The genus Capsicum belongs to the Solanaceae family and is native to the tropical and subtropical regions of America. Currently, this genus includes 33 species (GRIN 2022), of which five have been domesticated: C. annuum L., C. baccatum L., C. chinense J., C. frutescens L., and C. pubescens Ruiz Pav. (Hernández-Verdugo et al. 1999, Kraft et al. 2013). Capsicum annuum is an important species as it is consumed in large amounts worldwide (Olatunji & Afolayan 2018). Mexico is considered one of the main domestication centers of this species due to the diversity of the wild variants that are located in the country (Loaiza-Figueroa et al. 1989), where they show great adaptability to different environmental conditions, including the solar radiation received.
In southeastern Mexico, among the wild chili peppers present and culturally appreciated are C. frutescens, commonly known as Pico paloma (PIP) (De la Cruz-Lázaro et al. 2017) and C. annuum var. glabriusculum (Dunal) Heiser & Pickersgill, with the variants amashito (AMA) and garbanzo (GAR) (Castañón-Nájera et al. 2008, Velázquez-Ventura et al. 2018). These peppers grow in diverse environments under shade or direct sun. They are mainly found in lowland forest ecosystems known as “acahuales” or mountains, and they frequently occur in different agrosystems, such as cocoa, coconut, banana, and citrus plantations and home gardens, where they receive substantial shade, and in pastures and along roads in full sun (Gutiérrez-Burón et al. 2020).
The adaptability of these wild chili species to different environments has an effect on their morphology, growth cycles, and perhaps the quality of their fruits. Among the characteristics that have been related to plant responses to the quantity and quality of light received are the elongation or shortening of the stem and the decrease or increase in the leaf area and branches, in addition to the modification of phenological cycle durations, fruit yields, and fruit quality (Taiz & Zeiger 2006). Additionally, anatomical changes, such as changes in the thickness of spongy parenchyma and palisade of leaves and in the thickness and lignification of the pericarp of fruits, have been observed in leaves and fruits exposed to different incident radiation conditions, and these changes are greater in plants grown under open field conditions than in those grown under shade conditions (Ilić et al. 2017). In C. annuum, greater plant heights, leaf areas, and yields have been observed in plants grown under different shade materials and colors compared to in those grown in open fields (Zermeño-González et al. 2019). Specifically, C. annuum var. glabriusculum growing under 35, 50, and 80 % shade favors vegetative development, and 35 % shade increases its yield compared to the yield of these plants grown in open fields (Valiente-Banuet & Gutiérrez-Ochoa 2016).
In their fruits, these wild species of Capsicum present high concentrations of bioactive compounds such as polyphenols, flavonoids (FLV), carotenoids (CAT), and capsaicinoids (De la Cruz-Ricardez et al. 2020a). The concentration of these compounds can be affected by the amount of light intercepted by plants, altering their biological potential. In C. annuum genotypes that grow under low incident radiation, it has been shown that the chlorophyll and carotenoid contents are higher than in plants grown under conditions of high incident radiation (Alkalai-Tuvia et al. 2014). In contrast, the contents of polyphenols, FLV, and total soluble solids are higher in the fruits of C. annuum grown under open field conditions than in those grown under shade (Díaz-Pérez et al. 2020). Shading by colored nets also influence the quality of C. annuum fruits. For example, in C. annuum cultivar “Cameleon” fruits, the vitamin C content increases under red coloured nets (Ilić et al. 2017). Regarding mineral concentrations, C. annuum fruits produced under shaded conditions have a higher content of N, P and K than those produced under full sun conditions (Díaz-Pérez 2014).
Given that C. annuum var. glabriusculum and C. frutescens are gastronomically important species in southeastern Mexico and their bioactive compounds have antifungal potential (Carole et al. 2019, De la Cruz-Ricardez et al. 2020b), it is necessary to understand the impacts that variations in incident radiation have on the growth, development, yield, and composition of the fruits of these wild species to develop strategies for crop cultivation and extraction of bioactive compounds. The objective of this study was to compare the phenological development, fruit yield, and total contents of phenols, flavonoids, proanthocyanidins, and carotenoids of two C. annuum var. glabriusculum genotypes and one C. frutescens genotype grown in an open field and under two shade levels.
Materials and methods
Plant material. The ripe (red) fruits of the C. frutescens genotype (PIP) and the two C. annuum var. glabriusculum genotypes (AMA and GAR) were collected in the morning in the Rafael Martínez de Escobar neighborhood of the municipality of Huimanguillo, Tabasco, Mexico (17° 43' 18.2'' N; 93° 23' 10.7” W). The seeds were extracted, washed with distilled water, dried in the shade at room temperature (26 ºC) and preserved for subsequent germination.
Germination and transplantation. A batch of 100 seeds of each genotype was treated with a solution of 500 mg L-1 gibberellic acid (GA3) for 24 h to break dormancy and promote germination. At the end of this process, these seeds were planted in germination trays using soil from the collection site. Forty-eight seedlings of each genotype were transplanted 40 days after germination to the experimental plot under three shade levels (35 and 70 % of shade, and in an open field). To establish the percentage of shade, a black shade mesh of high-density polyethylene with pigmentation and UV additives (Hydro Environment, SA de CV) was used. The percentage of shade was chosen to simulate the availability of light under the conditions in which the chili genotypes are found in the agricultural plantations and backyard orchards. The treatment without a shade mesh (open field) corresponded to the conditions under which some plants are found along roadsides and pastures. In each shade level, the experimental plot size was 4 × 4 m. The distance between seedlings was 1 m. The experiment was conducted in two consecutive years (2020 and 2021). In both years, planting and transplanting were performed on the same dates.
Phenological development and fruit yield. The days to the first bifurcation of the stem (DFB) were obtained by quantifying the days elapsed since the transplant until the time when each plant at each amount of shade presented this bifurcation. The height at the first stem bifurcation (FSB), height at flowering (FLO), height at fruit production (FRU) and height at fruit ripening or when the plants presented the first fruit with red coloration (FRR), characteristic of mature chili peppers were measured. The days to flowering (DFL) were determined by quantifying the days elapsed from the transplant to the time when each plant at each amount of shade presented anthesis, and the days to fruiting (DFR) by quantifying the days elapsed from the transplant to the time when each plant started fruit production. For the days to fruit ripening (DFRR) the days elapsed from the transplant to the time when the plants presented fruits with red coloration were quantified, and this was recorded for each level of shade. The duration of the fruit ripening period (DFRP) was determined by quantifying the days elapsed from anthesis to the time when the plants presented red fruits at each shade level. To determine this variable, at the time of anthesis, 5 flowers were marked on two occasions per plant and shade level, which were followed until the maturity of each fruit. At that time, the fruits were harvested.
To determine the fruit yield, weekly harvests of red fruits from each plant (16 plants) were carried out for each shade amount. At each harvest, the weight of the fruits per plant was obtained. At the end of each harvest season, the yield was obtained by season: dry season yield (DSY) and rainy season yield (RSY). At the end of the year, the total annual yield (TAY) was obtained. With the number of plants per area, the yield per hectare (t ha-1) was calculated. Immature and mature fruits were harvested separately, dried at room temperature, and stored in paper bags at 5 °C for further analysis.
Analysis of secondary metabolites. The complete immature (green) and mature (red) fruits of the AMA, GAR, and PIP genotypes in the shade and open field treatments were crushed in a coffee bean mill (KRUPS®, GX410011, Mexico) until a fine powder was obtained, and this powder was stored in the dark at 5 °C until its use.
Determination of total phenols (TPC).- Prior to the extraction of the TPC, the samples were degreased with 95 % hexane in a sample/hexane ratio of 1:10 (w/v). The obtained dry precipitate was stored at 4 °C and protected from light until use. A total of 250 mg was used for the extraction and quantification of TPC according to the method described by Singleton et al. (1999) with 80 % methanol. Quantification was performed with 50 % Folin-Denis reagent and 15 % anhydrous sodium carbonate. The absorbance was read at 765 nm in a UV-vis spectrometer (Multiskan Go 51119300; Thermo Fisher Scientific, Finland). A standard solution of 100 µg mL-1 of gallic acid (GA) (Sigma-Aldrich®) was used to prepare the calibration curve from 0 to 100 µg mL-1. The TPC content was expressed in mg of GA g-1 of dry weight.
Determination of total flavonoids (FLV).- The extraction of all the FLV was performed according to the methodology of Alvarez-Parrilla et al. (2011) with 80 % methanol. The quantification of the FLV was performed according to Menichini et al. (2009) with sodium nitrite (NaNO2) at 5 %, aluminum chloride (AlCl3) at 10 % (w/v), and sodium hydroxide (NaOH) at 1 M. The absorbance was read at 510 nm in a UV-vis spectrometer (Multiskan Go 51119300; Thermo Fisher Scientific, Finland). A standard solution of 1,000 µg mL-1 of 95 % quercetin (QE) (Sigma-Aldrich®) was used to prepare the calibration curve from 100 to 1,000 µg mL-1. The total FLV content was expressed in mg of QE g-1 of dry weight.
Determination of total proanthocyanidins (PAN).- The total PANs were determined with the method in Olatunji & Afolayan (2019), with modifications. To 250 mg of a fat-free sample, 1 mL of 80 % methanol was added; this sample was incubated in a water bath at 50 °C for 15 min and centrifuged at 10,000 rpm for 5 min; and the supernatant was recovered. The residue was washed again with 0.5 mL of 100 % methanol, and again, the supernatant was recovered in the same tube, which was stored at 4 °C and protected from light until use. To a 0.5 mL of each sample, 3 mL of 4 % vanillin solution in methanol (w/v) and 1.5 mL of concentrated HCl were added to 5 mL glass tubes. The mixture was vortexed and incubated at 27 °C for 15 min. The absorbance was read at 500 nm. A standard solution of 500 µg mL-1 of 95 % catechin (Sigma-Aldrich®) was used to prepare the calibration curve from 50 to 500 µg mL-1. The total PAN content was expressed in mg of CA g-1 of dry weight.
Determination of total carotenoids (CAT).- The total CAT were extracted with the method proposed by Talcott & Howard (1999) using a solution of acetone/ethanol (1:1) mixed with 200 mg L-1 of butylhydroxytoluene (BHT) (Sigma-Aldrich®). For quantification, the absorbance was read at 470 nm in a UV-vis spectrometer (Multiskan Go 51119300; Thermo Fisher Scientific, Finland). The total CAT content was calculated according to Gross (1991) using the equation (AV × 10 6)/(A1% × 100 G), where A is the absorbance at 470 nm, V is the total volume of extract, A1% is the extinction coefficient for a mixture of solvents arbitrarily set at 2,500, and G is the weight of the sample in g of dry weight. The CAT content was expressed in mg g-1 of dry weight.
Climatic data and soil analysis. Information on the rainfall and temperatures during the study period was downloaded from the National Meteorological Service website based on data from the automatic station at Paredón in 2020 and that at Cárdenas in 2021. The amount of photosynthetically active radiation (PAR) at the canopy of the chili plants under the different shade conditions was measured in µmoles of photons m-2 s-1 with a PAR sensor model BQM (Apogee Instruments, Inc. USA). The measurements were conducted every week during the entire growth and production period of the chili peppers at 10 h. Additionally, the PAR was measured throughout the day on July 13 and 14, and October 19 and 20, 2021. In terms of soil analysis, soil texture, fertility, and cation exchange capacity (CEC) were determined by Fertilab laboratory from Celaya, Guanajuato, Mexico.
Statistical analysis. The experimental data were analyzed as a completely randomly subsplit plot design. The main plot was the year of study (2020 and 2021), the mean plot was the level of shade (35 and 70 % of shade, and open field), and the small plot were the chili genotypes (AMA, GAR, and PIP). These genotypes were established with a random block structure with four replicates. With the obtained data, analysis of variance was performed, and when significant differences were found, multiple comparisons were made using the Tukey multiple range test (α = 0.05). R (R Core Team 2022) and RStudio v. 1.2.5033 was used for all the statistical analyses performed.
Results
Climatic conditions and soil fertility. The rainfall (mm) and maximum and minimum temperatures (°C) during 2020 and 2021 at the study site are shown in Figure 1. The lowest rainfall amounts were recorded in December, January, and February 2020 in comparison to that in those months in 2021, and the highest amount of precipitation was recorded from May to December 2020. The average of the PAR measured in the open field and under the two levels of shade over the two years (2020 and 2021) was correlated with the established percentages of shade (Figure 2A). During the day, the highest amount of PAR occurred between 11:00 and 15:00 h (Figure 2B). The highest amount of PAR (1,635.55 µmol m-2 s-1) was recorded in the open field at 13 h, while at the same time, it ranged between 1,098.3 and 586.2 µmoles m-2 s-1 under the shade meshes of 35 and 70 %, respectively. The soil at the study site has a clay loam texture, pH of 6.8, a very high level of organic matter, very high copper and iron contents, and low sodium contents, in addition to a moderate CEC value.

Figure 1 Precipitation and maximum and minimum temperatures at the experimental site for the two years of the study
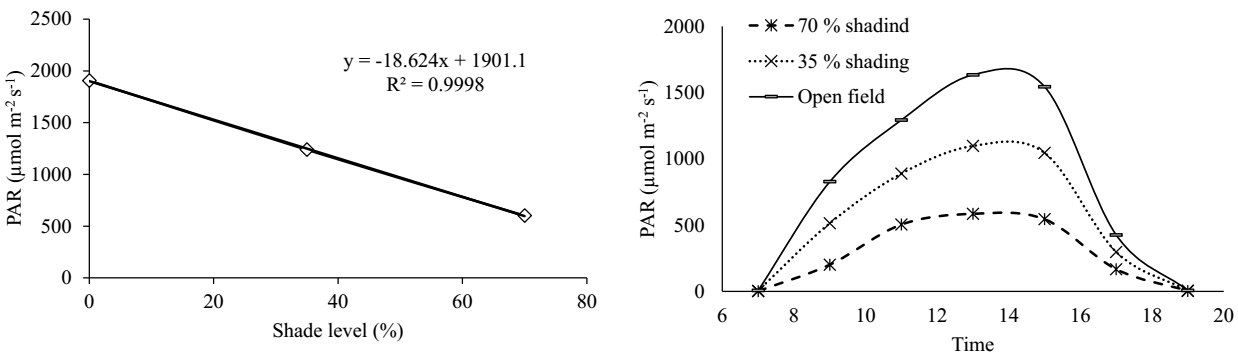
Figure 2 Behavior of photosynthetically active radiation (PAR) in the open field and under two levels of shade mesh. Left, PAR (µmol m-2 s-1) and shade level (35 and 70 %). Right, Relationship of the PAR and the time of day
Phenological development and yield. Significant differences due to the effect of shade were observed for most of the phenological variables evaluated, with the exception of DSY (Table 1). The genotype effect was significant for all variables. The effect of year was also significant for all variables, except for height at FSB. In the case of interactions, these were significant for most variables, with the exception of DFB, height at FSB, DFRP, and DSY.
Table 1 Significance levels for the analysis of variance of the growth and yield variables of Amashito, Garbanzo and Pico paloma chili pepper genotypes grown under two percentages of shade mesh and in an open field
| Variables | Shade (Ms) | Genotype (Ge) | Year | (Ms × Ge × Year) |
|---|---|---|---|---|
| Days to first stem bifurcation (DFB) | ** | *** | *** | NS |
| Height at first stem bifurcation | ** | *** | NS | NS |
| Days to flowering (DFL) | * | *** | *** | *** |
| Height at flowering | *** | *** | *** | *** |
| Days to fruiting (DFR) | * | *** | *** | ** |
| Height at fruiting | *** | *** | *** | *** |
| Days to fruit ripening (DFRR) | * | *** | *** | *** |
| Height at fruit ripening | *** | *** | *** | *** |
| Duration of the maturation period (DFRP) | ** | * | *** | NS |
| Yield in dry season (DSY) | NS | *** | *** | NS |
| Yield in rainy season (RSY) | *** | *** | *** | *** |
| Total annual yield (TAY) | ** | *** | *** | *** |
*** P ≤ 0.001, ** P ≤ 0.01, * P ≤ 0.05, NS Not significant.
The height at FSB, which on average for the two years and the three genotypes was 30.92 cm, was not significantly different in the two years of study; however, for the height at FLO, FRU and FRR, significant differences were observed among the three genotypes with the effect of year and shade (Table 1). In the first year, the plants showed greater growth from the first bifurcation of the stem until flowering, continuing with slight growth until the fruits changed to the red color that is characteristic of mature fruits. This rapid growth was not observed in the second year, perhaps being influenced by the observed precipitation (Figure 1), which was more abundant in 2021 than in 2020, and cloudier days and higher soil moisture. The plants of the three genotypes under the 70 % shade mesh reached greater heights, followed by the plants under the 35 % shade mesh compared to the plants that were in the open field (Figure 3). The effect of shade on height was mainly observed in the AMA (Figure 3A) and GAR (Figure 3B) genotypes in the first year.

Figure 3 Height at different phenological stages of the three chili genotypes by the shade effect in two years. A, Amashito; B, Garbanzo; and C, Pico paloma. First stem bifurcation (FSB), flowering (FLO), fruiting (FRU), and fruit ripening (FRR).
In general, for the variables DFB, DFL and DFRR, greater precocity (fewer days) was observed due to shade for the three genotypes in the two years (Figure 4). However, the effect was significant only for the AMA genotype in 2021 (Figure 4A). The AMA plants under 70 % shade showed phenological changes over fewer days (63, 74, and 79 days for DFB, DFL, and DFR, respectively) than those in the open field (82, 90, and 95 days, respectively). On the other hand, for DFRR, the shade effect was significant for the three genotypes in the second year, where the plants under 70 % shade had mature fruits in fewer days (124, 151, and 146 for AMA, GAR and PIP, respectively) than those in the open field (148, 160 and 152, respectively).
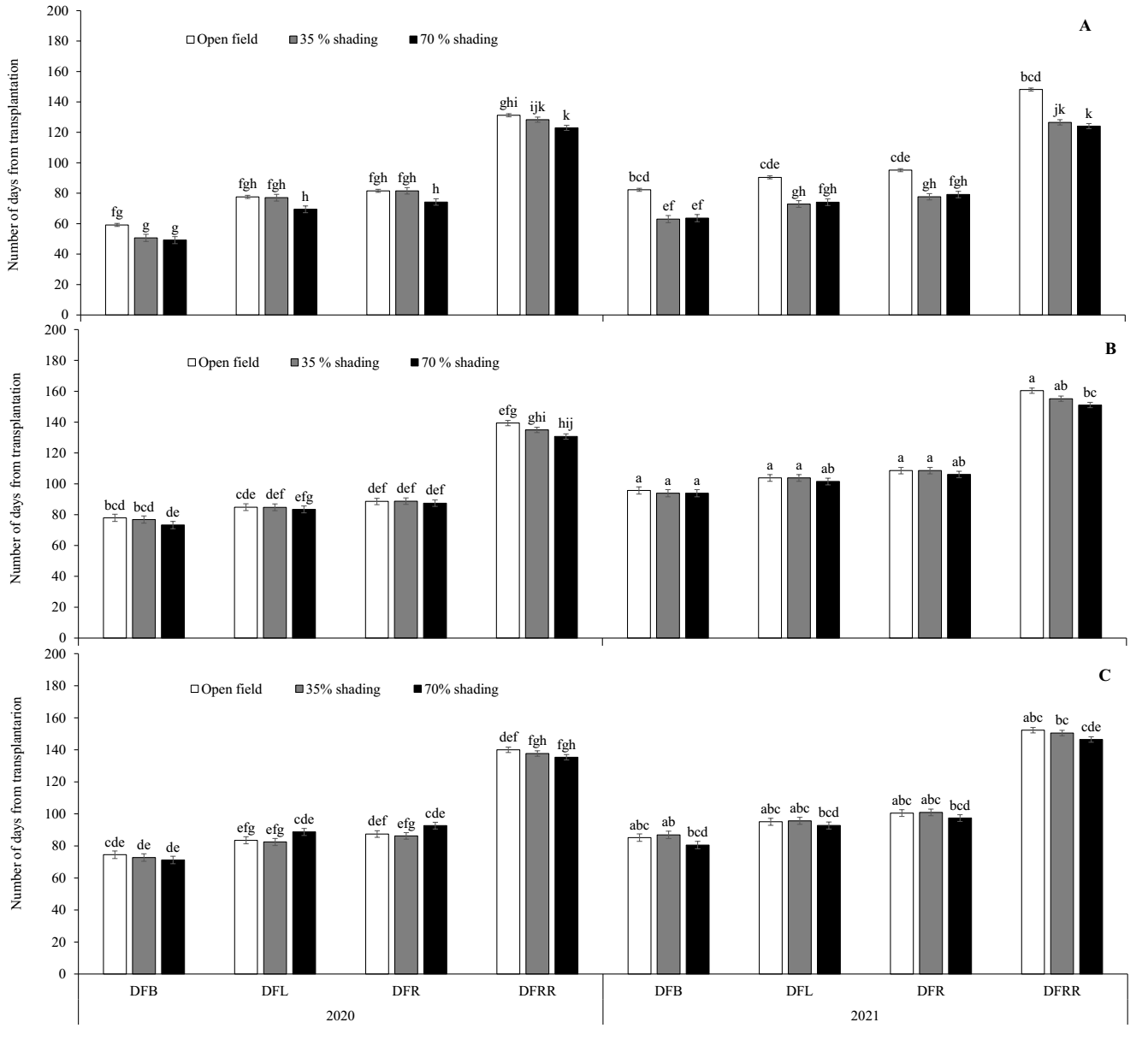
Figure 4 Number of days from transplantation to first stem bifurcation, to flowering, to fruiting and to fruit ripening of the three chili pepper genotypes due to shade in two years. A, Amashito; B, Garbanzo; and C, Pico paloma. Days to first bifurcation (DFB), days to flowering (DFL), days to fruiting (DFR), and days to fruit ripening (DFRR). Different letters between the bars show significant differences.
Regarding the DFRP, the shade effect was significant for the three genotypes in the two years (Figure 5). In comparison to those in the open field, the plants under the 70 % shade level were the first to present fruits with the red coloration characteristic of chili peppers. This result indicates that shade accelerated the ripening process of fruits from anthesis for the three genotypes in this study.

Figure 5 Duration of the fruit ripening period (DFRP) of the three chili pepper genotypes with shade in two years. Different letters between the bars show significant differences.
During the phenological development of the evaluated genotypes, there were two harvest peaks, one in the dry season from April to June and the other in the rainy season from August to October. The highest yield occurred in the rainy season of both years for the three genotypes (Table 2). The AMA genotype had its highest yield in the rainy season of the second year with 70 % shade, while the PIP genotype had its highest yield the second year in the open field; however, the GAR genotype had its highest yield in the first year with 35 % shade. Regarding the TAY, the GAR genotype reached 3.92 t ha-1 the first year with 35 % shade, PIP reached 3.49 t ha-1 the second year under open field conditions, and AMA reached 0.96 t ha-1 with 70 % shade in 2021.
Table 2 Fruit yields (t ha-1) of the Amashito, Garbanzo and Pico paloma chili genotypes based on shade effect and time of year during 2020 and 2021.
| Variable (t ha-1) | Year | Genotype | Open field | Shade 35 % | Shade 70 % |
|---|---|---|---|---|---|
| Dry season yield (DSY) | 2020 | Amashito | 0.017 i | 0.037 hi | 0.100 def |
| Garbanzo | 0.169 bc | 0.260 a | 0.221 ab | ||
| Pico paloma | 0.079 efg | 0.066 fgh | 0.059 fgh | ||
| 2021 | Amashito | 0.043 ghi | 0.046 fgh | 0.056 fgh | |
| Garbanzo | 0.057 fgh | 0.094 def | 0.069 efg | ||
| Pico paloma | 0.118 cd | 0.111 de | 0.090 efg | ||
| Rainy season yield (RSY) | 2020 | Amashito | 0.061 j | 0.071 j | 0.079 j |
| Garbanzo | 2.839 c | 3.656 a | 1.972 d | ||
| Pico paloma | 3.121 bc | 1.924 d | 1.422 ef | ||
| 2021 | Amashito | 0.688 hi | 0.718 ghi | 0.899 gh | |
| Garbanzo | 1.090 fg | 0.779 ghi | 0.487 i | ||
| Pico paloma | 3.369 ab | 2.870 c | 1.661 de | ||
| Total annual yield (TAY) | 2020 | Amashito | 0.078 k | 0.107 jk | 0.179 jk |
| Garbanzo | 3.007 c | 3.916 a | 2.193 d | ||
| Pico paloma | 3.199 bc | 1.990 de | 1.481 fg | ||
| 2021 | Amashito | 0.731 i | 0.764 hi | 0.955 hi | |
| Garbanzo | 1.147 gh | 0.873 hi | 0.555 ij | ||
| Pico paloma | 3.487 b | 2.981 c | 1.751 ef |
Different letters between fruit yields of the three chili genotypes based on shade effect and time of year (t ha-1) during 2020 and 2021 show statistically significant differences (P < 0.05).
Secondary metabolites of Capsicum spp. The shade effect was only significant (P ≤ 0.01) for the total TPC and total FLV contents in the rainy season; the effect of genotype was highly significant (P ≤ 0.001) for the TPC, FLV, and CAT contents in the two seasons of the year; and the effect of year was visible for TPC during the rainy season (Table 3).
Table 3 Significance levels for the analysis of variance of phytochemicals of mature and immature fruits of the Amashito, Garbanzo and Pico paloma chili pepper genotypes, harvested in the dry and rainy seasons under two percentages of shade mesh and in an open field in two years.
| Variable | Shade (Ms) | Genotype | Year | (Ms × Ge × Year) |
|---|---|---|---|---|
| TPC in ripe fruits in dry season | NS | *** | *** | * |
| TPC in immature fruits in dry season | NS | *** | *** | * |
| TPC in ripe fruits in rainy season | ** | *** | *** | NS |
| TPC in immature fruits in rainy season | ** | *** | *** | NS |
| FLV in ripe fruits in dry season | NS | *** | NS | NS |
| FLV in immature fruits in dry season | NS | *** | NS | NS |
| FLV in ripe fruits in rainy season | ** | *** | *** | * |
| FLV in immature fruits in rainy season | ** | *** | *** | * |
| CAT in ripe fruits in dry season | * | *** | NS | ** |
| CAT in immature fruits in dry season | * | *** | NS | ** |
| CAT in ripe fruits in rainy season | NS | *** | NS | * |
| CAT in immature fruits in rainy season | NS | *** | NS | * |
| PAN in ripe fruits in dry season | NS | NS | NS | NS |
| PAN immature fruits in dry season | NS | NS | NS | NS |
| PAN in ripe fruits in rainy season | NS | NS | NS | NS |
| PAN in immature fruits in rainy season | NS | NS | NS | NS |
*** P ≤ 0.001, ** P ≤ 0.01, * P ≤ 0.05, NS Not significant.
In general, the TPC content varied from 12.55 mg g-1 in the AMA genotype fruits harvested during a drought in 2021 to 18.32 mg g-1 in GAR genotype fruits harvested during the rainy season in 2020 (Table 4). The effect of shade on the TPC content was significant for the PIP genotype during the drought in the first year, where the concentration was higher in the fruits from plants that were in the open field (17.89 mg g-1) than in the fruit that were under the 70 % shade mesh (15.87 mg g-1). This effect was also observed in the GAR genotype in the rainy season of the first year, where the highest content was found in the fruits harvested in the open field (18.32 mg g-1) than in fruits harvests from 70 % shade (16.96 mg g-1).
Table 4 Total polyphenol content in fruits of the Amashito, Garbanzo and Pico paloma chili genotypes based on the shade effect and time of year in 2020 and 2021.
| Genotype | Shade level (%) | TPC Dry season (mg GA g-1 DW) | TPC Rainy season (mg GA g-1 DW) | ||
|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | ||
| Amashito | Open field | 16.84 abc | 14.03 cde | 16.92 abc | 14.24 bcd |
| 35 | 16.03 bcd | 12.55 e | 17.44 ab | 15.49 bcd | |
| 70 | 16.66 abc | 13.44 cde | 17.45 ab | 14.00 bcd | |
| Garbanzo | Open field | 15.83 bcd | 13.65 cde | 18.32 a | 15.19 bcd |
| 35 | 14.68 bcd | 13.82 cde | 15.09 bcd | 13.29 cd | |
| 70 | 12.96 de | 13.31 cde | 16.96 abc | 12.87 d | |
| Pico paloma | Open field | 17.89 a | 16.73 abc | 16.57 bcd | 16.29 bcd |
| 35 | 17.23 ab | 14.42 bcd | 17.13 ab | 15.81 bcd | |
| 70 | 15.89 bcd | 16.19 bcd | 16.98 abc | 14.53 bcd | |
Different letters between periods of precipitation show statistically significant differences (P < 0.05); TPC, total polyphenols; DW, dry weight; and GA, gallic acid.
The variation in the total FLV content between the three genotypes ranged from 5.06 mg g-1 in the GAR genotype fruits harvested in the rainy season of the second year to 14.54 mg g-1 in the PIP genotype fruits harvested in the rainy season of the first year (Table 5). The FLV concentration was highest in PIP, followed by that in AMA and GAR. The shade effect on the FLV content was observed only in the PIP genotype in the rainy season of the second year. Compared to those grown under 70 % shade, the fruits grown in the open field increased the FLV concentration from 10.62 mg g-1 to 14.54 mg g-1 in the first year and from 7.96 mg g-1 to 8.90 mg g-1 in the second year.
Table 5 Total flavonoid content in fruits of the Amashito, Garbanzo and Pico paloma chili genotypes based on the shade effect and time of year in 2020 and 2021.
| Genotype | Shade level (%) | FLV Dry season (mg QE g-1DW) | FLV Rainy season (mg QE g-1 DW) | ||
|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | ||
| Amashito | Open field | 11.56 a | 9.72 abc | 11.25 bc | 6.04 fg |
| 35 | 11.09 ab | 10.59 abc | 11.24 bc | 7.76 ef | |
| 70 | 9.01 abc | 10.30 abc | 10.85 bcd | 6.08 fg | |
| Garbanzo | Open field | 9.62 abc | 8.44 abc | 7.79 ef | 6.94 efg |
| 35 | 6.27 c | 7.07 bc | 7.81 ef | 5.89 fg | |
| 70 | 7.40 bc | 7.63 bc | 7.13 efg | 5.06 g | |
| Pico paloma | Open field | 11.57 ab | 12.95 a | 14.54 a | 8.90 cd |
| 35 | 10.87 abc | 11.07 ab | 11.83 b | 8.33 def | |
| 70 | 9.10 abc | 10.47 abc | 10.62 bcd | 7.96 ef | |
Different letters between periods of precipitation show statistically significant differences (P < 0.05); FLV, total flavonoids; DW, dry weight; and QE, quercetin equivalents.
For the PAN content in mature fruits, the range of variation was from 42.57 mg g-1 to 65.57 mg g-1, and in immature fruits, it ranged from 22.24 to 40.27 mg g-1 (Table 6). The effect of shade and genotype was not significant for this variable. However, for the three genotypes studied, a higher content of PAN was observed in the mature fruits developed in the dry season under open field conditions (63.50, 64.75, and 74.36 mg g-1 for the AMA, GAR, and PIP genotypes, respectively).
Table 6 Proanthocyanidin content in fruits of the Amashito, Garbanzo and Pico paloma chili genotypes based on the shade effect and degree of fruit maturity in dry and rainy season.
| Genotype | Shade level (%) | PAN Dry season (mg CA g-1 DW) | PAN Rainy season (mg CA g-1 DW) | ||
|---|---|---|---|---|---|
| Ripe fruit | Immature fruit | Ripe fruit | Immature fruit | ||
| Amashito | Open field | 63.50 ab | 37.84 cde | 46.77 bcd | 40.27 cde |
| 35 | 54.95 abc | 36.80 cde | 46.42 bcd | 37.12 cde | |
| 70 | 51.77 bcd | 29.77 de | 43.03 bcd | 38.99 cde | |
| Garbanzo | Open field | 64.75 ab | 22.24 e | 56.56 ab | 28.86 e |
| 35 | 58.53 abc | 23.27 e | 42.77 bcd | 38.66 cde | |
| 70 | 62.60 ab | 25.86 e | 45.85 bcd | 33.84 de | |
| Pico paloma | Open field | 74.36 a | 23.87 e | 60.68 a | 30.93 e |
| 35 | 65.57 ab | 22.29 e | 52.30 abc | 35.40 cde | |
| 70 | 57.04 abc | 22.31 e | 49.77 bcd | 35.87 cde | |
Different letters between periods of precipitation show statistically significant differences (P < 0.05); PAN, proanthocyanidins; DW
For CAT, there was a highly significant effect (p ≤ 0.05) on the degree of maturity for the three genotypes (Table 7). Of genotype fruits, the mature fruits of the PIP and AMA genotypes collected in the open field in the rainy season showed the highest content of CAT (38.25 mg g-1 and 23.71 mg g-1, respectively); however, mature fruits of the GAR genotype showed the highest content of CAT (28.80 mg g-1) in the open field during the dry season. Regarding shade, the effect was highly significant for the AMA and GAR genotype mature fruits harvested in the dry season, where a decrease in the CAT content was observed under shade. For the AMA genotype, the CAT content was 22.23, 17.91, and 15.51 mg g-1 in the open field and 35 and 70 % of shade, respectively; similarly, the GAR genotype had CAT values of 28.80, 25.09 and 23.25 mg g-1.
Table 7 Total carotenoid content in fruits of the Amashito, Garbanzo and Pico paloma chili genotypes based on the shade effect and degree of maturity in in dry and rainy season.
| Genotype | Shade level (%) | CAT Dry season (mg g-1 DW) | CAT Rainy season (mg g-1 DW) | ||
|---|---|---|---|---|---|
| Ripe fruit | Immature fruit | Ripe fruit | Immature fruit | ||
| Amashito | Open field | 22.23 cd | 6.05 f | 23.71 bc | 6.26 d |
| 35 | 17.91 de | 5.72 f | 20.82 c | 6.99 d | |
| 70 | 15.51 e | 5.41 f | 19.33 c | 6.04 d | |
| Garbanzo | Open field | 28.80 ab | 3.71 f | 26.86 b | 5.47 d |
| 35 | 25.09 abc | 4.94 f | 22.60 bc | 5.19 d | |
| 70 | 23.25 cd | 5.07 f | 23.74 bc | 3.99 d | |
| Pico paloma | Open field | 30.41 a | 6.61 f | 38.25 a | 6.43 d |
| 35 | 27.27 abc | 7.15 f | 35.18 a | 6.46 d | |
| 70 | 30.54 a | 6.58 f | 35.85 a | 6.33 d | |
Different letters between periods of precipitation show statistically significant differences (P < 0.05); CAT, total carotenoids; and DW, dry weight.
Discussion
Shade affects the morphology and phenological development of Capsicum species by changing the quantity and quality of incident radiation on plants. In C. annuum var. glabriusculum, intermediate and low incident radiation increase plant survival and vegetative and reproductive development (Jiménez-Leyva et al. 2022). In the Capsicum genotypes in this study, the height FSB, FLO, and FRR were higher under shade (35 and 70 %) than under open field conditions, which is consistent with the results of other studies on this genus (Díaz-Pérez 2013, Ayala-Tafoya et al. 2015, Valiente-Banuet & Gutiérrez-Ochoa 2016, Paredes-Jácome et al. 2019). Specifically, in C. annuum var. glabriusculum, Paredes-Jácome et al. (2019) observed an increase of 35.5 % in the height of plants under black shade mesh. This increase in the height at FRR was also observed in the AMA genotype, which belongs to the same species, in the two years of this study under 70 % shade with a black shade mesh. In comparison to the other genotypes, this genotype showed a greater increase in the height at FRR (47.93, 30.31, and 18.17 % for the AMA, GAR, and PIP genotypes, respectively). This greater growth of Capsicum species under shade responds to the elongation of the stem internodes and the greater size of the leaves, although they are thinner, which gives them a greater photosynthetic surface and stomatal density (Fu et al. 2010).
Shade alters the quality and quantity of the spectrum of light that reaches the plants, increases the incidence of blue light (400 to 500 nm), and decreases the incidence of red light (600-700 nm), affecting photosynthesis and chlorophyll biosynthesis. These changes at the plant level are due to specialized photoreceptors, as the red (R) and far red (FR) light absorbing phytochromes (600-700 nm) and the blue light absorbing cryptochromes and phototropins (400 to 500 nm) (Briggs & Huala, 1999, Franklin & Whitelam 2005). Plants under a low light ratio (R:FR), increase the elongation of the stem and reduce the thickness of the leaves to escape these conditions; however, if these conditions persist, they accelerate flowering to ensure reproductive success (Franklin & Whitelam 2005). This acceleration in flowering was observed mainly in the AMA genotype, with the decrease in the DFL (16 days) as the percentage of shade increased. Paredes-Jácome et al. (2019) also observed this effect in C. annuum, with 62.95 DFL in an open field versus 53.66 DFL under a black mesh. Capsicum species increase their photosynthetic efficiency under intermediate incident radiation (450-500 μmol m-2 s-1) (Fu et al. 2010), incident radiation that corresponds to the 70 % shading applied in this study (Figure 2).
Several studies indicate that shade increase yield up to five times more in Capsicum (Ayala-Tafoya et al. 2015, Zermeño-González et al. 2019, Díaz-Pérez et al. 2020). In C. annuum var. glabriusculum, 52 % increases in fruit yield have been observed due to shade (Rodríguez-del Bosque et al. 2005), which is consistent with our results for the AMA genotype. On the other hand, Díaz-Pérez (2014) indicated that for C. annuum, its yield increases with an increase in shade up to a maximum of 35 % shade and then decreases as the shade level increases. This effect was observed for the GAR genotype, which showed the highest yield under the 35 % shade level in the first year. This scenario seems to indicate a different degree of adaptability of the genotypes to the shade levels in this study, given that the GAR and PIP genotypes were more tolerant to open field conditions than shade conditions, which may be related to their ecological requirements and their adaptation capacity acquired based on changes in the agroecosystems where they develop in southeastern Mexico; while some grow under the tree canopy, receiving less solar radiation, others are found in open-air sites (Gutiérrez-Burón et al. 2020).
Wild Capsicum species are susceptible to rainfall, not tolerant to flooding, and susceptible to water stress (Martínez-Acosta et al. 2020). Therefore, these two climatic conditions affect the phenology, yield, and metabolism of plants. Specifically, the water deficits to which these wild plants are subjected in the dry season are a factor that limits their productivity. Olarewaju et al. (2017) reported that drought significantly affected the growth, development, and yield of Capsicum. In this study, a negative drought effect on yield was also observed, as there were lower yields for the three genotypes during drought. Showemimo & Olarewaju (2007) reported a yield reduction in C. annuum from 1.37 to 0.01 t ha-1 under severe drought, and these yields are very similar to those reported in this study for the three genotypes in the open field conditions.
The quality of fruits and the compounds produced by secondary metabolism are among the characteristics that are affected by the response of plants to the light they receive (Darko et al. 2014). The immature and mature fruits of the wild Capsicum genotypes in this study were an important source of secondary metabolites such as phenols, flavonoids, carotenoids and capsaicinoids. The degree of fruit maturity did not affect the TPC, which agrees with the results of Menichini et al. (2009), Lutz et al. (2015) and De la Cruz-Ricardez et al. (2020a). The range of variation in the TPC content fluctuated between 12.55 and 18.32 mg g-1, and that in the FLV ranged between 5.06 and 14.54 mg g-1; these values are consistent those of other Capsicum species (Guzman et al. 2021, Chel-Guerrero et al. 2022, Razola-Díaz et al. 2022). Among the functions of secondary metabolites such as phenolic compounds and flavonoids, protection against predators and microbial pathogens (Mazid et al. 2011, Piasecka et al. 2015, Erb & Kliebenstein 2020), and abiotic factors as exposure to UV-B radiation stand out. This UV-B protection is expressed as an increase in secondary metabolites as a function of increased radiation (Lake et al. 2009, Saviranta et al. 2010). Perhaps because of the above scenario, an increase in these compounds was observed for the GAR and PIP genotypes under open field conditions. PAN is a complex flavonoid polymer naturally present in cereals, legume seeds, and often in some fruits, but its polymeric nature makes its analysis difficult (Vermerris & Nicholson 2006).
Gu et al. (2003) and Hellström et al (2009) did not detect the presence of PAN in samples of chili fruits extracted with acetone-acetic acid-water and acetone-methanol-water. However, in fruit samples of C. frutescens extracted with 1 % HCl in methanol, Bayili et al. (2011) observed 1.11 mg g-1 of PAN. More recently, Olatunji & Afolayan (2019) determined a high PAN content in fruit samples of C. annuum and C. frutescens (619-709 mg g-1) extracted with the same solvent used in this study (80 % methanol).
One of the main functions of PAN is to provide protection against microbial pathogens, insects, and larger herbivores. Therefore, the fruits of these species can be a source of these compounds considering their high content. Shade did not affect the content of these compounds, so the fruits collected from plants developed under any environment could be used for this purpose. For total carotenoids, the highest content was detected in ripe red fruits rather than in immature green fruits, which is consistent with the results of Gómez-García & Ochoa-Alejo (2013). Several studies have shown an increase in the content of carotenoids as a function of the progression of fruit ripening (Hornero-Méndez et al. 2000, Deli et al. 2001). On the other hand, Daood et al. (2014) observed a variation of 5.31 to 11.51 mg g-1 in carotenoids in 22 hybrids of C. annuum in Hungary. Corrêa et al. (2018) showed higher contents (10.08 to 59.68 mg g-1) of CAT in 16 hybrids from Brazil. In the fruits of the genotypes evaluated, the total content ranged between 15.51 and 38.25 mg g-1 in mature fruits and between 3.99 and 7.15 mg g-1 in immature fruits. These contents were not affected by harvest year, but they did decrease due to shade. This shade effect should be considered if these wild genotypes are used as a source of carotenoids in the industry due to their multiple biological functions.
Shade positively affected the phenological development of the three genotypes of Capsicum: it accelerated the phenological processes from the first bifurcation of the stem by increasing the height of the plants to the ripening of the fruits, and shade also decreased the duration of the fruit ripening period. Shade only increased the AMA genotype yield, while in the GAR and PIP genotypes, the effect was negative. Regarding the secondary metabolites, shade negatively affected the contents of TPC, FLV, and CAT, which increased under open field conditions.











 nueva página del texto (beta)
nueva página del texto (beta)



