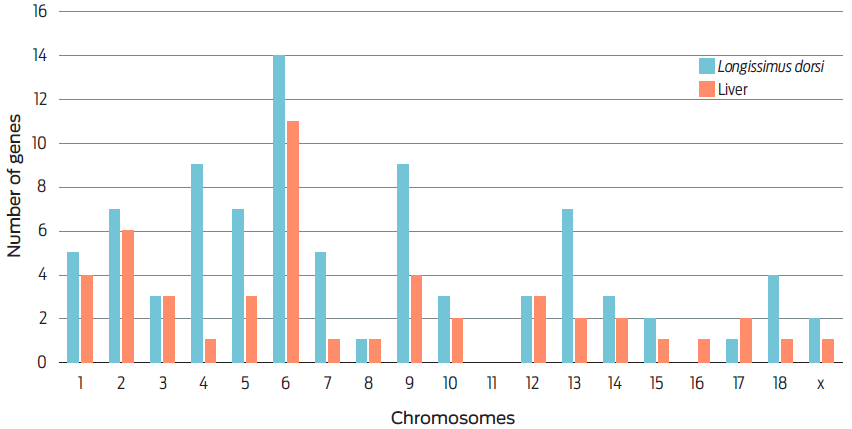
The transcriptome analysis revealed higher DGE for AM10 vs AM0 on L. dorsi muscle and liver (Table 3), and higher DGE in both tissues, with down log2 fold change by including 5 or 10 % AM in the diet. Furthermore, higher DGE in both tissues was observed in chromosomes (Chr) 6 and 2, but unidentified in Chr 11 (Figure 1).
Table 3 Transcriptome analysis in L. dorsi muscle and liver
| Tissues-AM1 | D2 | Max P<3 | Min P<3 | Up4 | diff up FC5 |
Maxup FC |
Minup FC |
Dow6 | diff dow FC7 |
MaxdowFC | Min dowFC |
| L. dorsi muscle | |||||||||||
| AM5vsAM0 | 10 | 1.0-02 | 6.8-17 | 4 | 2.7 | 3.44 | 2.2 | 6 | -3.85 | -2.13 | -6.52 |
| AM10vsAM0 | 77 | 9.6-03 | 1.0-45 | 30 | 2.1 | 3.88 | 1.5 | 47 | -2.55 | -1.52 | -8.13 |
| Liver | |||||||||||
| AM5vsAM0 | 19 | 4.1-02 | 3.2-22 | 3 | 3.9 | 5.62 | 2.8 | 16 | -5.75 | -2.99 | -9.74 |
| AM10vsAM0 | 35 | 9.0-03 | 1.4-12 | 9 | 2.5 | 3.46 | 1.9 | 26 | -3.40 | -1.79 | -5.4 |
1Avocado meal (AM) levels of 0, 5 and 10 % comparison; 2Total genes differential gene expression (D); 3Probability value (P <); 4Genes Up (UP); 5Differential up log2 Fold change (FC); 6Genes down (Dow); 7Differential down log2 Fold change (FC);
Tables 4 to 7 show higher DGE in L. dorsi muscle and liver when AM5 and AM10 diets were compared with the control diet. Higher DGE in AM10 was observed in the anatomical structure development and morphogenesis of L. dorsi muscle in, as was the effect identified in the biological processes: regulation of cellular component biogenesis and cell proliferation, regulation of the immune system process and system development, response to stress and cellular response, including lipid metabolism. With respect to the liver, AM5 and AM10 diets had higher DGE with down expression in anatomical structure development and morphogenesis. However, comparation of AM5 and AM0 diets, identified down DGE in liver biological process: Lipid metabolism, negative regulation of growth, response to stress and cellular response. AM10 and AM0 diets comparison showed down DGE for negative regulation of growth, regulation of cellular component biogenesis and cell proliferation, regulation of the immune system process and system development, response to external stimuli, response to stress and cellular response; in addition, to transport of fatty acids or hormones.
Table 4 Biological processes identified in L. dorsi muscle according to differential gene expression and diet AM5 vs AM01 comparison
| High level GO category biological process | Genes up DGE2 | Genes down DGE2 |
| Anatomical development and morphogenesis | CPXM2 RELT | ATF3 PTPRO TNIK |
| Cell component | TMEM238 | |
| Lipid metabolism | G0S2 | |
| Cellular biogenesis and Cell proliferation | IER5 PTPRO TNIK | |
| Regulation of immune system and development | OTUD1 | |
| Response to stress and celular response | UFL1 | |
| Thermogenesis and response to stimulus | G0S2 | IER5 PTPRO TNIK |
1Avocado meal (AM) levels of 0 and 5 %; 2Differential gene expression (DGE)
Table 5 Biological processes identified in L. dorsi muscle according to differential gene expression and diet AM10 vs AM0 comparison
| High level GO category biological process |
Genes up DGE2 | Genes down DGE2 |
| Anatomical development and morphogenesis | ARNTL ARRDC2 CRHR2 PVALB SUSD1 TRNP1 |
ABRA ATF3 BTG2 CEBPD CSRNP1 C1QC C4BPA FGF6 FOS F3 IER5 JUN LMOD2 MYC PCK2 RAMP3 SDC4 SERPINE1 TOB2 TRIB1 XIRP1-CMYA1 |
| Carbohydrate binding | CD209 | |
| Cell component | CCDC181 SNAI3 TMEM140 | |
| Circadian rhythms and behavior | ARNTL BHLHE40 | PER1 PER2 PER3 SIK1 |
| Lipid metabolism | MYLIP | PHGDH PLBD1 SDC4 |
| Cellular biogenesis and cell proliferation | AQP7 GJA9 LOC100518436 MICAL2 MLXIPL PTGER3 RGS14 RPRM |
CNN1 CTGF CYR61 C1QC FGF6 F3 LMOD2 MN1 PHGDH PSAT1 SCD4 SRPX TOB2 TRIB1 |
| Regulation of immune system and development | C1QTNF7 HP NCF4 NFIL3 |
ARMC12 CD163 CEBPD C1QC HSP70.2 JUN LOC100511275 OTUD1 PDE4B PPP1R15A SDC4 TOB2 TRIB1 |
| Response to stress and cellular response | RGS14 | ATF3 F3 PCK2 PNMT SDC4 SIK1 SRPX TRIB1 ZFP36 |
| Thermogenesis and stimulus | AQP7 | HSP70.2 |
| Transmembrane transport, localization | ITIH1 SLC43A2 SLC7A8 SLC9A1 | SLC38A2 |
| No information | C12H17orf53 KLHL38 ZNF672 | CRYBG3 FAM46B GPA33 |
1Avocado meal (AM) levels of 0 and 10 %; 2Differential gene expression (DGE).
Table 6 Biological processes identified in liver according with differential gene expression and diets AM5 vs AM01 comparison
| High level GO category biological process |
Genes up DGE2 | Genes down DGE2 |
| Anatomical development and morphogenesis | IGFBP1 LOC100524016 SOCS2 | |
| Cell component | TMEM52B | |
| Circadian rhythms and behavior | SIK1 | |
| Glycogen metabolic process | PPP1R3C PFKFB3 | |
| Lipid metabolism | ALOX15 ANO3 LIPG | |
| Negative regulation of growth | LOC100739663 LOC102166944 MT1A MT1D | |
| Protein metabolism | LOC100512873 | |
| Regulation of cellular biogenesis and cell proliferation | ANO3 | |
| Regulation of immune system and development | ADGRD2 | MCEMP1 TGM3 |
| Response to stress and celular response | LOC100739663 LOC102166944 MT1A MT1D SIK1 | |
| No information | LOC100518075 |
1Avocado meal (AM) levels of 0 and 5 %; 2Differential gene expression (DGE).
Table 7 Biological processes identified in liver according with differential gene expression and diets AM10 vs AM0 comparison
| High level GO category biological process |
Genes up DGE2 | Genes down DGE2 |
| Anatomical development and morphogenesis | ARNTL PLIN4 SLC30A10 | CSF3R HAVCR2 NOCT SYNDIG1 ZBTB16 |
| Circadian rhythms and behavior | ARNTL | DBP NOCT PER1 |
| Glycogen metabolic process | SDS | |
| Lipid metabolism | PLIN4 | ANO3 |
| Negative regulation of growth | LOC100739663 LOC102166944 MT1A MT1D | |
| Protein metabolism | EMILIN2 SDS | |
| Regulation of cellular biogenesis and cell proliferation | ANO3 HAVCR2 NNMT NOCT PDXP PEBP4 SULT1A3 SYNDIG1 ZBTB16 | |
| Regulation of immune system and development | CISH | CSF3R HAVCR2 TGM3 ZBTB16 CRP |
| Response to external stimulus | RNF125 SLC30A10 | ARHGEF6 CRP CSF3R CXCR4 FKBP5 GADD45B HAVCR2 NOCT PDXP |
| Response to stress and celular response | LOC100739663 LOC102166944 MT1A MT1D | |
| Transport of fatty acids or hormones | DBP | |
| No information | LOC100518075 |
1Avocado meal (AM) levels of 0 and 10 %; 2Differential gene expression (DGE).
Eleven genes were identified with DGE in more than one comparison (Table 8). Stand out genes associated with biological processes related to growth, response to stress, lipid metabolism and circadian rhythms with down expression, most of them in the liver. The ARNTL gene was up expressed in both tissues evaluated in pigs fed the AM10 diet, associated with anatomical structure development and morphogenesis, as well as circadian rhythms of locomotor activity and behavior.
Table 8 Top differential gene expression with biological processes identified in more than one diet comparison
| GENE | Comparison1 | FC2 | Comparison1 | FC2 | Chr3 | Biological process |
| ANO3 | LiAM5-LiAM0 | -6.87 | LiAM10- LiAM0 | -4.28 | 2 | Lipid metabolism |
| ARNTL | LDAM10-LDAM0 | 1.73 | LiAM10-LiAM0 | 1.99 | 2 | Anatomical structure |
| LOC100518075 | LiAM5-LiAM0 | -4.04 | LiAM10- LiAM0 | -3.8 | No information | |
| LOC100739663 | LiAM5- LiAM0 | -3.8 | LiAM10- LiAM0 | -5.4 | 6 | Negative regulation of growth |
| LOC102166944 | LiAM5-LiAM0 | -8.2 | LiAM10- LiAM0 | -5.1 | 6 | Negative regulation of growth |
| MT1A | LiAM5-LiAM0 | -7.9 | LiAM10- LiAM0 | -4.5 | 6 | Negative regulation of growth |
| MT1D | LiAM5-LiAM0 | -7.9 | LiAM10- LiAM0 | -4.6 | 6 | Negative regulation of growth |
| OTUD1 | LDAM5-LDAM0 | -6.5 | LDAM10-LDAM0 | -4.8 | 10 | Regulation of immune system |
| PER1 | LDAM10-LDAM0 | -4.3 | LiAM10- LiAM0 | -2.5 | 12 | Circadian rhythms |
| SIK1 | LDAM10-LDAM0 | -2.3 | LiAM5-LiAM0 | -3.2 | 13 | Circadian rhythms |
| TGM3 | LiAM5-LiAM0 | -3.9 | LiAM10- LiAM0 | -5.3 | 17 | Regulation of immune system |
1Liver (Li); Avocado meal (AM) levels of 0, 5 and 10 %; L. dorsi muscle (LD); 2 log2 Fold change (FC); 3Chromosome (Chr).
Growth, carcass, L. dorsi muscle, fatty acid composition and blood metabolites
The effect of diets on growth, carcass, L. dorsi muscle fatty acid composition and blood metabolites are shown in Tables 9, 10, and 11, respectively. Including 5 and 10 % AM in the diet increased daily gain, daily feed intake, slaughtered body weight, carcass weight and ham. The highest back fat mean was observed for the AM10 diet. All measured traits increased with AM, present significant quadratic regression effects. The AM diets had a negative linear regression effect on IMF. The AM10 diet had the highest values of linoleic acid, total fatty acids Ω6, ∑ polyunsaturated fatty acids, PUFA/SFA, and PUFA/MUFA; all variables with a positive linear regression effect. The inclusion of AM in the diet did not affect glucose, total cholesterol, lipoproteins, metabolites, or enzymes of liver function. Interestingly, while the 5 % AM diet decreased the triglycerides and VLDL content, the AM10 diet did not.
Table 9 Means and effects by level of avocado meal in the diet for growth and carcass
| Variables/Diets1 | AM0 | AM5 | AM10 | SEM2 | bo | b1 | b2 | P-level |
| Initial body weight (kg) | 54.62 | 55.65 | 56.41 | 0.72 | ||||
| Average daily gain(kg) | 0.74b | 1.07a | 1.02a | 0.04* | 0.74 | 0.1 | -0.01 | * |
| Daily feed intake (kg) | 2.81b | 3.63a | 3.76a | 0.06*** | 2.81 | 0.23 | -0.01 | *** |
| Feed/gain ratio | 3.8 | 3.45 | 3.71 | 0.14 | ||||
| Slaughtered weight (kg) | 101.4b | 115.8a | 109.5ab | 1.89* | 101.4 | 4.93 | -0.41 | * |
| Hind carcass weight (kg) | 53.13b | 64.87a | 58.28ab | 1.49* | 53.13 | 4.18 | -0.37 | * |
| Carcass percent (%) | 52.37 | 55.99 | 53.33 | 1.02 | ||||
| Ham (kg) | 8.95b | 11.50a | 10.07ab | 0.36* | 8.95 | 0.91 | -0.08 | * |
| Back fat (mm) | 23.40b | 16.91c | 31.67a | 0.9*** | 23.4 | -3.42 | 0.43 | *** |
| Intramuscular fat (%) | 8.1a | 7.31ab | 5.94b | 0.46* | 8.15 | -0.21 | * |
1Avocado meal (AM) levels of 0, 5 and 10 %; 2Pooled standard error (SEM); abcDifferent literals by row indicate differences between diets; *P < 0.05; ***P < 0.001; Regression interception (bo); Linear coefficient (b1); Quadratic coefficient (b2).
Table 10 Means and effects by level of avocado meal in the diet for fatty acids profile (%)
| Variables/diets1 | AM0 | AM5 | AM10 | SEM2 | P< | bo | b1 | b2 | P< |
| ∑ Saturated fatty acids | 33.14 | 32.55 | 31.12 | 0.51 | |||||
| Oleic | 48.98 | 48.6 | 48.26 | 0.4 | |||||
| ∑ Monounsaturated | 55.78 | 55.94 | 55.07 | 0.31 | |||||
| Linoleic | 10.09b | 10.06b | 12.91a | 0.47 | *** | 10.09 | -0.29 | 0.06 | *** |
| Total fatty acids Ω6 | 10.43b | 10.74b | 13.35a | 0.45 | *** | 10.44 | -0.17 | 0.05 | *** |
| α Linolenic | 0.45 | 0.5 | 0.36 | 0.04 | |||||
| Total fatty acids Ω3 | 0.64 | 0.77 | 0.47 | 0.07 | |||||
| ∑ Polyunsaturated | 11.08b | 11.51b | 13.82a | 0.43 | *** | 10.77 | 0.274 | *** | |
| PUFA/SFA relation | 0.33b | 0.35b | 0.45a | 0.02 | * | 0.322 | 0.011 | * | |
| MUFA/SFA relation | 1.69 | 1.72 | 1.78 | 0.04 | |||||
| PUFA/MUFA relation | 0.20b | 0.21b | 0.25a | 0.01 | *** | 0.19 | 0.005 | *** |
1Avocado meal (AM) levels of 0, 5 and 10 %; 2Pooled standard error (SEM); abcDifferent literals by row indicate differences between diets; *P < 0.05; ***P < 0.001; Regression interception (bo); Linear coefficient (b1); Quadratic coefficient (b2).
Table 11 Means and effects by level of avocado meal in the diet for blood metabolites
| Variables (mg/dL)/diets1 | AM0 | AM5 | AM10 | SEM2 | P< | bo | b1 | b2 | P< |
| Glucose | 91.25 | 160 | 115 | 12.5 | 91.25 | 25.1 | -2.28 | ||
| Cholesterol total | 85.5 | 84.2 | 89 | 2.73 | |||||
| Triglycerides | 99.25a | 77.80b | 90.00ab | 3.39 | * | 99.25 | -7.65 | 0.67 | * |
| HDL | 52.25 | 55.04 | 57.85 | 4.37 | |||||
| LDL) | 21.3 | 18.98 | 13.15 | 2.67 | |||||
| VLDL | 19.85a | 15.56b | 18.00ab | 0.68 | * | 19.85 | -1.53 | 0.14 | * |
| HDL/LDL ratio | 2.75 | 3.73 | 4.44 | 0.6 | |||||
| Urea | 60.5 | 38.2 | 43.5 | 6.03 | |||||
| Uric acid | 0.95a | 0.45ab | 0.21b | 0.1 | * | 0.91 | -0.08 | * | |
| TGO2 | 61.5 | 82.5 | 56.5 | 12.6 | |||||
| TGP2 | 50.75 | 63.25 | 55 | 5.37 | |||||
| GGT2 | 31.5 | 41.5 | 47 | 2.46 | 32.14 | 1.61 |
1Avocado meal (AM) levels of 0, 5 and 10 %; 2Pooled standard error (SEM); abcDifferent literals by row indicate differences between diets; *P < 0.05; ***P < 0.001; Regression interception (bo); Linear coefficient (b1); Quadratic coefficient (b2). 2TGO: oxalacetic glutamic transaminase; TGP: pyruvic glutamic transaminase; GGT: gamma-glutamil transferase.
Discussion
Transcriptome analysis in L. dorsi muscle and liver
The inclusions of 5 or 10 % of AM in the diet caused higher DGE in L. dorsi muscle and liver, identified with down log2 fold change. It is considered that the liver plays an important role in the lipid metabolism of IMF.14 However, L. dorsi muscle also had a high number of DGE. This effect is observed in the decrease in IMF and in the changes in fatty acids of pigs fed the AM10 diet. In both tissues, higher DGE was identified in chromosomes 6 and 2 of pigs on AM5 and AM10 diets.
The above referred chromosomes are involved in growth and meat quality in pigs (https://www.animalgenome.org/cgi-bin/QTLdb/SS/browse). With top DGE and down log2 fold, comparison of AM5 or AM10 vs AM0 diets identified the ANO3 gene, located in Chr 2, which is involved in lipid metabolism, associated in this study with a decrease in IMF. The down DGE of the PHGDH gene in L. dorsi muscle, involved in muscle growth, and in the liver,15 the down DGE of LOC100739663, LOC102166944, MT1A, and MT1D genes, involved in the negative regulation of growth, could be associated with the AM10 diet. Moreover, slaughter weight, hind carcass weight and ham decreased in the AM5 diet. Other DGEs with up expression in L. dorsi muscle involved in lipid metabolism were GOS2 in AM5 vs AM0, and MYLIP in AM10 vs AM0 diets; and in the liver the PLIN4 gene with AM10 vs AM0.
The up expression of the GOS2 gene in L. dorsi muscle from Laiwu pigs, was associated with fat metabolism and changes in fatty acids.6 The MYLIP gene that regulates myosin, LDL and VLDL, acts as a sterol-dependent inhibitor of cellular cholesterol uptake by mediating ubiquitination and subsequent degradation of LDL, and may be associated with the low value of LDL in AM10. The PLIN4 gene located in Chr 2 is strongly associated with fatty acid composition in the Longissimus thoracis muscle of Iberian pigs,16 which could influence the AM10 diet to have more changes in the fatty acid profile. Important genes related to anatomical structure development and morphogenesis were identified here, showing a higher down DGE in the AM10 diet, in L. dorsi muscle and liver. The ARNTL gene located in Chr 2 showed up DGE in both tissues (AM10 vs AM0 diet).
Other auhors found that this gene is a master regulator in the Alentejano obese breed,17 being activated and involved in the proliferation of fibroblast cell lines and the activation of myeloid cells. This is an indication of connective tissue development and an active state of the innate immune system, probably due to inflammation associated with obesity. The ARNTL gene could be associated with the results obtained when feeding AM10, since backfat was increased.
We found a down expression of the FOS gene in the Chinese Debao pig compared with Landrace, which influenced the pork quality between those breeds, being better for Debao pigs.18 The FOS gene, which is associated with the differentiation of adipocytes, in this work, had a down DGE in L. dorsi muscle (AM10 vs AM0 diet), as well as in the other 21 genes identified. The genes PER1, PER2, PER3, and SIK1 that are important for circadian rhythms of locomotor activity and behavior,19 showed down DGE in L. dorsi muscle for the AM10 diet vs AM0; and in the liver, the SIK1 gene (AM5 vs AM0), and PER1, NOCT, and DBP genes (AM10 vs AM0).
The ARNTL gene, which is associated with the circadian clock, had the opposite result: up DGE in L. dorsi muscle and liver for AM10 diet, but did not affect the circadian clock. In the pig muscle, nutrition modulates the circadian clock and those genes change their expression in response to nutrient intake.19 These authors reported that in pigs given food after fasting, the expression of ARNTL gene increased and the expression of PER1, PER2, PER3, SIK1 decreased. The ARNTL gene is a regulator of the circadian clock repressor genes PER1, PER2, and PER3, whose expression decreases due to environmental effects, while increasing that of repressor genes,20 which did not happen for AM10.
The NOCT gene is also associated with the circadian clock as it promotes adipogenesis and resistance to high-fat diets when knockout.21 Therefore, in the AM10 diet, the NOCT gene is down expressed, which could favor an increase in backfat. Higher down DGE for regulation of the immune system process were identified by feeding AM10 vs AM0 in both tissues. Within those, OTUD1, CD163 and CRP genes had similar reports where the low expression was favorable to the animal. The OTUD1 gene is a crucial negative regulator of innate antiviral immunity, in knockout cells and mice, where more resistance to lethal infections is reported.22 The CD163 gene in knockout pigs is fully resistant to high-pathogenic porcine reproductive and respiratory syndrome virus.23 The CRP gene also increases its levels as an indicator of inflammatory processes.24
Other up DGE elements, favorable for regulating the immune system process, identified when animals were fed AM10 and AM0 diets were C1QTNF7 gene, related to the immune response; HP, an antioxidative molecule that prevents the hemoglobin driven generation of hydroxyl radicals and lipid peroxides; NCF4 superoxide-generating NAD(P)H oxidase activity, and NFIL3 involve in the cellular response to interleukin-4 (www.ensembl.org/Sus_scrofa/Gene/Summary).25
Growth, carcass, L. dorsi muscle, fatty acid composition and blood metabolites
Feeding pigs before slaughter improved feed consumption when AM in the diet was included. This increased the daily consumption of metabolizable energy, oleic, linoleic and linolic fatty acids since AM contains a high amount of these monounsaturated and polyunsaturated fatty acids. However, the inclusion of 5 to 10 % AM in the diet showed a quadratic regression effect suggests a tendency to decrease as the inclusion of AM increases, which limits the increase in the levels of inclusion of AM in the diets. This could be due to the high content of tannins in AM that could have depressed feed consumption.11,26 Therefore, although the growth and carcass of pigs increased from 0 to 5 % AM the rate of improvement was less o declined when 10 % AM was fed. A different situation was found with diets rich in sunflower in Duroc pigs, where no effects on growth and fattening traits were found.3,4 However, sunflower affected the constitution of fatty acids with higher PUFA and the activation of the de novo lipid synthesis, as observed here in pigs fed AM.
Feeding AM5 and AM10 increased the average daily gain, slaughtered body weight, hind carcass weight and ham; with higher back fat and lower IMF in L. dorsi in the AM10 diet. All these variables increased with dietary AM, with a significant quadratic regression effect, suggesting a tendency to decrease as the inclusion of AM increases; caused by the fact that the consumption of AM does not have a linear effect, which limits the nutrients available for growth. Our results for growth and carcass traits were higher than those reported for Yorkshire and Duroc,3,27 where a linear decrease in IMF was observed.
The pigs fed the AM10 diet had higher values of linoleic acid, total fatty acid Ω6, ∑ polyunsaturated fatty acids, PUFA/SFA and PUFA/MUFA, which is suggestive of a greater activation of the de novo lipid synthesis with this diet. Those traits had a positive linear regression effect, although the feed consumption was not linear. This trend indicates that the greater nutrient content in the AM10 vs AM5 diets (i.e. Mcal/kg ME, oleic, linoleic and arachidonic) influenced results, which is further supported by the fact that there is a greater amount of tocopherol and antioxidants in the AM.8,9 Therefore, it is reasonable to conclude that the profile of fatty acids in adipose tissue could be modified by the source of lipids since similar results for MUFA and PUFA were reported28 in pigs fed diets with 10 % sunflower oil, linseed oil, or a combination of fish oil-linseed.
Additionally, including avocado paste in the diet of Yorkshire-Landrace pigs was reported to increase PUFA in the L. dorsi muscle and decrease IMF.29 These authors concluded that breed differences exist between Chinese and Yorkshire pigs in the fatty acids,27 in accordance with previous studies.3,4,7,30 This experimental evidence agrees with the fact that local pig breeds had a higher content of MUFA and lower PUFA, and that both could be modified with lipid sources in the diet of commercial pig breeds.2
In this study, the inclusion of AM5 and AM10 in the diet did not affect MUFA content, but the decrease in IMF with higher PUFA is possibly associated with a decrease in triglycerides. According to investigations,7,31 the balance between synthesis, degradation and uptake of triglycerides is reflected in IMF content as intramuscular triglycerides are not only stored in the adipocytes but also as droplets in the myofiber cytoplasm. It has been reported that a high glycolytic activity reduces the deposition of IMF.31 This phenomenom could explain the increase of muscle deposition in pigs fed the AM10 diet at the expense of a lower IMF content. IMF fatty acid composition is a complex polygenic trait whose variability depends on feeding aspects and the genetic background of the pig population1,2,6,17. It is necessary to identify the factors that affect the composition of the fatty acids in meat, to find a balance between the demands of consumers and the industry.
Conclusions
Transcriptome analysis revealed higher DGE for AM10 vs AM0 diet for L. dorsi muscle and liver associated with lipid metabolism, growth, circadian clock, immune system and antioxidative activity. Adding AM to the diet increased daily gain, daily feed intake, slaughtered body weight, carcass weight and ham, with a negative linear effect on IMF. The AM10 diet had the highest values of back fat mean, linoleic acid, total fatty acids Ω6, ∑ polyunsaturated fatty acids, PUFA/SFA, and PUFA/ MUFA. Glucose, total cholesterol, lipoproteins, as well as metabolites and enzymes of liver function were not affected by the inclusion of AM. It is possible to include 10 % AM in the diet of pigs and modify the gene expression and quality of the IMF.
Data availability
All relevant data are included in this manuscript. Data sets used and analyzed in this experiment are available from the corresponding author on reasonable request.
Acknowledgments
We especially thank Veronica Jimenez Jacinto for her technical assistance in transcriptome analysis.
Conflicts of interest
The authors have no conflicts of interest to declare in regard to this publication.
Author contributions
Conceptualization: C Lemus-Flores.
Funding acquisition: C Lemus-Flores, JO Bugarín, F Grageola, K Mejía, R Valdivia.
Formal analysis: C Lemus-Flores, JC Segura.
Investigation: C Lemus-Flores, JO Bugarín, K Mejía.
Methodology: C Lemus-Flores, JO Bugarín, K Mejía.
Software: C Lemus-Flores, JC Segura.
Writing - original draft: C Lemus-Flores, JO Bugarín, F Grageola.
Writing- review and editing: C Lemus-Flores, JC Segura, R Valdivia.
All authors have read and agreed to the published version of the manuscript.











 nueva página del texto (beta)
nueva página del texto (beta)