Introduction
Throughout the years, agricultural processes have generated very important quantities of hardly degradable by-products or residues (Castro Granado, 2018) in the environment (vegetable, plastic or pesticide container residues, etc.), derived from the use and maintenance of agricultural exploitations (Saval, 2012).
Biotechnology allows for the bio-conversion of agro-industrial residues (Blanco, 2017) in products of commercial interest by means of processes of direct extraction or transformation by chemistry or microbiology (Moldes et al., 2002). In Mexico, a great quantity of agro-industries generates organic residues with a great potential for use (Mejías-Brizuela et al., 2016), such as the case of grape bagasse which is used for elaborating wines in different regions of the country. Grape crops represent 0.83 % of the national GDP with a production in 2018 of 444.45 thousand tons (SIAP, 2018), estimating a production of 415.43 thousand tons by 2024 (SAGARPA, 2017). Regarding worldwide grape production during the 2018/2019 period, 22.15 million tons were generated (Shahbandeh, 2019). For the case of pomegranate crops, 8,073.88 tons were generated in 2018 in the national territory (SIAP, 2018). Meanwhile around 3 million tons are annually produced worldwide (Financial-Tribune, 2016).
The origin of the issue lies in the dumping of waste and surpluses generated as vegetable residues in the rural environment, causing problems by creating an important focal point of infection for the crops, as well as pollution during the burning. In this context, the use of complex carbohydrates making up the cell structure of the mentioned residues, such as cellulose and hemicellulose was investigated. In order to manage the use of these complex carbohydrates, they firstly have to be simplified in monomeric sugars, thus creating enriched broths for vegetables cultivation, and, by means of fermentation processes, they can generate diverse products of interest on the market (Mestries, 2015).
The pretreatment of the residues consists in fractioning the biomass into their main component (cellulose, hemicellulose and lignin) to make the subsequent enzymatic and microbial degradation easier. The hydrolysis, or breakup of the molecules in an aqueous medium (Sun et al., 2016) has the purpose of transforming glucose polymers (starch and cellulose) into simple sugars (Álvarez et al., 2016). The most used physicochemical strategies are liquid hot water (LHW), steam explosion, wet oxidation, sulfite pretreatment to overcome recalcitrance of lignocellulose (SPORL), ammonia pretreatment and microwave pretreatment (Kumar & Sharma, 2017). The LHW pretreatment is performed only with water and without catalyzers nor additional chemical products, which is advantageous since it is eco-friendly. Water is heated up at high temperature, while a pressure high enough to maintain water in its liquid state is applied. This process causes alterations in the lignocellulose structure.
The objective of this work was to analyze the possibility of using hydrothermal hydrolysis for breaking up vegetable polymers and for increasing monomeric sugars availability for its use in diverse industries of the biotechnological field.
Material and Methods
The present research work was performed at the department of Food Sciences and Technologies, in the laboratory of Fermentations of the Antonio Narro Agrarian Autonomous University. This research study consists of two stages, in the first one, a drying pretreatment was performed on raw material and its physicochemical characterization was achieved. Raw material was classified by particles size and total sugars and reducing sugars concentrations and antioxidant capacity were determined. The second stage consists of performing thermal hydrolysis on grape bagasse (merlot variety) and pomegranate (wonderful variety) for releasing monomeric sugars in “slurry” (hydrolyzed grape bagasse) and in the broth obtained from it. Three factors were evaluated in three levels each. These factors were: time (5, 10, 15 minutes), pressure (10, 15 and 20 psi) and particles size (0.85-1.00, 1.40-1.70 and 2.36-2.80 mm in diameter). The selection of factors was determined by considering diverse previous studies (Dos Santos Rocha et al., 2017; Kumar & Sharma, 2017; Moure et al., 2017) and by adjusting the levels for the study of the materials of the present experiment.
Obtaining and pre-processing raw material
Grape (merlot variety) and pomegranate (wonderful variety) residues were obtained in the regions of Parras and Cuatro Ciénegas, Coahuila, respectively. Since these residues were perishable, a dehydration pretreatment was performed on them in a convection drying stove (Biobase Biodustry BOV-T70C) at 60 °C for 24 hours. Grape and pomegranate dry residues were ground and classified by means of Montinox sieves (Mexico) of three sizes, #8 mesh (2.36 mm), #12 mesh (1.40 mm) and #20 mesh (0.85 mm). One gram of each vegetable sample was weighted, placed in 40 mL of distilled water and stirred for 20 min. Then the mixture was filtered and collected in glasses with ice, which was used for further analysis, considered as the filtered sample.
Determining total sugars
For quantifying total sugars (Dubois et al., 1956) in the extract, 500 μL of the filtered sample was placed in a tube with 500 μL of 5 % phenol (Fermont, Mexico) and it was placed in an ice water bath for 5 minutes. With the tubes inside of the ice water bath, 1000 μL sulfuric acid (Jalmek, Mexico) were slowly added on the walls of the tubes and the mixture was let rest for 15 minutes. To stop the reaction, tubes were softly stirred and brought in a hot water bath at 50 °C for 5 minutes. Then, they were let cool at room temperature for 5 minutes. Absorbances were read in a BIOBASE EL-10 A ELISA microplate reader at 470 nm. Calibration curve was performed by using 0.1 % glucose (Jalmek, Mexico).
Quantifying reducing sugars
For determining reducing sugars (Miller, 1959), 1000 μL of the filtered mixture were added to 1000 μL of the DNS (3,5-Dinitrosalicylic acid) prepared and the mixture was placed in a water bath (Thermo Scientific), at 50 °C for 5 minutes. 5 mL of distilled water were added, it was let rest for 5 minutes at room temperature. Absorbances were read in a BIOBASE EL-10 A ELISA microplate reader at 520 nm. Calibration curve was performed by using 0.1 % glucose (Jalmek, Mexico). For preparing DNS reactive, 10 mL distilled water were heated at 50 °C in a water bath (Thermo Scientific), stirring and 3,5-Dinitrosalicylic acid (Sigma-Aldrich, U.S.A.), sodium hydroxide (Jalmek, Mexico), sodium and potassium tartrate (Jalmek, Mexico), phenol (Fermont, Mexico) and sodium sulphate (Fermont, Mexico) were added in this order, it was diluted to 100 mL with distilled water, for conservation it was kept in dark and refrigerated.
Analyzing antioxidant compounds
For determining antioxidant compounds (Molyneux, 2004), 100 mg of sample were weighted and 1.5 mL methanol (Hycel, Mexico) were added in a 2 mL conic tube. The mixture was stirred in vortex for 20 seconds, sonicated for 5 minutes and then centrifuged at 12,000 rpm for 10 minutes. The supernatant was extracted and filtered with nylon syringe filter with pore size of 0.45 μm for further analysis. 2,2-Diphenyl-1-picrylhydrazyl (DPPH; Sigma-Aldrich, U.S.A.) was prepared at a concentration of 60 % w/v. The vegetable material sample and the DPPH radical were placed in a 50:50 ratio. Absorbances were read in a BIOBASE EL-10 A ELISA microplate reader at 540 nm.
Optimizing the thermal hydrolysis process
Optimization process was performed according to a fractional factorial design with orthogonal arrays, by using Minitab 17.1.0 software. Three factors were selected (time, pressure and particles size) and an orthogonal matrix was established with three levels of variation per factor (Table 1).
Table 1 Taguchi L-9 design with three factors in three levels.
| Treatment | Time (min) | Pressure (psig)* | Size (mesh) |
|---|---|---|---|
| T1 | 5 | 10 | 8 |
| T2 | 5 | 15 | 12 |
| T3 | 5 | 20 | 20 |
| T4 | 10 | 10 | 12 |
| T5 | 10 | 15 | 20 |
| T6 | 10 | 20 | 8 |
| T7 | 15 | 10 | 20 |
| T8 | 15 | 15 | 8 |
| T9 | 15 | 20 | 12 |
*Hydrolysis temperatures were 115, 120 and 125°C, corresponding to pressures of 10, 15 and 20 psig respectively.
Thermal hydrolysis process
For the hydrolysis, 2 g of sample were used of each size of vegetable material and were placed in fiber filter bags (Ankom Technology F57), with pore size of 0.25 μm, with 500 mL distilled water. Then, the bags were placed inside a Molded Aluminum Pressure Reactor. Samples were classified as “slurry” referring to grape bagasse after the hydrolysis treatment; and the liquid fraction derived from the hydrolysis treatment was called hydrolyzed broth. Total sugars were determined in these two fractions. After obtaining results of each of the samples, they were compared and a factorial design analysis was performed using Minitab (17.1.0 version) statistical software to be able to select the best values.
Results and Discussion
The initial analysis of total and reducing sugars in the raw material, for both grape and pomegranate, showed that the highest concentration of total sugars in grape bagasse. Classified by particles size, was obtained with a size ranging from 1.40 to 1.70 mm with 2.682 mg/ml (Table 2). The highest concentration of reducing sugars in grape bagasse was obtained with a diameter of particles ranging from 0.85 to 1.00 mm (1.333 mg/mL). Regarding the antioxidant activity (AOx) represented by the DPPH content, the highest concentration was obtained with diameter of particles ranging from 2.36 to 2.80 mm (2.43 mg/mL).
Table 2 Concentration of total and reducing sugars in grape bagasse and pomegranate bagasse, classified by particle size.
| Grape | Pomegranate | |||||
|---|---|---|---|---|---|---|
| Particle size | TS (mg/mL) | RS (mg/mL) | AOx (mg/mL) | TS (mg/mL) | RS (mg/mL) | AOx (mg/mL) |
| 2.36-2.80 mm | 1.976±0.077c | 0.821±0.044c | 2.43±0.11a | 2.070±0.105c | 1.656±0.187b | 2.77±0.64b |
| 1.40-1.70 mm | 2.682±0.104a | 1.017±0.054b | 2.08±0.08b | 4.215±0.212a | 1.646±0.086b | 4.59±0.66a |
| 0.85-1.00 mm | 2.297±0.089b | 1.333±0.070a | 2.31±0.10a | 3.938±0.198b | 2.058±0.107a | 4.95±0.60a |
Averages with same letters between columns, are not statistically equals (Tukey p ≤ 0.05). TS: Total sugars; RS: Reducing sugars; AOx: Antioxidant activity.
For pomegranate peel, the highest of total sugars was obtained in particles ranging from 1.40 to 1.70 mm (4.215 mg/mL). The highest concentration of reducing sugars was obtained for diameters ranging from 0.85 to 1.00 mm (2.058 mg/mL). The highest concentration of DPPH was obtained with diameters of particles ranging from 0.85 to 1.00 mm (4.95 mg/mL).
The above mentioned reflected that the particles size is a factor affecting the quantification of analytes of interest. This variation can be attributed to the easiness of interaction of the raw material with the chemical compound, since it allows a better surface distribution. Jiménez Islas et al. (2012) showed the effect of the concentration of the acid used in the hydrolysis processes and mentioned that there can be an interference due to the variation in the size of particles of the material, mainly its interaction between the surface area and the acid attack towards the particles. To improve sugars extraction by thermal hydrolysis processes, a lower particles size supposes lower changes in the adhesion forces among particles of different sizes (Polachini et al., 2019).
Experimental analysis of the thermal hydrolysis treatment
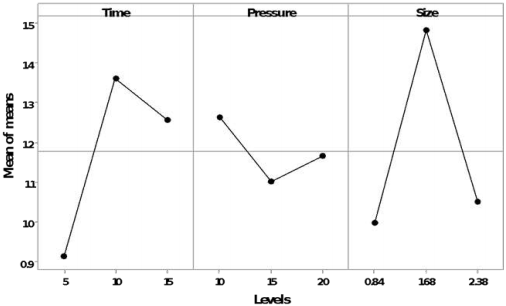
The levels experimentally evaluated to be able to study the thermal hydrolysis process, presented heating and cooling profiles with times of 13±2 min and 22±2 min, respectively. Table 3 shows the analysis of variance performed on reducing sugars data obtained from the treatments derived from the L-9 orthogonal design. For grape liquid hydrolysate, time, pressure and particles size factors in the individual levels evaluated did not showed significant effects (p ≤ 0.05). However, the model estimated by Taguchi’s analysis achieved to determine that the best effect for releasing reducing sugars for the hydrolysate was given by the particles size, followed by the duration time of the process (Figure 1).
Table 3 ANOVA of the Taguchi design for reducing sugars from grape residues.
| Hydrolysate | Source | DF | Seq SS | Adj SS | Adj MS | F | P |
| Time | 2 | 0.32786 | 0.32786 | 0.16393 | 1.24 | 0.447 | |
| Pressure | 2 | 0.03980 | 0.03980 | 0.01990 | 0.15 | 0.869 | |
| Size | 2 | 0.42639 | 0.42639 | 0.21319 | 1.61 | 0.383 | |
| Residual error | 2 | 0.26502 | 0.26502 | 0.13251 | |||
| Total | 8 | 1.05906 | |||||
| Slurry | Source | DF | Seq SS | Adj SS | Adj MS | F | P |
| Time | 2 | 0.47264 | 0.47264 | 0.236319 | 26.00 | 0.037 | |
| Pressure | 2 | 0.35601 | 0.35601 | 0.178004 | 19.58 | 0.049 | |
| Size | 2 | 0.37008 | 0.37008 | 0.185042 | 20.35 | 0.047 | |
| Residual error | 2 | 0.01818 | 0.01818 | 0.009091 | |||
| Total | 8 | 1.21691 |
DF: degrees of freedom; Seq SS: Sequential Sum-of-Squares; Adj SS: Adjusted Sum-of-squares; Adj MS: Adjusted Mean Square..
For the slurry of grape residues, the three factors evaluated showed a significant effect for the release of reducing sugars (p ≤ 0.05), being the time the factor which most benefits the process, followed by the pressure in the system (Figure 2).
In the case of the liquid hydrolyzed and the slurry of pomegranate residues, time, pressure and particles size factors in the individual levels evaluated, did not show significant effects (p ≤ 0.05) in either treatment.
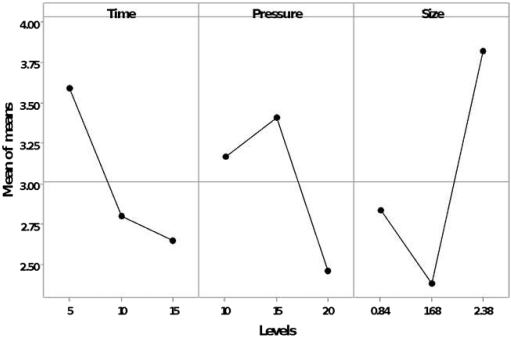
The greatest effect for the release of reducing sugars for the hydrolysate was given by the pressure during the process, followed by the duration time of the process (Figure 3); and the greatest effect for the release of sugars in the slurry was the particles size, followed by the time of the process (Figure 4).
Table 4 ANOVA of the Taguchi design for reducing sugars from pomegranate residues.
| Hydrolysate | Source | DF | Seq SS | Adj SS | Adj MS | F | P |
| Time | 2 | 1.2535 | 1.2535 | 0.6268 | 0.53 | 0.652 | |
| Pressure | 2 | 1.1289 | 1.1289 | 0.5644 | 0.48 | 0.675 | |
| Size | 2 | 0.5560 | 0.5560 | 0.2780 | 0.24 | 0.808 | |
| Residual error | 2 | 2.3454 | 2.3454 | 1.1727 | |||
| Total | 8 | 5.2838 | |||||
| Slurry | Source | DF | Seq SS | Adj SS | Adj MS | F | P |
| Time | 2 | 1.549 | 1.549 | 0.7745 | 0.24 | 0.808 | |
| Pressure | 2 | 1.463 | 1.463 | 0.7317 | 0.22 | 0.817 | |
| Size | 2 | 3.238 | 3.238 | 1.6189 | 0.50 | 0.668 | |
| Residual error | 2 | 6.521 | 6.521 | 3.2604 | |||
| Total | 8 | 12.771 |
DF: degrees of freedom; Seq SS: Sequential Sum-of-Squares; Adj SS: Adjusted Sum-of-squares; Adj MS: Adjusted Mean Square.
The difference detected in ANOVAs and the impact of the factors evaluated in the release of monomeric sugars can be due to the characteristics of the vegetable material. When using residues of industrial processes where sugars are required as raw material, they lack of hydro-soluble available sugars for their aqueous extraction at low time and temperature conditions.
Analyzing and optimizing thermal hydrolysis conditions for grape residues showed that time-size and pressure-size interactions presented the highest severity indexes (27.41 % and 20.76 % respectively). For pomegranate residues, the most critical interaction with 34.29 % of severity was the pressure-size one. The particles size makes the hydrolysis easier, due to the extension of the contact area, in addition to improve solubility for the smaller particles sizes (Grajales Martínez et al., 2018).
Therefore, the conditions that most favored the release of reducing sugars of grape residues hydrolysates can be determined, such as: time of 10 minutes, pressure of 10 psi and size of 1.40-1.70 mm; while for slurry, they were: time of 15 minutes, pressure of 15 psi and size of 2.36-2.80 mm. The conditions for pomegranate residues hydrolysates were time of 15 minutes, pressure of 20 psi and size of 1.40-1.70 mm; while for slurry, they were: time of 15 minutes, pressure of 15 psi and size of 2.36-2.80 mm.
The conditions achieved a better extraction of reducing sugars content (Table 5), allowing to generate an added value to these residues, and being able to use them as a substrate in diverse processes for obtaining biomolecules or biofuels.
Table 5 Sugars and antioxidant (AOx) content comparison from the grape and pomegranate and the thermo -hydrolysis properties.
| Glucose (mg/ml) | AOx (mg/ml) | |
|---|---|---|
| Grape-Initial | 1.31±0.04b | 2.27±0.18a |
| Grape-Hydrolysate | 1.77±0.02a | 0.99±0.53b |
| Grape-Slurry | 1.76±0.04a | 1.64±0.14b |
| Pomegranate-Initial | 2.08±0.02c | 4.10±0.17a |
| Pomegranate-Hydrolysate | 3.18±0.03a | 2.24±0.13b |
| Pomegranate-Slurry | 2.33±0.04b | 2.21±0.40b |
In both vegetable residues, the hydrolysis process achieved to increase reducing sugars extraction present in vegetables, compared to initial values of the respective grape and pomegranate values. The quantity of water absorbed in the particles allows to give access to water molecules, helping swell the vegetable material, generating amorphous regions and favoring the hydrolysis process (Kapoor et al., 2019).
Hydrolysis treatments achieved to convert recalcitrant vegetable wall, mainly made up by lignin, into hemicellulose compounds of higher availability for monomeric sugars. Zietsman et al. (2017) identified that the thermal treatment to which their grape residues samples were submitted managed to eliminate pectin and to increase released carbohydrates (xylan, xyloglucan, cellulose and mannan polymers) in the supernatants obtained. Dos Santos Rocha et al. (2017), when performing hydro-thermal treatments studies on sugarcane bagasse, reported that thermal treatments can remove hemicellulose up to 85 %, favoring the conversion of complex carbohydrates into oligomers and monomers.
Pressure (temperature) conditions and particles size evaluated achieved to remove lignin from the liquid phase (hydrolysate), being more evident in pomegranate residues than in grape residues (Table 4). The low quantity of glucose in hydrolysate grape can be due to the conversion of initial monomeric sugars into hydroxymethylfurfural and furfural (Dos Santos Rocha et al., 2017).
The analysis of the antioxidant capacity of materials of grape bagasse and pomegranate residues were observed to be related to times of residence and temperature, due to the characteristics presented by proanthocyanidins, anthocyanins and flavonoids when being submitted to hydrolysis and when increasing hydrolysis times (Petrović et al., 2016), which represents a decrease in its antioxidant activity when being submitted to higher times and temperatures of the thermal process. The antioxidant activity is related to phenolic compounds present in the vegetable material and to the derivatives of lignin degradation by thermal treatment. In the case of grape and pomegranate hydrolysates, reductions of the antioxidant effect were shown in samples treated with thermal hydrolysis. The concentration and the type of phenolic compounds present in the hydrolysates depend on operating conditions (temperature and residence times), as well as on the treated lignocellulosic material and its initial phenolic compounds content (Moure et al., 2017).
Conclusion
The thermal hydrolysis process managed to release a higher quantity of sugars in grape and pomegranate samples, compared with the initial ones. The release of reducing sugars indicated that the thermal hydrolysis process as a pretreatment managed to remove the vegetal lignin and to give availability to the present sugars.
The time of the process and the particles size are determining factors in the hydrolysis process. The exposure time is clearly a variable to be considered in the future, above all if higher concentrations are wanted in sugars extraction of agro-industrial residues.











 texto en
texto en