Introduction
Gastric cancers are one of the most common gastrointestinal system tumors in the world1. In spite of advancements in the treatment modalities such as chemotherapy and radiotherapy, surgery for gastric cancers is still the most important and primary treatment. Lymph node metastasis is undoubtedly the most important independent risk factors for survival in gastric cancer2-5. The performants of D2 or D3 lymph node dissection (LND) in addition to radical gastrectomy is still controversial. Far Eastern surgeons perform gastrectomy plus D2 LND as the standard practice, whereas Western surgeons believe that D1 LND is sufficient since D2 LND and D1 LND are not superior to each other not only in terms of higher morbidity and mortality rates of D2 LND but also in terms of survival rates of patients undergone D2 LND and D1 LND6-10. However, in recent years, western centers have reported that D2 LND can be performed with low mortality and morbidity in selected patients11-13. Randomized control studies have shown that D2 LND are linked to increased survival14-16. However, the study by Japan Clinical Oncology Group (JCOG) reported no significant difference in survival rate between patients that underwent D2 or D2 plus para-aortic LND17,18. Many of these studies added para-aortic lymph node group to D2 LND. Therefore, only a few studies explaining the clinical role of lymph nodes other than para-aortic lymph nodes, especially No. 13 lymph node dissection. Wu et al. reported a significantly increase in survival in patients undergoing D3 LND15. According to the Japanese classification, D2 plus No. 13 lymph node dissection is recommended when the tumor has invaded the duodenum. Dissection of posterior pancreatic head lymph nodes in combination D2 LND and D2 plus No. 13 lymph node dissection has been reported to be beneficial for survival in advanced stage tumors with antrum localization19. The incidence of No. 13 lymph node metastasis is around 6.7%20.
Considering that the incidence rate of retropancreatic lymph node metastasis is not negligible, it is important to determine the clinical significance and risk factors of this condition in terms of metastasis. The aim of this study was to determine the risk factors for retropancreatic lymph node metastasis and to investigate its clinical significance.
Materials and methods
After obtaining the approval from the Clinical Research Ethics Committee of the Gazi University (2018/107), the treatments of a total of 315 patients with the diagnosis of gastric cancer were planned at the Gazi University Faculty of Medicine Hospital between June 2012 and June 2017. Patients with histopathologically diagnosed with gastric adenocarcinoma, tumors located in the cardia, corpus or antrum, history of no gastrectomy or other malignancy, at least D2 lymph node dissection and R0 resection were included in the study. The exclusion criteria were determined as follows according to the clinical records including the retrospectively scanned pathology reports:
− Those who have received neoadjuvant chemotherapy
− Those who death within the first postoperative 30 days
− Those who were found not to have 13th lymph node dissected
− Those who have distant organ metastasis
− Those who have positive peritoneal lavage cytology.
Finally a total of 237 patients met inclusion were enrolled and 78 patients were excluded from the study. Radical subtotal or total gastrectomy along with D2 and/or D2 plus lymph node dissection according to the Japanese Classification of Gastric Cancer Association was performed in all patients21. The lymph node stations in our study were defined as followed: No. 3 (lesser curvature LNs), No. 7 (LNs along the trunk of left gastric artery between its root and the origin of its ascendary branch), No. 8 (LNs along the common hepatic artery), No. 9 (celiac artery LNs), No. 12p (hepatoduodenal LNs along the portal vein), and No. 13 (LNs on the posterior of the pancreatic head cranial to duodenal papilla).
Study design
No. 13 lymph nodes are defined as lymph nodes located in the retropancreatic area. Whether or not No. 13 LN dissection was performed was retrospectively determined from the patients' pathology reports. Patients with No. 13 LN metastases were included in the No. 13 LN (+) group. Patients with No. 13 LN dissection determined in the pathology report No. 13 LN (-) were included in the patient group. No. 13 LN dissection was performed in cases where the tumor was thought to have invaded the duodenum. There were no predefined indications for the dissection of the retropancreatic lymph node. The removal of retropancreatic lymph nodes was based on the surgeons' opinion due to the tumor invasion to duodenum. No. 3, 7, 8, 9, 12p, and 13 lymph nodes dissected were determined according to the pathology reports. The total number of dissected lymph nodes and metastatic lymph nodes were determined based on the above-mentioned stations. The patients were classified according to the presence of metastasis in No. 13 lymph nodes (13+ and 13-). Age (≤ 60 and > 60 year), gender, tumor localization (cardia, corpus, and antrum), tumor diameter (≤ 4, 4-8, and ≥ 8 cm), differentiation type (differentiated and undifferentiated), presence of lymphovascular invasion, Bormann classification (type I/II, III/IV), Lauren's classification (intestinal, diffuse, mixed type), tumor invasion depth (T stage), nodal stage (N stage), total number of dissected and metastatic lymph nodes, post-operative pathological stage (according to the 7th edition of the AJCC gastric cancer guidelines), and total survival time of the patients were retrospectively analyzed.
The patients were followed up every 3 months in the first 1-year, every 6 months in the next 2 years, and then every following year. Tumor markers, endoscopic evaluation, abdominal, and thoracic computed tomography were measured during patients' check-ups.
The families of these cases were contacted through phone and were asked to participate after being verbally informed of the aim and methods of the study. The study was conducted in accordance with the Declaration of Helsinki. Informed contents form was obtained from all patients.
Statistical analysis
The Chi-square test was used to compare differences in the categorical data. Mann–Whitney U test was used to compare differences in the non-categorical data. Survival was compared using the log-rank test, and survival curves were generated using the Kaplan-Meier method. The life-table was used to calculate survival time. Multivariate analyses were conducted using the Cox proportional hazards regression model and forward logistic regression. Logistic regression analysis was used to determine factors associated with No. 13 LN metastasis. p < 0.05 was considered statistically significant. All data were analyzed using the Statistical Package for the Social Sciences (SPSS 22.0, Armonk, NY, USA).
Results
Clinicopathological factors
The study enrolled 166 (70%) male and 71 (30%) females with an average age of 59.6 ± 12.4 years. Fourteen patients (5.9%) were diagnosed with No. 13 LN metastasis. The tumor localization was as followed: cardia (n = 59, 24.9%), corpus (n = 86, 36.3%), or antrum (n = 92, 38.3%). Table 1 shows the clinicopathological characteristics of the sample. According to without No. 13 lymph node metastasis; the Bormann type (p = 0.014), tumor size (p < 0.001), Lauren classification (p = 0.006), histological tumor type (p = 0.003), presence of angiolymphatic invasion (p < 0.001), depth of tumor invasion (p = 0.035), N stage (< 0.001), and exitus (p = 0.011) were significantly different between the two groups. No statistically significant difference was found in terms of age, gender, tumor location, or number of removed lymph nodes between the groups (Table 2).
Table 1 The demographical and clinicopathological features
| n (%) | |
|---|---|
| Age (year), median | 61.0 (23-87) |
| Gender | |
| Female | 71 (30) |
| Male | 166 (70) |
| Tumor location | |
| Cardia | 59 (24.9) |
| Corpus | 86 (36.3) |
| Antrum | 92 (38.8) |
| Tumor size (cm), mean ± SD | 5.0 ± 2.8 |
| Bormann Classification | |
| I/II | 83 (35) |
| III/IV | 140 (56.1) |
| Unknown | 14 (5.9) |
| Lauren Classification | |
| Intestinal type | 138 (58.2) |
| Diffuse type | 82 (34.6) |
| Mixed type | 2 (0.8) |
| Unknown | 15 (6.3) |
| Histological type | |
| Differentiated | 143 (60.3) |
| Undifferentiated | 94 (39.7) |
| pT stage | |
| pT1 | 42 (17.7) |
| pT2 | 24 (10.1) |
| pT3 | 79 (33.3) |
| pT4 | 92 (38.8) |
| N stage | |
| N0 | 82 (34.6) |
| N1 | 36 (15.2) |
| N2 | 43 (18.1) |
| N3 | 76 (32.1) |
| Stage | |
| I | 51 (21.5) |
| II | 64 (27) |
| III | 122 (51.5) |
| Harvested LN, median (range) | 43 (15-94) |
Table 2 Comparison of clinicopathological parameters between patients with (13+) or without (13-) No. 13 LN metastasis
| Parameters | 13 (+) | 13 (-) | p value |
|---|---|---|---|
| Age (year) | 0.784* | ||
| ≤ 60 | 6 (42.9) | 111 (49.8) | |
| >60 | 8 (57.1) | 112 (50.2) | |
| Age (year), median (range) | 61.5 (42-80) | 61 (23-87) | 0.838† |
| Gender, n (%) | 1.000* | ||
| Male | 10 (71.4) | 156 (70.0) | |
| Female | 4 (28.6) | 67 (30.0) | |
| Tumor location | 0.281* | ||
| Cardia | 1 (7.2) | 58 (26) | |
| Corpus | 6 (42.8) | 80 (35.9) | |
| Antrum | 7 (50) | 85 (38.1) | |
| Tumor size (cm) | < 0.001* | ||
| ≤ 4 | 3 (21.4) | 106 (47.5) | |
| 4-8 | 3 (21.4) | 89 (39.9) | |
| ≥ 8 | 8 (57.1) | 28 (12.6) | |
| Tumor size, cm | 8.25 (3.5-12) | 4.5 (0.2-18) | 0.001† |
| Bormann classification | 0.014* | ||
| Type I/II | 1 (7.1) | 82 (36.8) | |
| Type III/IV | 11 (78.6) | 129 (57.8) | |
| Unknown | 2 (14.3) | 12 (5.4) | |
| Lauren classification | 0.006* | ||
| Intestinal type | 2 (14.3) | 136 (61) | |
| Diffuse type | 10 (71.4) | 72 (32.3) | |
| Mixed type | - | 2 (0.9) | |
| Unknown | 2 (14.3) | 13 (5.8) | |
| Histological type | 0.003* | ||
| Differentiated | 3 (21.4) | 140 (62.8) | |
| Undifferentiated | 11 (78.6) | 83 (37.2) | |
| Angiolymphatic invasion | < 0.001* | ||
| Yes | 14 (100) | 119 (53.4) | |
| No | - | 104 (46.6) | |
| Harvested LN | 0.373* | ||
| 15-25 | - | 25 (11.2) | |
| 25 | 14 (100) | 198 (88.8) | |
| Harvested LN, median | 41.5 (28-85) | 43 (15-94) | 0.438† |
| T stage | 0.035* | ||
| pT1 | - | 42 (18.8) | |
| pT2 | 2 (14.3) | 22 (9.9) | |
| pT3 | 2 (14.3) | 77 (34.5) | |
| pT4 | 10 (71.4) | 82 (36.8) | |
| N stage | < 0.001* | ||
| N0 | - | 82 (36.8) | |
| N1 | - | 36 (16.1) | |
| N2 | 1 (7.1) | 42 (18.8) | |
| N3 | 13 (92.9) | 63 (28.3) | |
| TNM stage | 0.001* | ||
| I | - | 51 (22.9) | |
| II | - | 64 (28.7) | |
| III | 14 (100) | 108 (48.4) | |
| Exitus | 0.011* | ||
| Yes | 10 (71.4) | 80 (35.9) | |
| No | 4 (28.6) | 143 (64.1) |
*Chi-squared test.
†Mann-Whitney U test.
Factors affecting No. 13 LN metastasis
Univariate and multivariate logistic regression analysis was carried out to test the prognostic factors. The results of logistic regression analyses are presented in table 3. Univariate logistic regression analysis showed that tumor diameter, Bormann type, Lauren's type, histological type, angiolymphatic invasion, tumor invasion depth, and nodal stage were associated with No. 13 LN metastasis respectively (p = 0.001, p = 0.014, p = 0.001, p = 0.002, p = 0.001, p = 0.015, p = 0.0001) (Table 3). In addition, the status of all LN stations (No. 3, 7, 8, 9, and 12p) involved in the D2 dissection site affected the No. 13 lymph node in terms of metastatic (p < 0.05). The results of multivariate logistic regression analysis showed that tumor size of ≥ 8 cm, Bormann type III/IV, undifferentiated histological type, T3-T4 stage, N3 stage, and No. 9 and No. 12p LN metastases were independent factors for No. 13 lymph node metastasis (Table 3).
Table 3 Univariate and multivariate analysis of variables for No. 13 LN metastasis
| Variables | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| n | r value | p value | OR (95% CI) | p value | |
| Age (year), n (%) | |||||
| ≤ 60 | 117 | 0.033 | 0.617 | ||
| > 60 | 120 | ||||
| Gender, n (%) | |||||
| Male | 166 | 0.008 | 0.908 | ||
| Female | 71 | ||||
| Tumor location | |||||
| Cardia | 59 | 0.089 | 0.171 | ||
| Corpus | 86 | ||||
| Antrum | 92 | ||||
| Tumor size (cm) | |||||
| ≤ 4 | 109 | 0.206 | 0.001 | 1.00 | |
| 4-8 | 92 | 0.307 (0.065-1.447) | 0.136 | ||
| ≥ 8 | 36 | 1.079 (1.012-5.283) | 0.009 | ||
| Bormann classification | |||||
| Type I/II | 83 | 0.159 | 0.014 | 1.00 | 0.007 |
| Type III/IV | 140 | 1.312 (1.004-2.577) | |||
| Unknown | 14 | ||||
| Lauren classification | 0.218 | 0.001 | |||
| Intestinal type | 138 | ||||
| Diffuse type | 82 | ||||
| Mixed type | 2 | ||||
| Unknown | 15 | ||||
| Histological type | |||||
| Differentiated | 143 | 0.199 | 0.002 | 1.00 | |
| Undifferentiated | 94 | 1.576 (1.020-2.871) | 0.001 | ||
| Angiolymphatic invasion | |||||
| + | 133 | 0.222 | 0.001 | ||
| - | 104 | ||||
| T stage | |||||
| pT1 | 42 | 0.157 | 0.015 | 1.00 | 0.786 |
| pT2 | 24 | 1.002 (0.074-1.210) | 0.022 | ||
| pT3 | 79 | 2.541 (1.043-4.438) | 0.0001 | ||
| pT4 | 92 | 4.767 (1.940-5.787) | |||
| N stage | |||||
| N0 | 82 | 0.292 | 0.0001 | 1.00 | |
| N1 | 36 | 1.080 (1.002-1.743) | 0.743 | ||
| N2 | 43 | 2.546 (1.121-9.987) | 0.659 | ||
| N3 | 76 | 4.054 (2.879-5.981) | 0.0001 | ||
| TNM stage | 0.231 | 0.0001 | |||
| I | 51 | 1.00 | |||
| II | 64 | 0.986 (0.046-1.457) | 0.089 | ||
| III | 122 | 5.271 (3.469-9.419) | 0.0001 | ||
| No. 3 LN metastasis | 0.189 | 0.003 | |||
| + | 133 | ||||
| - | 106 | ||||
| No. 7 LN metastasis | 0.416 | 0.0001 | |||
| + | 33 | ||||
| - | 204 | ||||
| No. 8 LN metastasis | 0.416 | 0.0001 | |||
| + | 33 | ||||
| - | 204 | ||||
| No. 9 LN metastasis | |||||
| + | 31 | 0.487 | 0.0001 | 1.118 (1.021-3.665) | 0.015 |
| - | 206 | 1.00 | |||
| No. 12p LN metastasis | |||||
| + | 23 | 0.704 | 0.0001 | 1.008 (1.010-2.174) | 0.0001 |
| - | 214 | 1.00 | |||
Survival significance of No. 13 LN metastasis
The survival rate between patient with and without No. 13 LN metastasis was significantly different (Fig. 1A). No 5-year survival rate was found in the patients with No. 13 LN metastasis, while 9% survival rate was found in the patients without No. 13 LN metastasis. Furthermore, patients with or without No. 13 LN metastasis were compared in terms of survival time, the survival results were poor in patients with No. 13 LN metastasis (Table 4 and Fig. 1A). Likewise, the survival time of the patients with No. 12p LN metastasis was found to be significantly poor (Table 4 and Fig. 1B). No. 13 and No. 12p LN metastasis was found to be an independent prognostic factor for survival, affecting overall survival (p = 0.034 and p = 0.009) (Table 4).
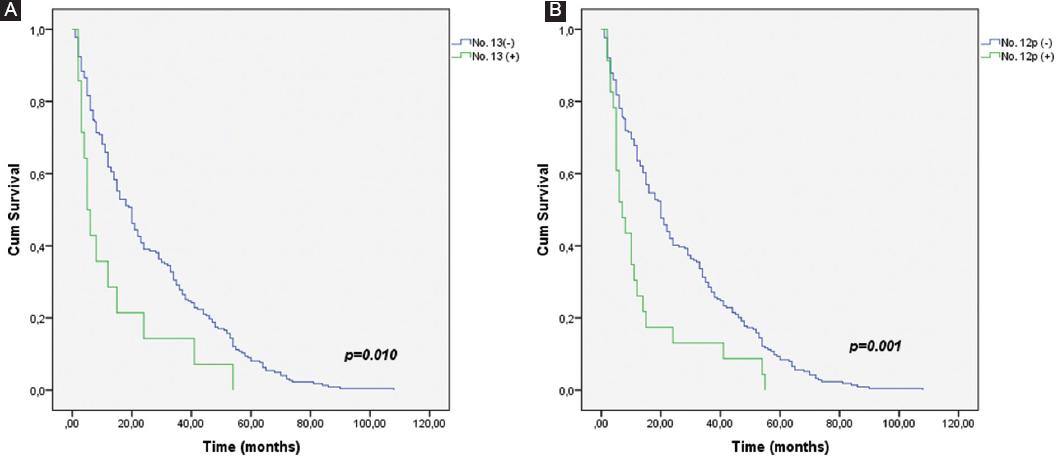
Figure 1 A: survival in 237 gastric cancer patients with and without No. 13 lymph node (LN) metastasis. There were significant differences in the 5-year survival rate between patients with and without No. 13 LN metastases (0% vs. 9%, p = 0.010). B: survival in 237 gastric cancer patients with and without No. 12p LN metastases. There were significant differences in the 5-year survival rate between patients with and without No. 12p LN metastases (0% vs. 8.4%, p = 0.001).
Table 4 Univariate and multivariate Cox regression survival analysis of the 237 patients with gastric cancer
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| n | Median OS (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Age (year), n (%) | 0.058 | ||||
| ≤ 60 | 117 | 24 (18.1-29.8) | |||
| > 60 | 120 | 13 (10.1-15.8) | |||
| Gender, n (%) | 0.683 | ||||
| Male | 166 | 18 (13.7-22.2) | |||
| Female | 71 | 20 (15.4-24.5) | |||
| Tumor location | 0.277 | ||||
| Cardia | 59 | 15 (8.5-21.4) | |||
| Corpus | 86 | 20 (12.7-27.2) | |||
| Antrum | 92 | 19 (14.7-21.2) | |||
| Tumor size (cm) | 0.023 | ||||
| ≤ 4 | 109 | 20 (15.2-24.7) | - | 0.215 | |
| 4-8 | 92 | 20 (14.8-25.1) | 0.8 (0.6-1.1) | 0.177 | |
| ≥ 8 | 36 | 12 (6.1-17.8) | 1.3 (0.9-2.0) | ||
| Bormann classification | 0.219 | ||||
| Type I/II | 83 | 22 (13.8-30.1) | |||
| Type III/IV | 140 | 15 (10.3-19.6) | |||
| Unknown | 14 | 8 (1.8-14.1) | |||
| Lauren classification | 0.076 | ||||
| Intestinal | 138 | 21 (17.4-24.5) | |||
| Diffus | 82 | 14 (11.5-16.4) | |||
| Mixed | 215 | 9 (4.2-13.7) | |||
| Unknown | |||||
| Histological type | 0.234 | ||||
| Differentiated | 143 | 20 (15.1-24.8) | |||
| Undifferentiated | 94 | 14 (10.5-17.4) | |||
| Angiolymphatic invasion | |||||
| + | 133 | 14 (10.8-17.1) | 0.064 | ||
| - | 104 | 22 (12.7-31.2) | |||
| T stage | 0.003 | ||||
| pT1 | 42 | 35 (26.8-43.1) | - | ||
| pT2 | 24 | 16 (2.7-29.2) | 1.7 (1.0-2.9) | 0.026 | |
| pT3 | 79 | 15 (9.1-20.8) | 1.6 (1.0-2.4) | 0.019 | |
| pT4 | 92 | 14 (10.5-17.4) | 1.9 (1.3-2.9) | 0.001 | |
| N stage | 0.004 | ||||
| N0 | 82 | 22 (13.8-30.1) | - | 0.930 | |
| N1 | 36 | 20 (0.8-39.1) | 0.9 (0.6-1.4) | 0.902 | |
| N2 | 43 | 20 (9.2-30.7) | 0.9 (0.6-1.4) | 0.012 | |
| N3 | 76 | 11 (8.4-13.5) | 1.3 (1.1-2.7) | ||
| TNM stage | 0.006 | ||||
| I | 51 | 33 (25.1-40.8) | - | 0.770 | |
| II | 64 | 15 (12.0-17.9) | 0.8 (0.3-2.0) | 0.711 | |
| III | 122 | 14 (10.8-17.1) | 0.8 (0.2-2.4) | ||
| No. 3 LN metastasis | 0.018 | ||||
| + | 133 | 14 (9.6-18.3) | 1.2 (0.9-1.6) | 0.710 | |
| - | 106 | 21 (12.5-29.4) | |||
| No. 7 LN metastasis | 0.034 | ||||
| + | 33 | 11 (3.1-18.8) | 1.0 (0.5-1.9) | 0.924 | |
| - | 204 | 20 (15.9-24.0) | |||
| No. 8 LN metastasis | 0.019 | ||||
| + | 33 | 12 (5.4-18.5) | 1.2 (0.6-2.4) | 0.572 | |
| - | 204 | 20 (15.9-24.0) | |||
| No. LN 9 metastasis | 0.132 | ||||
| + | 31 | 14 (3.0-24.9) | 0.8 (0.4-1.5) | 0.545 | |
| - | 206 | 18 (14.6-21.3) | |||
| No. LN 12p metastasis | 0.001 | ||||
| + | 23 | 7 (3.4-10.5) | 1.8 (1.1-2.8) | 0.009 | |
| - | 214 | 20 (16.4-23.5) | |||
| No. LN 13 metastasis | 0.010 | ||||
| + | 14 | 5 (2.5-7.4) | 1.7 (1.1-2.4) | 0.034 | |
| - | 223 | 20 (16.3-23.6) | |||
ALI: angiolymphatic invasion; OS: overall survival; CI: confidence interval; LN: lymph node.
Lymph node metastasis
Table 5 shows the metastasis rates for each lymph node. The difference found between the patients with and without No. 13 LN metastasis in terms of metastasis presence in No. 7 (p = 0.0001), No. 8 (p = 0.0001), No. 9 (p = 0.0001), and No. 12p (p = 0.0012) lymph nodes was found to be statistically significant. Furthermore, the logistic regression analysis showed that the metastasis to No. 9 lymph node (p = 0.015) and No. 12p lymph node (p = 0.001) increased the risk of metastasis to No. 13 lymph nodes by 8.4 and 12.3 times, respectively, (Table 6).
Table 5 Regional LN metastasis in patients with No. 13 LN metastasis
| Lymph node | LNs metastasis (n) | No. 13 LN metastasis (n = 14) | p value |
|---|---|---|---|
| No. 3 | 131/237 | 13/14 | 0.0001 |
| No. 7 | 33/237 | 10/14 | 0.0001 |
| No. 8 | 33/237 | 10/14 | 0.0001 |
| No. 9 | 31/237 | 11/14 | 0.0001 |
| No. 12p | 23/237 | 10/14 | 0.012 |
No. 3: lesser curvature; No. 7: along left gastric artery; No. 8: along hepatic artery group; No. 9: around celiac axis; No. 12p: along portal vein in the hepatoduodenal ligament.
Table 6 Logistic regression analysis of characteristics for No. 13 LN metastasis
| Lymph node | B value | SE | Wald | p value | OR (95% CI) |
|---|---|---|---|---|---|
| No. 3+ | 0.260 | 1.665 | 0.024 | 0.876 | 1.296 (0.050-33.876) |
| No. 7+ | 2.969 | 2.010 | 2.181 | 0.140 | 19.469 (0.379-1001.118) |
| No. 8+ | 0.832 | 1.578 | 0.278 | 0.598 | 0.435 (0.020-9.601) |
| No. 9+ | 2.133 | 1.069 | 29.616 | 0.015 | 8.442 (1.502-47.460) |
| No. 12p+ | 4.816 | 1.126 | 18.293 | 0.001 | 12.324 (1.274-112.977) |
A logistic regression analysis showed that each of the metastasis to the No. 9 and No. 12p LNs were a risk factor for No. 13 LN metastases (p = 0.015 and p = 0.001, respectively).
Discussion
The extent of lymphadenectomy in gastric cancer surgery remains controversial. Compared to D1 dissection, D2 dissection is performed by Western surgeons leading to increase survival with acceptable mortality and morbidity rates. Today, D2 and D2 plus LN dissections can be performed with acceptable morbidity and mortality rates in experienced centers17,22. However, only few studies have investigating the risk factors and clinical significance for retropancreatic LN metastasis after D2 plus lymph node dissection in gastric cancers. This study investigated the risk factors for No. 13 LN metastasis and the clinical significance in a patient population undergoing retropancreatic LN dissection. Retropancreatic LN metastasis is a poor prognostic factor in patients with gastric cancer. We found that there might be metastasis to the retropancreatic lymph node in the case of a tumor diameter of ≥ 8 cm, Bormann type III/IV, undifferentiated tumor, pT4, N3 stage, and No. 9 and No. 12p LN metastasis.
Kumagai et al.23 emphasized that survival rates could increase in patients with stage 3 gastric cancer when No. 13 lymph node is dissected. Although our study did not found a significantly correlation (p = 0.277) between tumor localization and No. 13 LN metastasis in patients with No. 13 LN metastasis, retropancreatic LN metastasis was found in tumors located in the corpus with a rate of 42.9% in addition to tumors located in 1/3 distal. In the patients without No. 13 LN metastasis, the rate of distal tumor localization was 38.1%, whereas rate of corpus localization for tumor was 35.9%. Based on the literature, this result supports the necessity of dissecting No. 13 lymph node in corpus tumors.
The previous studies have demonstrated a correlation between tumor size and lymph node metastases around the hepatoduodenal and mesenteric arteries and veins23,24. In this present study, the rate of No. 13 lymph node positivity was 57.1% in tumors with a size ≥ 8 cm, independently of localization. A tumor diameter > 8 cm is an independent prognostic factor for retropancreatic lymph node metastasis (p = 0.009). These results are similar to other studies25,26. Based on our study, we believe that retropancreatic LN dissection is needed in tumor sizes greater 8 cm.
The rates of LN positivity around the hepatoduodenal and mesenteric artery/vein have been reported to be higher in type III/IV tumors according to the Bormann classification and in diffuse type tumors according to the Lauren's classification24,27. In addition, Xue et al.26 showed that the differentiation stage was associated with macroscopic type No. 13 LN metastasis. In our study, the rate of No. 13 LN metastasis in patients with Bormann III/IV stage was 78.6%. Likewise, the rate of No. 13 LN metastasis was found to be high in diffuse type tumors according to the Lauren classification (71.4%). The correlation between retropancreatic LN metastasis and Bormann III/IV and diffuse type gastric cancers may be due to the higher prevalence of Bormann type III/IV and diffuse type tumors in advanced stage cancers26. Moreover, considering histopathological tumor types, besides studies reporting that distant lymph node metastases might be higher, especially in undifferentiated tumors, there are also studies reporting low rates19,23,24,27. In our study, the rate of No. 13 LN metastasis was considerably higher in patients with undifferentiated tumors than in patients with differentiated tumors (78.6% vs. 21.4%, p = 0.003). Thus, we believe that D2 plus retropancreatic LN dissection is required in Bormann type III/IV, diffuse type, and undifferentiated tumors.
As the tumor depth and the rate of LN metastasis increase independently of the localization, the rate of metastasis to distant lymph nodes such as hepatoduodenal, mesenteric artery/vein, and retropancreatic LN is also increasing19,23,24,27. In our study, the rate of No. 13 LN positivity was high, especially in pT4 patients (71.4%). Furthermore, we also showed that the rate of No. 13 LN positivity was 7.1% in the case of N2 positivity, while the rate of No. 13 LN positivity was 92.9% in the case of pN3 positivity. Thus, pT and pN were an independent risk factor for No. 13 LN metastasis. Furthermore, the median survival time was 14 months (p = 0.003) for pT4 tumors and 11 months for pN3 tumors (p = 0.004). These results demonstrate that increased tumor invasion depth and advanced nodal stage are associated with a poor prognosis and a risk of retropancreatic LN metastasis. Thus, LN dissection should be extended in such patients.
There was a difference between the patients with and without No. 13 LN metastasis in terms of metastasis to No. 3, 7, 8, 9, and 12p lymph nodes. However, only No. 9 and 12p lymph nodes were independent risk factors for retropancreatic lymph node metastasis (p = 0.015 and p = 0.0001). The intricate interactions among the lymph nodes around the stomach might explain these results. The probability of metastasis to No. 13 lymph node was closely linked to No. 9 and 12p lymph nodes and supported by other studies26,28. This is possibly due to the communicating branches of lymphatic vessels among the regional lymph nodes. Especially in the case of 7, 8, 9 and 12p LN positivity, the rate of No. 13 LN metastasis was high. This result shows that 1/3 of the patients with No. 9 LN positivity have No. 13 LN positivity in advanced stage gastric cancers. Thus, if metastasis is detected in No. 9 lymph node intraoperatively, there is a possibility that metastasis is present in retropancreatic lymph node, suggesting that D2 plus lymph node dissection should be performed. Studies have shown 4.9% to 14.8% in No. 9 LN positivity, whereas we found 35.5% positivity19,27. The high rate of No. 9 LN metastasis in this present study can be explained by the advanced stage of tumors in our study.
Our logistic regression analysis showed that tumor size (> 8 cm), depth of tumor invasion (pT4), higher number of LN metastasis (pN3), advanced stage, No. 3, 7, 8, 9, and 12p lymph node metastasis were risk factors for retropancreatic LN metastasis, whereas depth of tumor invasion (OR:4.767, CI:1.940-5.787, p = 0.00021), nodal stage (OR:4.054, CI:2.879-5.981, p = 0.0001), and presence of No. 9 and 12p lymph node metastasis were separately found to be an independent risk factors for retropancreatic lymph node metastasis. Furthermore, this study showed that the rate of retropancreatic lymph node positivity increased 8.4 times in the case of No. 9 lymph node positivity and 12.2 times in the case of No. 12p lymph node positivity which is similar to previous studies27. The high rates in our study can be explained by the fact that the majority of our patients were diagnosed with corpus, distal and advanced stage tumors. Although it is not possible to eliminate this heterogeneity completely, the study can be made more homogeneous using patients at a single stage. Retrospective characteristic of our study, low number of patients, not specifying duodenum invasion of tumor and not indicating the status of preoperative and postoperative complications of the patients can be regarded as the limitations of our study.
Conclusions
We found a 5.9% of incidence of metastasis in No. 13 lymph node linked to a decline in survival rates. In advanced stage gastric cancers, D2 plus LN dissection may be required in tumors located in the corpus in addition to tumors located in 1/3 distal. Moreover, it has been shown that No. 9 and 12p lymph node metastases are independent risk factors for retropancreatic lymph node metastasis, therefore suggesting that No. 13 lymph node should also be added to the lymph node dissection. In experienced centers, retropancreatic LN dissection can be performed without any increase in morbidity, especially in the case of T3, T4 and N positive tumors located in the corpus and distal, as revealed in this study. The lower survival rates of the patients with lymph node metastasis and the higher mortality rates can be explained by poor prognostic criteria such as Diffuse type, Bormann III-IV, large tumor diameter, more T3-T4, and N2-N3 tumors.











 nueva página del texto (beta)
nueva página del texto (beta)


