Introduction
Diabetes is defined as a metabolic disorder of multiple etiology characterized by chronic hyperglycemia with alterations in the metabolism of carbohydrates, fat, and proteins resulting from defects in the secretion, action of insulin, or both (Ballali & Lanciai, 2012). The elevation and accumulation of glucose in the bloodstream is progressive and is associated with a high risk of atherosclerosis, kidney damage, neuronal and blindness, which makes it one of the main causes of morbidity and mortality in the world (Neeland & Patel, 2019).
The prevalence of type 2 diabetes mellitus has increased by 30 % globally in the past decade, with the number affected increasing from 333 million in 2005 to 435 million in 2015 (Dunlay et al., 2019). This also implies an increase in the related risk factors, such as overweight or obesity (WHO, 2017).
In type 2 diabetes mellitus there is an increase in oxidative stress and a decrease in antioxidant defense systems, which have been implicated in the etiopathogenesis of the disease and in the appearance of chronic complications. The mechanisms that can contribute to the deterioration of the balance in diabetic patients are different, particularly in subjects with inadequate glucose control (hypo and hyperglycemia) and elevated triglycerides (hypertriglyceridemia) (Teodoro et al., 2018).
A high glucose concentration on bloodstream often react binding proteins, and this process is called protein glycation. The products from glycation may accumulate and bind to plasma membrane, circulating and structural proteins. The formation of reduced sugars-amino groups of protein complexes (Amadori products) induces further oxidative modifications to generate Advanced Glycation Endproducts (AGEs) which are associated with the oxidative stress, the increased expression of extracellular matrix proteins, cytokines and inflammation, whose leads to development and acceleration of diabetes complications (Fournet et al., 2018). These mechanisms participate in the formation of free radicals in diabetic patients including the increase of non-enzymatic glycation, auto-oxidative and metabolic stress as a result of changes in metabolism, level of mediators of inflammation and state of the antioxidant defense system (Fuentes et al., 2015).
Antioxidants are compounds or systems that delay oxidation by preventing the formation of free radicals or interrupting their propagation. One of the most common components attributed to antioxidant capacity in plants are phenolic compounds. The phenolic compounds can be divided into several groups such as phenolic acids, phenolic diterpenes, flavonoids, and volatile oils (Najafian & Moradi, 2017).
These compounds constitute a broad group of secondary plant metabolites widely distributed in plants that are fundamental in the signaling and defense mechanism. Its importance lies in the prevention of damage due to stress by pathogenic and predatory organisms; also, it has a function as precursors of compounds of greater complexity, in the intervention in processes of regulation and control of plant growth (Santos-Sánchez et al., 2019).
Phenolic compounds in food play an essential role in the defense against aging and chronic diseases such as diabetes mellitus type 2, cancer and cardiovascular disease among others (Ighodaro & Akinloye, 2018). These compounds inactivate free radicals involved in oxidative stress and prevent their propagation. Therefore, the supplementation with natural antioxidants could have a beneficial effect on diabetic’s health, preventing and delaying development of chronic complications (Lobo et al., 2010).
Besides the multiple phytochemicals with antioxidant capacity (such as flavonoids, alkaloids, hydroxycinnamic acids, vitamins, phytosterols, and essential oils), there is another group of compounds known as steviol glycosides (diterpene glycosides responsible of the sweet taste in S. rebaudiana Bertoni) which have been reported to display antioxidant properties (Bender, 2017; Ribeiro et al., 2019).
In addition of the antioxidant capacity of Stevia rebaudiana Bertoni, several studies have reported hypoglicemic effects attributed to steviol glycosides. Traditional applications of stevia and its steviosides in Latin America include the regulation of blood glucose levels, among others (Misra et al., 2011).
Therefore, S. rebaudiana leaves are a potential source of natural antioxidants (Ruiz-Ruiz et al., 2017) and several compounds which can be helpful in the treatment of diabetes like glycol steviosides such as stevioside, rebaudioside A, rebaudioside B, and dulcoside A. The aim of the present study was to evaluate the antioxidant effect and the ability to inhibit the formation of Advanced Glycation End Products (AGES) in vitro of an aqueous extract of S. rebaudiana Bertoni (AES). In the EAS, the antioxidant capacity was determined by FRAP (ferric reducing power test), hydroxyl radical elimination activity (OH·) and DPPH free radical elimination assay, in addition to inhibition of in vitro AGEs and identification of the main steviosides by UHPLC MS/MS.
Material and methods
Dehydrated Stevia rebaudiana Bertoni leaves were obtained from a greenhouse in Xochimilco, Mexico City. Other chemicals were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise specified.
Aqueous Extract of Stevia (AES)
The dried leaves of S. rebaudiana Bertoni were ground. 1 g of the dried leaves was weighed in a beaker, 10 mL of water was added and it was left in a water bath at 40 °C for 60 minutes with constant agitation. The suspension was filtered under vacuum to obtain EAS and frozen at -20 °C until use (Shukla et al., 2012a).
Total Phenolic Content (TPC)
TPC was determined by the Folin-Ciocalteu method (Lemus-Mondaca et al., 2018). Gallic acid (GA) calibration curve was used for quantification of 10 a 100 mg de GA. All measurements were performed in triplicate. Results were expressed as mgGAE/100 g dry mass.
In vitro antioxidant assays
To evaluate the antioxidant capacity of AES, three different methods were used at concentrations of 2.5, 5 and 10 mg/mL.
Ferric reducing power assay (FRAP)
1 g of sample was weighed in the test tube, adding 2.5 mL of phosphate regulator and 2.5 mL of 1 % potassium ferricyanide solution, and incubated for 20 minutes at 50 °C in an Isotherm® General Purpose Incubator, ESCO. Then, 2.5 mL of 10 % trichloroacetic acid was added to each test tube and centrifuged at 8000 rpm for 10 minutes. 2.5 mL of the supernatant was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1 % ferric chloride solution. The absorbance was read at 700 nm (Vijayalakshmi & Ruckmani, 2016).
Hydroxyl radical (OH·) scavenging activity
The assay was performed evaluating the scavenging properties against OH· generated from Fenton’s reaction (Brands et al., 2019) by adding 1 mL of 1-10 phenanthroline, 2 mL of phosphate buffer pH 7.4, 1 mL of FeSO4, 1mL H2O2 0.12 %, 1 mL of AES (2.5, 5 y 10 mg/mL) each dissolved in distilled water; the mixture was incubated at 37 °C for 90 min and measured at 536 nm.
DPPH free radical scavenging assay
The ability to eliminate 2,2-diphenyl-1-picrylhydrazyl radicals (DPPH) was determined according to what was reported before (Di Maro et al., 2013). 1.5 mL of the aqueous AES extracts (2.5, 5 and 10 mg/mL) were added with 1.5 mL of 0.1 mM DPPH in 95 % ethanol. The mixture was stirred and kept standing for 30 minutes at room temperature. The effect of DPPH free radical inhibition was measured at 517 nm.
Advanced Glycation End-products (AGEs) Inhibition: Formation of AGEs in the Bovine Serum Abumin (BSA)/Glucose and BSA/ Fructose Systems.
2 mL of AES (2.5, 5 and 10 mL) were placed in 15 mL conical tubes, adding 0.67 mL of a BSA/ glucose solution (10 mg / mL), plus NaCl and phosphate regulator with azide sodium 0.02 % pH 7.4 (to reach a total volume of 5 mL), this solution is incubated at 37 °C for 21 days. This same procedure was carried out with BSA / fructose (Hori et al., 2012). Metformin (50 mg/mL) was used for this experiment as a positive control, in addition to a blank. The determination was carried out in triplicate. The formation of AGEs was evaluated by excitation wavelength (370 nm) and emission (440 nm) in a fluorescence spectrophotometer (Variouskan Lux microplate reader, Thermo Fisher, 2000) (Aranda-González et al., 2015).
UHPLC MS/MS steviosides identification and quantification
For the identification of steviol glycosides, 5 µL of AES (2.5, 5 y 10 mg/mL) was injected into a UHPLC RP-C18 column (2.1x100 mm particle size 1.7 microns). The elution was performed by varying the proportion of solvent A (Deionized water) to solvent B (Acetonitrile 100 %) as follows: H2O:Acetonitrile 100 % (60:40), at 15 min; H2O: Acetonitrile 100 % (60:40), at 22 min; H2O: Acetonitrile 100 % (50:50), at 25 min, and finally, H2O:Acetonitrile 100 % (0:100), at 28 min. The total running time was 37 min. The column temperature was 30 ºC. The flux was 0.7 mL/min.
Each glycoside was identified by registering the mass and MS/MS spectra of each chromatographic peak obtained, applying the FIND BY FORMULA algorithm (Aranda-González et al., 2015). The registration conditions of the mass spectra were the following: a logging interval between 100-1700 m/z; scan speed: 1 m/z; electrospray ionization (ESI) as ionization source. Mode: negative, collision energy: 0, gas temperature 320 °C and variable fragmentation voltages.
To obtain the calibration curves and to quantify the stevioside and rebaudioside A concentrations, both standards were run in triplicate on a RP-C18 column (0.25-3.00 mg/mL, injected 5 µL of both standards). The same separation gradient applied previously was used. The corresponding chromatograms were recorded; the calibration curves were performed with the mass signals at 803.370 m/z for the stevioside and 965.422 m/z for the rebaudioside A. The conditions of the mass spectra registration were the same as previously used. The quantification of stevia extracts samples were prepared by taking 200 µL of extract and diluting them with distilled water for a final volume of 700 µL. Finally, 5 µL of each sample in triplicate was injected into the equipment with the same separation and recording conditions as those used for the calibration curve.
Results and Discussion
Total phenolic content (TPC)
Most research has been directed towards the identification of plants with antioxidant ability. Available synthetic antioxidants have shown toxicity in contrast to those from natural sources (Shukla et al., 2012a). Figure 1A show that phenolic content was directly proportional to aqueous extract concentration, suggesting that the AES may have high levels of antioxidant activity (Figure 1A). The total polyphenol content of extracts of S. rebaudiana varied in average 20.1 ± 0.4 mg GAE/g (Grozeva et al., 2015). The amount of the polyphenols found in water extracts was higher compared to 95 % ethanol extract (15.6 ± 0.4 vs 12.75 ± 0.55) and even higher than the stevia methanolic leaves extract reported by other authors (Jahan et al., 2015).
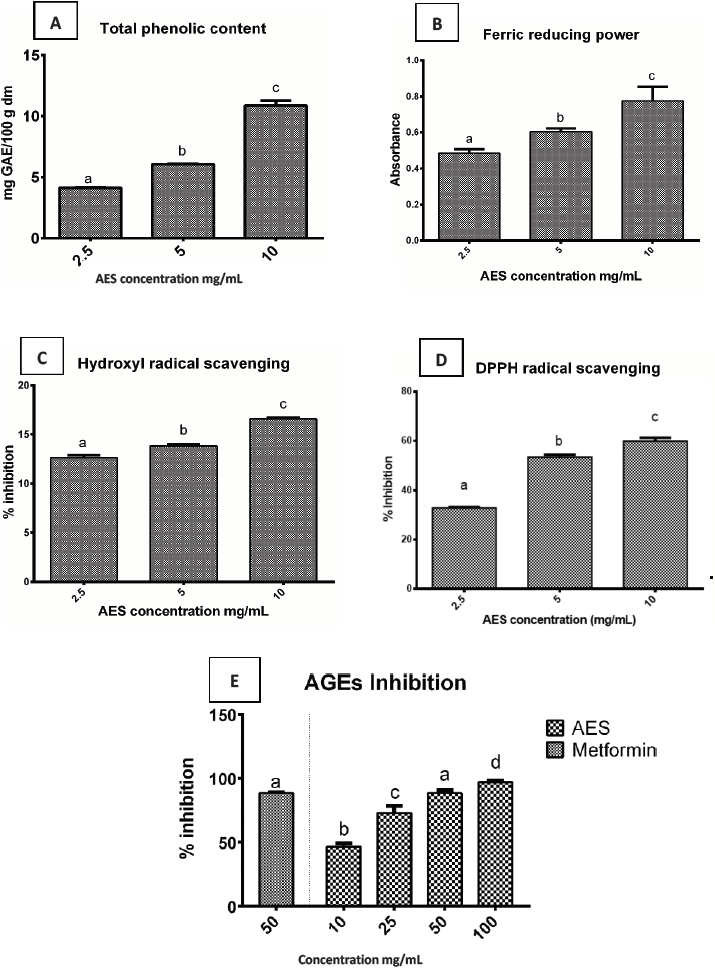
A) Amount of total phenolic content, B) Ferric antioxidant power activity, C) Hydroxyl radical scavenging activity, D) DPPH radical scavenging activity, E) Advanced glycation end products (AGEs) inhibition. Metformin as control. AES: aqueous leaf extract of S. rebaudiana. Values are expressed as mean ± SD of three independent determinations. Different letters indicate a significant difference (p<0.05), as determined by Tukey’s multiple range test.
Figure 1 Aqueous leaf extract of S. rebaudiana.
There are several phenolic compounds reported in extracts of S. rebaudiana Bertoni, the most relevant being chlorogenic acid, ellagic acid, coumarin, hesperidin and rosmarinic acid in addition to eugenol, coumarin and vanillin, depending on the culture conditions (Jahan et al., 2015; Grozeva et al., 2015). Dietary consumption of these polyphenolic compounds can prevent the development of some types of dysplasia, cardiovascular diseases, neurodegenerative diseases, diabetes and osteoporosis, as evidenced by studies in in vitro models and animal models (Santos-Sánchez et al., 2019).
Ferric reducing power assay (FRAP)
Antioxidant compounds donate electrons to reactive radicals reducing them into more stable species; a higher absorbance indicates a higher ferric reducing power (Bursal & Köksal, 2011). A concentration-dependent absorbance reaches the highest optical density at 10 mg/mL (Figure 1B). These results are consistent with those documented before (Rao, 2014), where the highest concentrations showed the higher absorbance in S. rebaudiana Bertoni leaves. The ferric reducing capacity is generally associated with the ability to break the free radical chain by donating a hydrogen atom and is widely used to evaluate the antioxidant properties of dietary phytochemicals (Najafian & Moradi, 2017).
DPPH free radical-scavenging
The effect of AES on the DPPH radical scavenging as the percentage of inhibition is reported (Figure 1D). The AES antioxidant activity showed a significant difference between concentrations (p≤0.05) with an inverse concentration-dependent behavior. The higher AES concentration (10 mg/mL) showed higher activity, with 60 % of DPPH inhibition, value below 72 % reported before (Ruiz-Ruiz et al., 2017). Free radical DPPH can react with antioxidant compounds through a process characterized by the transfer of a hydrogen atom provided by the antioxidant agent and its response depends on the concentration. Low concentrations of this reagent for reliable results it’s recommended (Santos-Sánchez et al., 2019).
Other authors (Periche et al., 2015) reported that the drying conditions applied in fresh stevia leaves have a significant impact on steviol glycosides and antioxidants through an increase in antioxidant capacity but a decrease in steviosides; on this research the AES showed an antioxidant capacity, which may be due to other compounds with antioxidant activity reported in stevia leaves as phenolic compounds such as phenolic acids (chlorogenic acid, caffeic acid, and trans-ferulic acid) and flavonoids (rutin) which are present in aqueous extracts of stevia leaves (Lemus-Mondaca et al., 2018).
Finally, it is important to emphasize that DPPH and FRAP are classified as methods based on individual electron transfer. Although these tests are similar in terms of results, they focus on different mechanisms: the DPPH test is carried out in alcoholic media, and FRAP in acidic and non-liquid reaction media. Therefore, both can be used to corroborate the data obtained and evaluate, for example, the possible practical applications of the analyzed samples (Álvarez-Robles et al., 2016).
Hydroxyl radical (OH·) scavenging activity
The hydroxyl radical is an extremely reactive free radical formed in biological systems; it can damage almost every molecule found in living cells. This radical can join nucleotides in DNA and cause strand breakage leading to carcinogenesis, mutagenesis, and cytotoxicity (Kim et al., 2011). OH· radicals generation is particularly dangerous for cellular membranes. In the present research, all extracts had scavenging activity on hydroxyl radicals in a dose-dependent manner; where the high inhibition percentage was found at 10mg/mL (Figure 1C). On prior studies (Stoyanova et al., 2011; Hajihashemi & Geuns, 2013; Shukla et al., 2012b) it has been documented that S. rebaudiana Bertoni possess a high ROS (OH·) scavenging activity.
The percent inhibition on hydroxyl radical scavenging was found to be 13.03 %, 14.36 % and 17.51 %, for each concentration in a dose-dependent manner, respectively; therefore the extract is capable of inhibit and reduce free radicals by terminating the radical chain reaction, acting as reducing agent (Kim et al., 2011). All studies published in this regard show that the extracts themselves exert purifying effects of reactive oxygen species (ROS) (Hajihashemi & Geuns, 2013). Plants contain many phenolic compounds; whose hydroxyl group contained on an aromatic ring can interrupt chain oxidation reactions by donation of a hydrogen atom or chelating metals: they act as reducing agents and antioxidants. High amounts of phenolic compounds indicate high antioxidant capabilities (Jahan et al., 2010). In S. rebaudiana the content of phenols and rebaudioside A has been significantly and positively correlated with antioxidant capacity (Tavarini & Angelini, 2013).
Advanced Glycation End-products (AGEs) Inhibition
The modification of some crucial physiological proteins caused by AGEs formation is an important factor in the development of age-related diseases such as atherosclerosis, diabetes and its complications. Figure 1E shows the effect of AES extract at different concentrations in the formation of fluorescent AGEs in glycation BSA/glucose, BSA//fructose model system for 21 days. AES affect the formation of AGEs in a dose-dependent rate. At 100 mg/mL AES exhibit a better antiglycation activity (96.5 %) in comparison to the metformin control (89 %). Several edible plants or its extracts have shown antiglycation activities in BSA-glucose-fructose models (Kaewnarin et al., 2014).
The major components of edible plants contain phenolic compounds and flavonoids such as catechin, epicatechin, quercetin, rutin, ellagic acid, cumaric acid, between others who exhibit antioxidant capacity. Aqueous leaf extract of S. rebaudiana contains high levels of total phenolic, which results in a high antioxidant capacity that is capable of inhibiting or reduce free radicals to terminate the oxidative estate. Since the EAS exhibits a high antiglication activity, it can be inferred that the antioxidant compounds it contains could suppress the formation of AGEs and protein oxidation. The high content of phenolic compounds and an important ability to eliminate reactive oxygen species in hydroxyl and DPPH radicals present in the EAS could explain the reduction in protein oxidation measured by the formation of AGEs.
Regarding the role of steviosides in preventing the formation of AGEs, the possible mechanism of antiglication activity has been reported. It seems to be related to inhibition of the interaction between glucose and amino acids. It has been reported that steviol glycosides compete with glucose to bind to the carrier thus inhibiting the entry of glucose into cells, decreasing glycosylation (Rizzo et al., 2013).
AES may inhibit AGEs formation by its ROS scavenging capacity on hydroxyl and DPPH radicals during the auto-oxidation of sugar and oxidative degradation of Amadori products, leading to reduced protein oxidation. Other mechanisms of antiglycation, particularly for inhibiting the formation of late-stage Amadori products, breaking the cross-linking structures in the intracellular formed AGEs has been proposed (Díaz-Casasola & Luna-Pichardo, 2016). Further comprehensive studies of Stevia rebaudiana are required to confirm the antiglycation mechanisms described above.
UHPLC MS/MS steviosides identification and quantification
The Figure 2 shows the spectra of steviosides from Stevia rebaudiana extract obtained from UHPLC identification. The notorious peaks were 9, whose identification are presented in Table 1. During identification, we found two peaks with the same molecular weight despite being different compounds (steviolbioside and rubososide). It is possible to distingue them by the difference in retention time (Gardana et al., 2010). Regarding the results of the steviosides quantification, our results differ from those previously reported (Aranda-González et al., 2015). The value for stevioside was 5.018 ± 0.18 mg GAE/100 g which is higher than reported before (Gardana et al., 2010), however, the value for rebaudioside A was lower (7.79 ± 2.05 mg GAE/100 g vs 15.15 ± 0.02 mg GAE/100 g). On the other hand, Aranda-González et al., (2015), reported a content of rebaudioside A of 2 % to 54 % in dried leaves of S. rebaudiana Bertoni This variability may be due to variety of Stevia, weather conditions and farming, since these factors contribute to the amount and type of glycosides in the plant.
Table 1 Characteristics of the steviosides identified in the aqueous extract of Stevia rebaudiana Bertoni by UHPLC MS/MS
| 1 | Rebaudioside A | C44H70O23 | 966.4309 | 14.172 | 965.4218 | 7.79 ± 2.05 |
| 2 | Rebaudioside D | C50H880O28 | 1128.485 | 11.886 | 965.4136 | ND |
| 3 | Rebaudioside C | C44H70O22 | 950.4359 | 15.033 | 949.4289 | ND |
| 4 | Rebaudioside F | C43H68O22 | 936.4206 | 14.87 | 935.4151 | ND |
| 5 | Rebaudioside B | C38H60O18 | 804.3778 | 14.384 | 803.3703 | ND |
| 6 | Stevioside | C38H60O18 | 803.3704 | 16.912 | 803.3703 | 5.018 ± 0.18 |
| 7 | Dulcoside A | C38H60O17 | 78.3838 | 15.282 | 787.3751 | ND |
| 8 | Esteviolbioside | C32H50O13 | 642.3151 | 16.175 | 641.3181 | ND |
| 9 | Rubososide | C32H50O13 | 642.3151 | 17.238 | 641.3181 | ND |
It has been discovered that the same variety may have higher glycoside content if it is exposed to high solar radiation or if it is grown with a specific density from 12.5 to 25 plants per square meter (Jarma-Orozco et al., 2011). Regarding the total content of steviosides it has been published that can vary from 4 % to 22 % depending on the conditions (Aranda-González et al., 2015).
Conclusion
Aqueous leaf extract of S. rebaudiana contains high levels of total phenolic, which results in a high antioxidant capacity that is capable of inhibiting or reduce free radicals to terminate the cellular oxidative estate. AES exhibit a high anti-glycation activity since antioxidant compounds can suppress the effect on AGEs formation and protein oxidation. Significant antioxidant activity of AES provides an experimental validation for the traditional use of this plant as a source of natural antioxidants with consequent health benefits. Additional studies are needed to investigate the bioactive compounds responsible for the observed activities.











 texto en
texto en 



