Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias pecuarias
versión On-line ISSN 2448-6698versión impresa ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.10 no.2 Mérida abr./jun. 2019
https://doi.org/10.22319/rmcp.v10i2.4652
Review
Oxidative stress and antioxidant use during in vitro mammal embryo production. Review
a Universidad Nacional de Colombia, Grupo de Investigación BIOGEM. Medellín, Colombia.
bUniversidad CES, Facultad de Medicina Veterinaria y Zootecnia, Grupo INCA-CES. Medellín, Colombia.
Of the many animal reproduction biotechnologies, in vitro embryo production has developed most over the past twenty years. Procedure success depends on many factors, including the presence of reactive oxygen species in adequate proportions. Both in vitro fertilization and gamete and embryo manipulation exposes cells to endogenous and/or exogenous factors that can affect antioxidant defense mechanisms and quality. This review discusses some sources of reactive oxygen species, the use of enzymatic, nonenzymati and polyphenolic antioxidants to reduce oxidative stress in in vitro embryo production processes, and their effects on oocyte and embryo quality, gene expression and embryo developmental competence.
Key words: Antioxidants; Reactive oxygen species; Oxidative stress; In vitro culture; Embryo development
La producción de embriones in vitro es una de las biotecnologías de la reproducción animal que ha presentado mayor desarrollo en las últimas dos décadas; sin embargo, los resultados exitosos en estos procedimientos dependen de múltiples factores, entre ellos la presencia de especies reactivas de oxígeno, debido a que el proceso de fertilización in vitro y la manipulación de los gametos y embriones expone a las células a factores endógenos y exógenos que pueden afectar los mecanismos de defensa antioxidante y por consiguiente la calidad de los gametos y embriones. En esta revisión se discutirán algunas fuentes de especies reactivas de oxígeno, el uso de antioxidantes enzimáticos, no enzimáticos y polifenólicos para disminuir el estrés oxidativo en los procesos de producción in vitro de embriones, y su efecto sobre la calidad de los oocitos y embriones, la expresión génica y su competencia para el desarrollo embrionario.
Palabras clave: Antioxidantes; Especies reactivas de oxígeno; Estrés oxidativo; Cultivo in vitro; Desarrollo embrionario
Introduction
In vitro embryo production (IVEP) involves three steps: 1) in vitro maturation (IVM) of oocytes obtained from antral follicles; 2) coincubation of male and female gametes, or in vitro fertilization (IVF); and 3) in vitro culture (IVC) of the presumed zygotes to blastocyst stages. Under normal physiological conditions mammal oocytes grow and are fertilized in the ideal protective environment, the ovary and the female reproductive tract. Under in vitro conditions, however, the gametes and embryos must be manipulated during maturation, fertilization and embryo development in environments that generate oxidative stress. The conditions causing this stress include high oxygen concentration (20 %) compared to the in vivo environment (3 to 5%)1; exposure to light2; culture medium composition3; changes in pH4; centrifugation processes5; and many others6. These can negatively affect both gametes and embryos, altering the functionality of biomolecules such as lipids, nucleic acids and proteins, and thus influencing embryo development7.
Cells have an enzymatic and non-enzymatic antioxidant defense system, but antioxidant molecules have been used to supplement culture media and thus decrease reactive oxygen species (ROS) production in gametes and embryos. This improves their quality and reproductive potential by reducing intracellular ROS8, and protects against damage to DNA and other biomolecules, raising embryo developmental competence9-11. The present review is aimed at analyzing the effect of oxidative stress and antioxidant use in in vitro production of embryos on gamete and embryo quality at the metabolic level, as well as gene expression and epigenetic marks.
Reactive oxygen species and oxidative stress
Reactive oxygen species (ROS) constitute a group of molecules generated through partial reduction of molecular oxygen. Most of these species (except hydrogen peroxide) have one or more unpaired electrons, a configuration called a free radical. Under basal conditions, aerobic metabolism is linked to production of ROS such as hydrogen peroxide (H2O2), the superoxide anion (O2-) and the hydroxyl radical (OH-), while reactive nitrogen species (RNS) such as nitric oxide (NO•) form through conversion of L-arginine to L-citrulline by the enzyme nitric oxide synthase (NOS). Oxidative stress occurs when ROS production exceeds cellular defenses12, generating oxidative damage to biomolecules such as lipids, proteins, carbohydrates and nucleic acids, and consequently inducing structural and functional changes such as lipid hydroperoxides13, carbonylated proteins14 and DNA with oxidized bases (7, 8 dihydro-8-oxoguanine)15.
The mitochondrial respiratory chain is susceptible to oxidative damage (mainly to complexes I and II), by production of superoxide and nitrile radicals. These can affect mitochondrial proteins and alter the function of many metabolic enzymes in the mitochondrial electron transporter chain16. Mitochondrial DNA (mtDNA) is also known to be more sensitive to oxidative stress than nuclear DNA17. Possible reasons are that it lacks histones, which protect against damage from free radicals, does not have a suitable repair system, and is located near the internal mitochondrial membrane, the largest ROS production site18,19. Oxidative damage to the mtDNA can induce mutations and alter mitochondrial function and integrity20. In humans this can manifest in degenerative mitochondrial diseases such as Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis21,22.
ROS production during in vitro embryo production
Some cellular processes in the reproductive tract are regulated by ROS, which act as second messengers generating a specific cellular response. Macromolecules sensitive to redox modifications (phosphatases, kinases, transcription factors) are important during cell development stages such as proliferation, differentiation and cell death. In the latter, different ROS levels generate different types of cell death; for example, low concentrations promote apoptosis, intermediate concentrations generate autophagocytosis and high concentrations promote cell necrosis12,23. During IVM, physiological levels of ROS are needed to reinitiate meiosis of the oocytes arrested in diplotene, and to stimulate release of intracellular CA2+ in the oocyte and the protein kinase activated by mitogen (MAPK)24,25.
Physiological levels of ROS are also required for the training, hyperactivation and acrosomal reaction of mammalian sperm26. During sperm training, ROS such as the anion peroxynitrate, H2O2 and NO• have a dose-dependent effect on sperm function. Low ROS concentrations are required to promote cholesterol flow, AMPc production, hyperactivation and oocyte-sperm fusion27,28. In contrast, excess ROS may affect sperm functionality because the spermatozoa cell membrane is rich in polyunsaturated fatty acids, making it susceptible to lipid peroxidation. It can also negatively affect mitochondrial function in the sperm-zona pellucida interaction by reducing sperm motility, and compromising sperm DNA and therefore male fertility29-31. Use of antioxidant molecules is thus vital to protecting cells from high ROS levels and their negative effects.
Simulation of in vivo conditions in assisted reproduction techniques has improved immensely although two main factors continue to contribute to in vitro ROS generation and accumulation: absence of endogenous defense mechanisms and gamete and embryo exposure to environments which generate ROS. There are two main ROS sources (Figure 1). Endogenous ROS are accumulated by oocytes, sperm and embryos via various metabolic pathways and enzymes, mainly oxidative phosphorylation, NADPH oxidase and xanthine oxidase32. Exogenous ROS sources include environmental factors such as cryopreservation, oxygen concentration, energy source, culture medium, and light6,33.
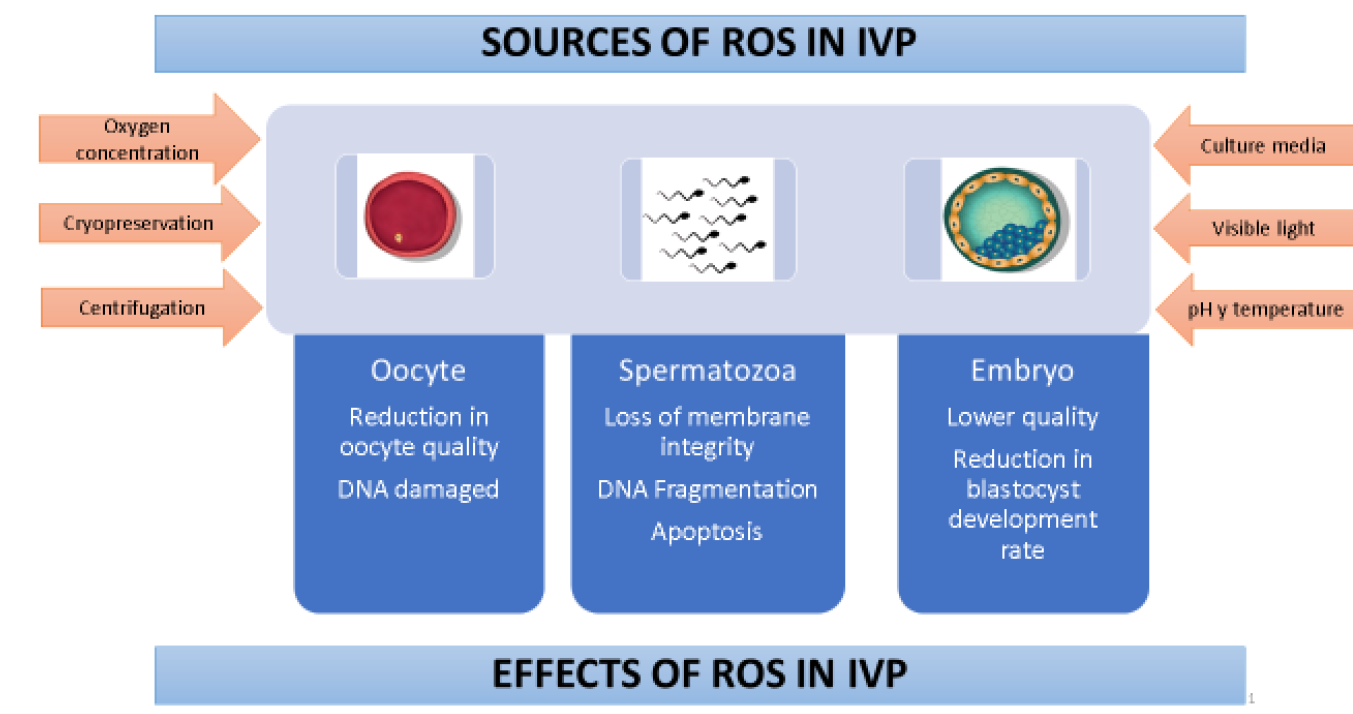
IVP = In vitro produced embryos
Figure 1 Effects of oxidative stress and sources of reactive oxygen species during embryo production
Oxygen is a vital component of oviduct and uterine environments and is involved in embryo development regulation, specifically through metabolism regulation. Oxygen tension found in the oviduct and uterus ranges from 5 to 8.7% in several species34. Levels used in oocyte maturation and cultivation with good results span from 5 to 20 %35,36. However, the trend is to use 20% O2 during oocyte maturation because the energy production route at low O2 concentrations (5 %) reduces the proportion of oocytes in IVM, which affects oocyte developmental competence1. In contrast, 5 % O2 during cultivation favors embryonic developmental competence, cellularity and gene expression related to oxidative stress37. However, exposure to high O2 concentrations (20 % in air) has been reported to intensify ROS level increases and thus reduce embryonic development percentages in rodents38, swine39,40, goats41, bovines42 and humans43. This in turn can cause arrested development, DNA damage, apoptosis and lipid peroxidation, which undermine embryo competence44. Studies evaluating the relative abundance of mRNA in oocytes have found a pattern of better quality when oocytes are matured at low O2 concentrations (5 %)45,46. Incorrect atmospheric oxygen concentrations can clearly have detrimental effects in mammalian embryo cultivation.
Depending on composition and supplements, culture media can contribute to ROS production in IVEP systems3,47. Culture media contain metal ions, such as Fe2+ and Cu2+, which are inducers of ROS formation through Fenton and Haber-Weiss reactions; iron can also act on lipids by generating lipid peroxidation initiated by free hydroxyl radicals48. Supplementation with biological fluids such as fetal bovine serum (FBS) may increase ROS levels more than other supplements such as bovine serum albumin (BSA). Presence of the enzyme amino oxidase in serum49, which participates in oxidation of primary amines, generates hydrogen peroxide as a secondary product50, which could explain the effect of serum amino oxidase concentration on apoptosis percentage51, cryotolerance and the gene expression pattern in bovine embryos produced in vitro52. However, this protein supplement improves bovine embryo production rate and quality53. Maintaining developmental competence in bovine oocytes during in vitro maturation requires control of medium glucose content. High glucose concentrations in the maturation medium raise ROS levels and lower intracellular glutathione (GSH) content in bovine oocytes. This inhibits the enzymes responsible for GSH synthesis, negatively affecting oocyte capacity to reduce ROS54,55, which, in early embryonic development, can lead to lipid peroxidation of the cell membrane, DNA fragmentation and improper protein synthesis32,56.
Visible light also induces ROS production by generating base oxidation, breaking down DNA chains, and causing oxidative damage in other biomolecules2. Sperm motility and hyperactivation are affected by excess ROS production caused by exposure to visible light57. Excess ROS production has also been reported in vitro in embryos transiently exposed to visible light. White fluorescent light, the most common type used in laboratories, was found to generate the most ROS in mouse and hamster zygotes, as reflected in the blastocyst apoptosis index, although use of filters helped to diminish these effects58. A study assessing the effects of daylight and laboratory light and different exposure times in culture media and porcine embryos found both types of light to reduce embryo quality and parthenogenetic blastocyst percentages59. This suggests that culture media and embryos need protection from light during in vitro production processes.
Centrifuging is a necessary step in semen preparation and training protocols for IVF. However, centrifuge time and force contribute to raising ROS levels, causing oxidative damage and affecting sperm function5,60. Centrifuging sexed and unsexed sperm for long periods (45 min) at 700 x g caused loss of plasma membrane integrity and DNA fragmentation61,62. This suggests that the sperm plasma membrane experiences a lipid peroxidation process in response to high ROS levels, thus reducing membrane fluidity and functionality for fertilization. For this reason different sperm preparation techniques have been developed (e.g. swim-up and density gradients) to obtain spermatozoa with higher motility and DNA integrity percentages to improve spermatozoa fertilization capacity during IVEP processes63.
Cryopreservation significantly increases ROS production in spermatozoa, affecting motility, viability and training, and enhancing lipid peroxidation of the spermatic membrane, affecting potential fertility64. Low fertilization rates in cryopreserved oocytes have been related to freeze damage, including hardening of the zona pellucida due to premature release of cortical granules, spindle disorganization and microtubule loss or agglutination65,66. Use of antioxidants may therefore be a vital factor in sperm survival and function before, during and after cryopreservation.
Enzymatic and non-enzymatic antioxidants
An antioxidant with biological function is defined as a substance that decreases or prevents substrate oxidation, resulting in a more potent reducing agent67. Reactive oxygen species (ROS) can be inactivated by a defense system consisting of antioxidant enzymes and molecules2. These antioxidant mechanisms can be mediated by iron- and copper-binding proteins such as transferrin, ferritin and albumin68, and small antioxidant molecules derived mainly from fruits and vegetables. Enzymatic mechanisms can also mediate them, and include enzymes such as superoxide dismutase (SOD), which catalyzes dismutation of the superoxide anion in oxygen and hydrogen peroxide; catalase (CAT) and glutathione peroxidase (GPX), which convert hydrogen peroxide into water (and oxygen for CAT reactions); hydrophilic molecules such as ascorbate, urate and GSH; and lipophilic molecules such as tocopherols, flavonoids, carotenoids and ubiquinol (Figure 2). Other enzymes involved in reduction of oxidized forms of small antioxidant molecules are also part of cell antioxidant mechanisms, including GSH reductase and dehydroascorbate reductase, as well as molecules responsible for maintenance of thiol groups in proteins (thioredoxin)12.
Reduced glutathione (GSH) is the largest non-protein sulfhydryl component in mammalian cells, and is known to protect the cell from oxidative damage and to regulate the intracellular redox balance69. Several studies suggest that GSH plays an important role in many biological processes, including DNA and protein synthesis and cell proliferation during embryo development70. In bovine oocytes, it is considered a vital biochemical marker of oocyte viability and quality71. Synthesis of GSH has been reported during IVM72,73, and is associated with formation of the male pronucleus after fertilization72,74, and early embryo development70. Intracellular GSH levels are therefore considered a marker of oocyte quality and embryo developmental competence after IVF.
The most evaluated antioxidants as culture media supplements are ascorbic acid (AA) and alpha tocopherol (AT). Ascorbic acid decreases ROS production in bovine oocytes, improving their potential to develop embryos9, while AT benefits blastocyst rate and cellularity75,76. Alpha tocopherol (AT) can prove useful in IVEP because its hydrophobicity allows it to cross the lipid bilayer, intersperse in it and decrease ROS in the cell. In contrast, AA is hydrosoluble which allows it to act synergistically with tocopherol in some conditions, regenerating tocopherol from tocopheroxyl radicals in a redox cycle77. It has also been reported to reduce ROS production in the culture medium and augment bovine embryo developmental competence by lowering intracellular ROS in oocytes matured with AA78. Ascorbic acid (AA) can also increase blastocyst rate and cellularity11, raise intracellular GSH levels and lower ROS production in bovine oocytes10. During the IVC, AA is also reported to decrease ROS production and expression of pro-apoptotic genes in pig embryos, thus enhancing embryo development79, and improving the survival rate and quality of vitrified embryos80,81. Embryo culture medium supplemented with AT or AA is reported to increase development and cellularity capacity, and reduce the proportion of apoptotic cells in porcine blastocysts derived from IVF or somatic cell nuclear transfer (SCNT); however, this effect was not observed with combined supplementation76.
Melatonin has been reported to have beneficial effects in IVEP due to its ability to trap free radicals, reduce ROS concentration, and increase expression of antioxidant enzymes (SOD and glutathione reductase)82,83, as well as suppress expression of pro-oxidant enzymes and improve mitochondrial function84,85. Like many antioxidants, melatonin can have positive or negative effects depending on the concentration at which it is administered in a culture medium. When supplemented in IVF medium at low concentrations it improves sperm quality and motility, decreases ROS levels and lipid peroxidation, and acts as an anti-apoptotic agent in bovine sperm86 and ejaculated human sperm87-89. In contrast, high concentrations induce fragmentation and oxidation of sperm DNA, decrease the number of viable spermatozoa and generate a decrease in blastocyst rates, without affecting embryo quality90,91. In embryo culture medium, melatonin augments cleavage, blastocyst and hatching rates, increases embryo cellularity and promotes activation of antioxidant enzymes92,93.
Phenolic antioxidants
Impressive progress has been made in identification, purification and evaluation of natural-origin antioxidant molecules94, such as phenolic antioxidants. Because their structure includes aromatic rings and hydroxyl groups they are very stable and can inhibit oxidation of biologically and commercially important compounds94,95. As a result, they have been suggested as potentially useful for prevention and treatment of diseases caused by free radicals, such as ischemia, atherosclerosis, and neuronal and cardiovascular diseases96,97.
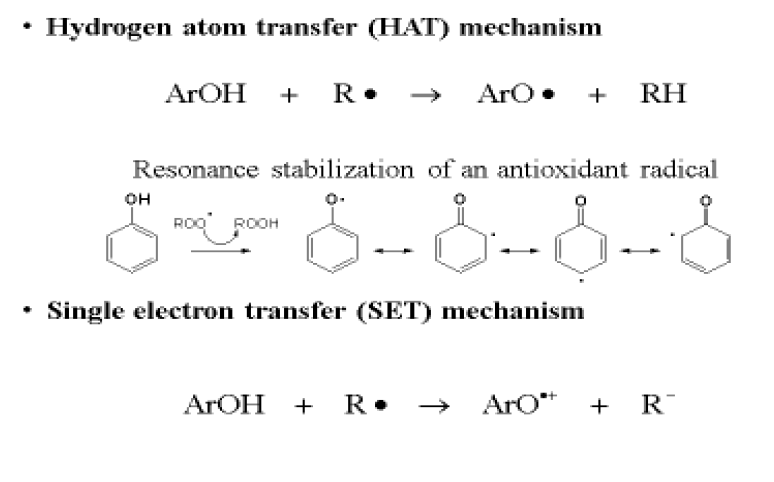
Phenolic antioxidants (ArOH) have two action mechanisms: hydrogen atom transfer (HAT) or electron transfer (SET). In the first (HAT), the free radical (R•) removes a hydrogen atom from the antioxidant (ArOH), transforming it the radical ArO•. This is more stable and more efficient because its hydrogen bonds, conjugation and resonance make it a non-reactive phenoxyl radical. In the second mechanism (SET), the antioxidant can donate an electron to the free radical, forming, among other products, a radical cation of the antioxidant (ArO•+) which is stable and does not react with substrates (Figure 3). Both mechanisms can always occur in parallel, although they have different reaction rates98.
As part of the search for new antioxidants and evaluation of their activity in in vitro reproduction, green tea extract (the principal components of which are polyphenols) has been evaluated in oocytes matured in vitro. It favored blastocyst rate and reduced glutathione concentrations within the oocyte, but exhibited limited repeatability probably due to variability in extract composition99. Anthocyanins are another type of biological molecule with antioxidant capacity. These were evaluated in pig and bovine oocytes in maturation medium100,101, and different oocyte quality parameters tested such as free radical production level, intracellular glutathione levels, relative mRNA abundance associated with embryo development, and capacity for in vitro embryo production. The benefits of phenolic compounds from grapes have also garnered attention. Resveratrol (3,5,4'-trans-trihydroxystilbene) and pterostilbene (natural antioxidant analog of resveratrol) are abundant in plants and fruits such as blueberries, blackberries, peanuts, grapes and red wine102,103. Both compounds have various in vivo and in vitro biological properties, such as antioxidant capacity, cardiac protection, anti-inflammatory, chemoprevention in some cancer models and some positive effects in metabolic diseases102,104. Given these properties, research has been done into their in vitro biological effects in other animal models and systems, such as IVEP.
When added to embryo culture medium, pterostilbene has been reported to reduce ROS levels and the percentage of lipids in embryos105. However, very few studies have been done on this type of antioxidant in reproductive biotechnology, highlighting the need for more studies on the molecular mechanism by which pterostilbene exerts its effect on embryo metabolism.
Various studies have been done on resveratrol in IVEP (Table 1). There are studies on the use of resveratrol in in vitro oocyte maturation in swine106, bovines10,107-109, and goats110, which indicate that it increases GSH concentration within the oocyte, decreases ROS production and raises the blastocyst rate. Use of resveratrol during in vitro embryo culture also has a positive effect on embryo development111, and increases blastocyst cryotolerance112,113, showing that its antioxidant capacity improves oocyte quality and resistance to cryopreservation processes. High resveratrol concentrations (20 and 40 µM), however, do not provide benefits and are reported to decrease the percentage of bovine oocytes capable of completing the maturation process up to metaphase II114.
Table 1 Studies of resveratrol as a supplement in culture media for in vitro embryo production
| Culture | Gametes | Species | Resveratrol Concentrations |
Results | Reference |
|---|---|---|---|---|---|
| IVM | Oocytes | Porcine | 0.1, 0.5, 2.0 and 10.0 Μm | Improved development in in vitro parthenogenic and fertilized embryos; increased intracellular GSH and decreased ROS levels. | (106) |
| Porcine | 20 µM | Increased SIRT1 expression; improved mitochondrial functioning and oocyte developmental capacity. | (142) | ||
| Porcine | 2 µM | Improved oocyte resistance to cryopreservation-induced damage. | (113) | ||
| Bovine | 0.1, 1 and 10 µM | Induced progesterone secretion; increased intracellular GSH; decreased ROS levels; promoted oocyte maturation and subsequent embryo development. | (107) | ||
| Bovine | 20 µM | Increased ATP content and expression of SIRT1 protein in mature oocytes; improved fertilization by reinforcing mechanisms for blocking polyspermia. | (143) | ||
| Bovine | 2 μM | Lowered ROS levels; raised embryo development rates and cellularity. | (10) | ||
| Bovine | 20 and 40 μM | Regulates expression of CYP1A1 gene involved in meiosis reinitiation. | (114) | ||
| Bovine | 1, 10, 20 and 40 μM | Increased embryo development and intracellular GSH, and decreased ROS levels | (108) | ||
| Bovine | 0.2 µM, 1 µM and 20 µM | Improved oocyte developmental competence; increased maturation and blastocyst rates. | (109) | ||
| Bovine | 2 µM | Affected expression of SIRT1 protein in oocytes and blastocysts of donors of different ages. | (144) | ||
| Bovine | 2 µM | Lowered ROS levels; increased GSH levels and cleavage and blastocyst rates; decreased expression of pro-apoptotis genes. | (145) | ||
| Caprine | 0.1, 0.25, 0.5, 2.0 and 5.0 µM | Decreased ROS levels; increased GSH levels and embryo development rates; decreased expression of pro-apoptosis genes in cumulus cells, mature oocytes and blastocysts. | (110) | ||
| IVF | Spermatozoids | Mouse | 15 µg/ml | Increased oocyte fertilization, decreased ROS generation, glutathione peroxidase activity and lipid peroxidation concentration. | (146) |
| Human | 0.1, 1.0 and 10.0 μM | Prevented damage to DNA caused by cryopreservation in sperm from fertile males. | (147) | ||
| IVC | Embryos | Porcine | 0.05, 0.1, 0.5, 1.0 and 25 µM | 0.5 µM in culture had positive effect on embryo development. | (111) |
| Bovine | 0, 0.25, 0.5 and 1 µM | 0.5 µM improved embryo quality and cryotolerance. | (112) |
IVM= in vitro maturation; IVF= in vitro fertilization; IVC= in vitro culture.
Resveratrol’s physiological effect appears to be related to its ability to enhance cellular processes dependent on Sirtuin 1 (SIRT1). This in turn is also associated with adenosine monophosphate-activated protein kinase (AMPK), an energy sensor which controls cell metabolism, including oxidative phosphorylation and fatty acid oxidation115. Activation of AMPK by resveratrol increases levels of NAD+ (the SIRT1 cofactor), which decreases the acetylation of SIRT1 substrates and activates PGC-1α (coactivator 1 α of the peroxisome proliferator-activated receptor gamma)116,117. However, despite AMPK activation for observation of resveratrol’s metabolic effects, this compound’s direct target is upstream from AMPK. One proposed mechanism is that resveratrol activates AMPK through competitive inhibition of phosphodiesterases (PDEs), thus increasing AMPc levels118. This second messenger plays a vital role in oocyte maturation in mammals, which occurs after bonding FSH and LH to their specific receptors in the plasma membrane of granulosa cells through activation of adenylate cyclase119. Intracellular AMPc levels are regulated by PDEs, which hydrolyze it to 5'-AMP. Use of PDE inhibitors delays meiosis reinitiation and slows cumulus expansion kinetics, which prolongs maintenance of the gap bonds between the oocyte and cumulus cells120. This extension of gap bonds during in vitro maturation in the presence of PDE4 inhibitors could allow passage of metabolites, ions, nucleotides and amino acids, which improve oocyte cytoplasm maturation, bringing it near synchronization of nuclear and cytoplasmic maturation, which would favor blastocyst production and quality.
Changes in gene expression and epigenetic disorders induced by ROS
Oocyte developmental competence is defined as the ability of an oocyte to reinitiate meiosis, be fertilized, divide and attain the blastocyst stage121. This competence or quality is acquired progressively during folliculogenesis as the oocyte grows and matures through a series of cellular (mitochondrial activity), molecular (gene expression profile) and functional (protein kinase activity) changes55,122. During oocyte growth and maturation mRNA and proteins are synthesized, which contribute to early development before and after activation of the embryo genome. This mRNA storage occurs during oocyte growth, and a polyadenylation event develops in each transcript, which is a key gene expression regulator and known to be an important step in mammalian embryo development123. However, IVM conditions can affect polyadenylation levels in maternal mRNA, with implications for embryo quality124. This suggests that deficiencies in developmental competence in most in vitro matured oocytes are reflected in the composition and abundance of specific RNA transcripts in the oocyte.
For this reason, different transcripts have been evaluated in the oocyte in search of associations between them and oocyte quality or embryo developmental competence. Among the most studied are NLRP5 (NLR Family Pyrin Domain containing 5, known as MATER), a maternal effect gene specific to the oocyte which is required for early embryo development in bovines, mice and humans125-127. Another is POU5F1 (POU domain Class 5, transcription factor 1, also known as OCT-4), which has been validated as a marker for epigenetic and pluripotency reprogramming, and is crucial for normal embryo development128; increased POU5F1 expression has been reported in pig embryos derived from SCNT treated with vitamin C129. In an effort to predict fertilization success and maintain oocyte viability, expression of genes such as hyaluronic acid synthetase 2 (HAS2), cyclooxygenase 2 (COX2; PTGS2) and gremlin (GREM1) have been studied in cumulus cells and correlated with oocyte competence and subsequent embryo development111,130.
In the development of bovine embryos, cell viability is determined by alterations in the expression of metabolism-related genes such as GLUT-1 (glucose transporter-1 transcripts)131, growth factors such as IGF-2 (insulin-like growth factor 2) and IGF-2R (insulin-like growth factor 2 receptor), early differentiation and trophoblastic functions such as IF (interferon tau) and Mash2 (mammalian achaete-scute homologue)132,133. Changes occur in gene expression during IVEP processes, and epigenetic disorders can arise that alter DNA methylation patterns in some genes (DNA methyltransferase, DNMT1a, DNMT3a and DNMT3b), affecting the gene expression profiles that encode for a specific tissue134.
Evaluations have been done on the effect of antioxidant supplements in culture media on gene expression regulation. Supplementation with resveratrol during IVM of oocytes from pigs106,111,135 and goats110 decreased the transcription levels of genes related to apoptosis (e.g. BAX, BAK and caspase-3) but caused no changes in expression of the BCL-2 gene. This suggests that resveratrol suppresses expression of pro-apoptotic genes in matured oocytes, and exerts a protective effect on embryos produced in vitro. Medium supplementation with AA has been reported to positively regulate pluripotent gene expression in porcine parthenogenetic blastocysts, and decrease expression of the pro-apoptotic gene Bax79. Ascorbic acid (AA) supplementation in culture medium and vitrification-thawing media increases expression of the GPX1 and SOD1 genes, both associated with oxidative stress, thus improving survival rates and decreasing peroxide levels 24 h post-thaw136. A study in bovines found that the relative abundance of GPX1 is higher in excellent quality blastocysts (Grade 1) than in good blastocysts (Grade 2), suggesting that less expression of GPX1 is associated with lower embryo quality137.
Reactive oxygen species (ROS) produced either endogenously or exogenously during IVEP may also induce epigenetic changes. Culture conditions that include changes in pH, osmolarity, temperature, visible light exposure, oxygen concentration and cell centrifuging can influence the epigenetic pattern during the in vitro process thus affecting gamete and embryo quality134. Oxidative stress may produce alterations in DNA methylation patterns and modification of histone proteins in the gametes; these are transmissible from gametes to embryos, and generate variations in the epigenome that could alter subsequent embryo development138-140. The mechanisms for transmission of these alterations to the embryo during fertilization and cleavage have not yet been elucidated. It has been suggested that oxidative stress damage in the gamete epigenome -which increases the adducts in DNA and alterations in the methylation profiles- is transferred to the embryo, manifesting in phenotypic alterations that can be observed in newborns141. For example, induction of oxidative stress in spermatozoa using H2O2 causes oxidative damage in the spermatozoa epigenome, which subsequently reduces embryo development rates and alters cell differentiation in blastocysts. This causes reductions in implantation rates, reduced fetal growth, increased adipose tissue, decreased lean mass and lower glucose tolerance. These findings implicate ROS as one of the mechanisms responsible for transmitting health signals from parents to children141.
Conclusions
In vitro embryo production techniques are used commercially in animal production, but myriad factors can generate oxidative stress and potentially affect the quality of matured oocytes and consequently embryo development rates. The environment and the procedures to which embryos and gametes are subjected generate increases in ROS levels that surpass the physiological levels required to regulate various cellular functions, thus affecting cell morphology and functionality. These factors can affect different biomolecules, causing damage to DNA, lipid peroxidation, changes in gene expression levels and epigenetic disorders. Use of molecules with antioxidant activity can ameliorate in vitro maturation conditions, but novel substances of natural origin are still needed to reduce oxidative stress during in vitro embryo production processes and improve oocyte quality and embryo developmental competence.
Acknowledgements
The research reported here was financed by the Banco de la República (Convenio 201633 Proyecto No. 3,862).
REFERENCES
1. Park JI, Hong JY, Yong HY, Hwang WS, Lim JM, Lee ES. High oxygen tension during in vitro oocyte maturation improves in vitro development of porcine oocytes after fertilization. Anim Reprod Sci 2005;87(1-2):133-141. [ Links ]
2. Du Plessis SS, Makker K, Desai NR, Agarwal A. Impact of oxidative stress on IVF. Expert Rev Obstet Gynecol 2008;3(4):539-554. [ Links ]
3. Martín-Romero FJ, Miguel-Lasobras EM, Domínguez-Arroyo JA, González-Carrera E, Alvarez IS. Contribution of culture media to oxidative stress and its effect on human oocytes. Reprod Biomed Online 2008;17(5):652-661. [ Links ]
4. Will MA, Clark NA, Swain JE. Biological pH buffers in IVF: help or hindrance to success. J Assist Reprod Genet 2011;28(8):711-724. [ Links ]
5. Li Z, Zhou Y, Liu R, Lin H, Liu W, Xiao W, et al. Effects of semen processing on the generation of reactive oxygen species and mitochondrial membrane potential of human spermatozoa. Andrologia 2012;44(3):157-163. [ Links ]
6. Agarwal A, Durairajanayagam D, du Plessis SS. Utility of antioxidants during assisted reproductive techniques: an evidence based review. Reprod Biol Endocrinol 2014;12:112. [ Links ]
7. Blanco MR, Demyda S, Moreno Millán M, Genero E. Developmental competence of in vivo and in vitro matured oocytes: A review. Anim Reprod Sci 2012;9(3):281-289. [ Links ]
8. Ali AA, Bilodeau JF, Sirard MA. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology 2003;59(3-4):939-949. [ Links ]
9. Vásquez NA, Torres V, Rojano BA. Efecto del ácido ascórbico durante maduración in vitro de oocitos bovinos en la producción de especies reactivas de oxígeno (ERO) y competencia para el desarrollo embrionario. Inf Tecnol 2014;25(2):141-150. [ Links ]
10. Sovernigo T, Adona P, Monzani P, Guemra S, Barros F, Lopes F, et al. Effects of supplementation of medium with different antioxidants during in vitro maturation of bovine oocytes on subsequent embryo production. Reprod Domest Anim 2017;52:561-569. [ Links ]
11. Kere M, Siriboon C, Lo N-W, Nguyen NT, Ju J-C. Ascorbic acid improves the developmental competence of porcine oocytes after parthenogenetic activation and somatic cell nuclear transplantation. J Reprod Dev 2013;59(1):78-84. [ Links ]
12. Covarrubias L, Hernández-García D, Schnabel D, Salas-Vidal E, Castro-Obregón S. Function of reactive oxygen species during animal development: Passive or active? Dev Biol 2008;320(1):1-11. [ Links ]
13. Rikans LE, Hornbrook KR. Lipid peroxidation, antioxidant protection and aging. Biochim Biophys Acta. 1997;1362(2-3):116-127. [ Links ]
14. Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 2003;329(1-2):23-38. [ Links ]
15. David SS, O’Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;21;447(7147):941-950. [ Links ]
16. Selivanov VA, Votyakova T V, Pivtoraiko VN, Zeak J, Sukhomlin T, Trucco M, et al. Reactive oxygen species production by forward and reverse electron fluxes in the mitochondrial respiratory chain. Beard DA, editor. PLoS Comput Biol 2011 Mar 31;7(3):e1001115. [ Links ]
17. Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997;21;94(2):514-519. [ Links ]
18. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000;9;408(6809):239-247. [ Links ]
19. Chinnery PF, Elliott HR, Hudson G, Samuels DC, Relton CL. Epigenetics, epidemiology and mitochondrial DNA diseases. Int J Epidemiol 2012;41(1):177-187. [ Links ]
20. Han Y, Chen JZ. Oxidative stress induces mitochondrial DNA damage and cytotoxicity through independent mechanisms in human cancer cells. Biomed Res Int 2013;2013:1-8. [ Links ]
21. Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res 2013;8(21):2003-2014. [ Links ]
22. Kirkinezos IG, Moraes CT. Reactive oxygen species and mitochondrial diseases. Semin Cell Dev Biol 2001;12(6):449-457. [ Links ]
23. Hernández-García D, Wood CD, Castro-Obregón S, Covarrubias L. Reactive oxygen species: A radical role in development? Free Radic Biol Med 2010;49(2):130-143. [ Links ]
24. Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci 2011;108(4):1462-1467. [ Links ]
25. Morado SA, Cetica PD, Beconi MT, Dalvit GC. Reactive oxygen species in bovine oocyte maturation in vitro. Reprod Fertil Dev 2009;21(4):608-614. [ Links ]
26. Park EJ, Pezzuto JM, Novelle MG, Wahl D, Diéguez C, Bernier M, et al. [Resveratrol: distribution, properties and perspectives]. J Reprod Dev 2011 Aug 1;1852(3):92-99. [ Links ]
27. Jin S-K, Yang W-X. Factors and pathways involved in capacitation: how are they regulated? Oncotarget 2017;8(2):3600-3627. [ Links ]
28. Gangwar DK, Atreja SK. Signalling events and associated pathways related to the mammalian sperm capacitation. Reprod Domest Anim 2015;50(5):705-711. [ Links ]
29. Henkel RR. Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian J Androl. 2011;13(1):43-52. [ Links ]
30. Kothari S, Thompson A, Agarwal A, du Plessis SS. Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol 2010;48(5):425-435. [ Links ]
31. Bromfield EG, Aitken RJ, Anderson AL, McLaughlin EA, Nixon B. The impact of oxidative stress on chaperone-mediated human sperm-egg interaction. Hum Reprod 2015;30(11):2597-2613. [ Links ]
32. Guerin P, Mouatassim S, Menezo Y. Oxidative stress and protection against reaction oxygen species in the pre-implantation embryo and its surroundings. Reprod Updat 2001;7(2):175-189. [ Links ]
33. Gupta S, Sekhon L, Kim Y, Agarwal A. The role of oxidative stress and antioxidants in assisted reproduction. Curr Womens Health Rev 2010;6(3):227-238. [ Links ]
34. Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. Reproduction 1993;99(2):673-679. [ Links ]
35. Oyamada T, Fukui Y. Oxygen tension and medium supplements for in vitro maturation of bovine oocytes cultured individually in a chemically defined medium. J Reprod Dev 2004;50(1):107-117. [ Links ]
36. Corrêa GA, Rumpf R, Mundim TCD, Franco MM, Dode MAN. Oxygen tension during in vitro culture of bovine embryos: Effect in production and expression of genes related to oxidative stress. Anim Reprod Sci 2008;104(2-4):132-142. [ Links ]
37. Balasubramanian S, Son WJ, Kumar BM, Ock SA, Yoo JG, Im GS, et al. Expression pattern of oxygen and stress-responsive gene transcripts at various developmental stages of in vitro and in vivo preimplantation bovine embryos. Theriogenology 2007;68(2):265-275. [ Links ]
38. Karagenc L, Sertkaya Z, Ciray N, Ulug U, Bahçeci M. Impact of oxygen concentration on embryonic development of mouse zygotes. Reprod Biomed Online 2004;9(4):409-417. [ Links ]
39. Kitagawa Y, Suzuki K, Yoneda A, Watanabe T, Agarwal A, Sharma RK, et al. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology 2004;1;62(7):1186-1197. [ Links ]
40. Booth PJ, Holm P, Callesen H. The effect of oxygen tension on porcine embryonic development is dependent on embryo type. Theriogenology . 2005;63(7):2040-2052. [ Links ]
41. Batt P, Gardner D, Cameron A. Oxygen concentration and protein source affect the development of preimplantation goat embryos in vitro. Reprod Fertil Dev 1991;3(5):601. [ Links ]
42. Takahashi M, Keicho K, Takahashi H, Ogawa H, Schultz RM, Okano A. Effect of oxidative stress on development and DNA damage in in-vitro cultured bovine embryos by comet assay. Theriogenology 2000;54(1):137-145. [ Links ]
43. Bontekoe S, Mantikou E, van Wely M, Seshadri S, Repping S, Mastenbroek S. Low oxygen concentrations for embryo culture in assisted reproductive technologies. Cochrane database Syst Rev 2012;(7):CD008950. [ Links ]
44. Takahashi M. Oxidative stress and redox regulation on in vitro development of mammalian embryos. J Reprod Dev 2012;58(1):1-9. [ Links ]
45. Bermejo-Álvarez P, Lonergan P, Rizos D, Gutiérrez-Adan A. Low oxygen tension during IVM improves bovine oocyte competence and enhances anaerobic glycolysis. Reprod Biomed Online 2010;20(3):341-349. [ Links ]
46. Rinaudo P, Giritharan G, Talbi S, Dobson A, Schultz R, Katoh Y, et al. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil Steril 2006;86(4):1265.e1-1265.e36. [ Links ]
47. Combelles CMH, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod Biomed Online 2009;18(6):864-880. [ Links ]
48. Guerin P. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001; (2):175-189. [ Links ]
49. Conklin DJ, Langford SD, Boor PJ. Contribution of serum and cellular semicarbazide-sensitive amine oxidase to amine metabolism and cardiovascular toxicity. Toxicol Sci 1998;46(2):386-392. [ Links ]
50. Stites TE, Mitchell AE, Rucker RB. Physiological importance of quinoenzymes and the O-quinone family of cofactors. J Nutr 2000;130(4):719-727. [ Links ]
51. Sudano MJ, Paschoal DM, da Silva Rascado T, Magalhães LCO, Crocomo LF, de Lima-Neto JF, et al. Lipid content and apoptosis of in vitro-produced bovine embryos as determinants of susceptibility to vitrification. Theriogenology 2011;75(7):1211-1220. [ Links ]
52. Rizos D, Gutiérrez-Adán A, Pérez-Garnelo S, De La Fuente J, Boland MP, Lonergan P. Bovine embryo culture in the presence or absence of serum: implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol Reprod 2003;68(1):236-243. [ Links ]
53. Almeida T, Tetzner D, Saraiva NZ, Perecin F, Cristina S, Niciura M, et al. The effects of ovalbumin as a protein source during the in vitro production of bovine embryos. Rev Bras Zootec 2011;40(10):2135-2141. [ Links ]
54. Hashimoto S, Minami N, Yamada M, Imai H. Excessive concentration of glucose during in vitro maturation impairs the developmental competence of bovine oocytes after in vitro fertilization: Relevance to intracellular reactive oxygen species and glutathione contents. Mol Reprod Dev 2000;56(4):520-526. [ Links ]
55. Krisher RL, Brad AM, Herrick JR, Sparman ML, Swain JE. A comparative analysis of metabolism and viability in porcine oocytes during in vitro maturation. Anim Reprod Sci 2007;98(1-2):72-96. [ Links ]
56. Hashimoto S, Minami N, Takakura R, Yamada M. Low oxygen tension during in vitro maturation is beneficial for supporting the subsequent development of bovine cumulus ± oocyte complexes. Mol Reprod Dev 2000;57:353-60. [ Links ]
57. Shahar S, Wiser A, Ickowicz D, Lubart R, Shulman A, Breitbart H. Light-mediated activation reveals a key role for protein kinase A and sarcoma protein kinase in the development of sperm hyper-activated motility. Hum Reprod 2011;26(9):2274-2282. [ Links ]
58. Takenaka M, Horiuchi T, Yanagimachi R. Effects of light on development of mammalian zygotes. Proc Natl Acad Sci U S A. 2007;104(36):14289-14293. [ Links ]
59. Li R, Liu Y, Pedersen HS, Callesen H. Effect of ambient light exposure of media and embryos on development and quality of porcine parthenogenetically activated embryos. Zygote 2015;23(03):378-383. [ Links ]
60. Lampiao F, Strijdom H, Du Plessis S. Effects of sperm processing techniques involving centrifugation on nitric oxide, reactive oxygen species generation and sperm function. Open Androl J 2010;2:1-5. [ Links ]
61. Urrego R, Ríos A, Ángel MO, Camargo O. Efecto de la centrifugación sobre la membrana plasmática y el ADN de espermatozoides bovinos. Rev Colomb Ciencias Pecu 2008;21(1):19-26. [ Links ]
62. Ángel D, Pérez N, Pareja A, Camargo O, Urrego R. Efecto de la preparación espermática previo a la fertilización sobre la membrana plasmática y el adn de semen bovino sexado in vitro. Rev CES 2009;4(2):29-37. [ Links ]
63. Jayaraman V, Upadhya D, Narayan PK, Adiga SK. Sperm processing by swim-up and density gradient is effective in elimination of sperm with DNA damage. J Assist Reprod Genet 2012;29(6):557-563. [ Links ]
64. Bilodeau JF, Chatterjee S, Sirard MA, Gagnon C. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Mol Reprod Dev 2000;55(3):282-288. [ Links ]
65. Hwang IS, Hochi S. Recent progress in cryopreservation of bovine oocytes. Biomed Res Int 2014;2014:1-11. [ Links ]
66. Carroll J, Depypere H, Matthews CD. Freeze-thaw-induced changes of the zona pellucida explains decreased rates of fertilization in frozen-thawed mouse oocytes. J Reprod Fertil 1990;90(2):547-53. [ Links ]
67. Tirzitis G, Bartosz G. Determination of antiradical and antioxidant activity: Basic principles and new insights. Acta Biochim Pol 2010;57(2):139-142. [ Links ]
68. Kirschvink N, Moffarts B De, Lekeux P. The oxidant/antioxidant equilibrium in horses. Vet J 2008;177(2):178-191. [ Links ]
69. Battin EE, Brumaghim JL. Antioxidant activity of sulfur and selenium: A review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem Biophys 2009;55(1):1-23. [ Links ]
70. De Matos DG, Gasparrini B, Pasqualini SR, Thompson JG. Effect of glutathione synthesis stimulation during in vitro maturation of ovine oocytes on embryo development and intracellular peroxide content. Theriogenology . 2002;57(5):1443-1451. [ Links ]
71. Zuelke KA, Jeffay SC, Zucker RM, Perreault SD. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos. Mol Reprod Dev 2003;64(1):106-112. [ Links ]
72. Miyamura M, Yoshida M, Hamano S, Kuwayama M. Glutathione concentration during maturation and fertilization in bovine oocytes. Theriogenology 1995;43(1):282. [ Links ]
73. De Matos DG, Furnus CC, Moses DF, Martinez AG, Matkovic M. Stimulation of glutathione synthesis of in vitro matured bovine oocytes and its effect on embryo development and freezability. Mol Reprod Dev 1996;45(4):451-457. [ Links ]
74. de Matos DG, Furnus CC, Moses DF. Glutathione synthesis during in vitro maturation of bovine oocytes: role of cumulus cells. Biol Reprod. 1997;57(6):1420-1425. [ Links ]
75. Olson SE, Seidel GE. Culture of in vitro-produced bovine embryos with vitamin E improves development in vitro and after transfer to recipients. Biol Reprod 2000;62(2):248-252. [ Links ]
76. Jeong YW, Park SW, Hossein MS, Kim S, Kim JH, Lee SH, et al. Antiapoptotic and embryotrophic effects of alpha-tocopherol and L-ascorbic acid on porcine embryos derived from in vitro fertilization and somatic cell nuclear transfer. Theriogenology 2006;66(9):2104-2112. [ Links ]
77. Halliwell B, Gutteridge JM. The antioxidants of human extracellular fluids. Arch Biochem Biophys 1990;280(1):1-8. [ Links ]
78. Vásquez NA, Torres V, Rojano BA. Efecto del ácido ascórbico durante maduración in vitro de oocitos bovinos en la producción de especies reactivas de oxígeno (ERO) y competencia para el desarrollo embrionario. Inf Tecnol 2014;25(2):141-50. [ Links ]
79. Hu J, Cheng D, Gao X, Bao J, Ma X, Wang H. Vitamin C enhances the in vitro development of porcine pre-implantation embryos by reducing oxidative stress. Reprod Domest Anim 2012;47(6):873-879. [ Links ]
80. Castillo-Martín M, Yeste M, Soler A, Morató R, Bonet S. Addition of l-ascorbic acid to culture and vitrification media of IVF porcine blastocysts improves survival and reduces HSPA1A levels of vitrified embryos. Reprod Fertil Dev 2015;27(7):1115-1123. [ Links ]
81. Castillo-Martín M, Bonet S, Morató R, Yeste M. Comparative effects of adding β-mercaptoethanol or L-ascorbic acid to culture or vitrification-warming media on IVF porcine embryos. Reprod Fertil Dev 2014;26(6):875-882. [ Links ]
82. Kaur H, Bhatla SC. Melatonin and nitric oxide modulate glutathione content and glutathione reductase activity in sunflower seedling cotyledons accompanying salt stress. Nitric Oxide 2016;59:42-53. [ Links ]
83. Li B, He X, Zhuang M, Niu B, Wu C, Mu H, et al. Melatonin ameliorates busulfan-induced spermatogonial stem cell oxidative apoptosis in mouse testes. Antioxid Redox Signal 2018;28(5):385-400. [ Links ]
84. Zhang HM, Zhang Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res 2014;57(2):131-146. [ Links ]
85. Loren P, Sánchez R, Arias ME, Felmer R, Risopatrón J, Cheuquemán C. Melatonin scavenger properties against oxidative and nitrosative stress: Impact on gamete handling and in vitro embryo production in humans and other mammals. Int J Mol Sci 2017;18(6):1-17. [ Links ]
86. Pang Y-W, Sun Y-Q, Jiang X-L, Huang Z-Q, Zhao S-J, Du W-H, et al. Protective effects of melatonin on bovine sperm characteristics and subsequent in vitro embryo development. Mol Reprod Dev 2016;83(11):993-1002. [ Links ]
87. Espino J, Ortiz Á, Bejarano I, Lozano GM, Monllor F, García JF, et al. Melatonin protects human spermatozoa from apoptosis via melatonin receptor- and extracellular signal-regulated kinase-mediated pathways. Fertil Steril 2011;95(7):2290-2296. [ Links ]
88. Espino J, Bejarano I, Ortiz Á, Lozano GM, García JF, Pariente JA, et al. Melatonin as a potential tool against oxidative damage and apoptosis in ejaculated human spermatozoa. Fertil Steril 2010;94(5):1915-1917. [ Links ]
89. Karimfar M, Niazvand F, Haghani K, Ghafourian S, Shirazi R, Bakhtiyari S. The protective effects of melatonin against cryopreservation-induced oxidative stress in human sperm. Int J Immunopathol Pharmacol 2015;28(1):69-76. [ Links ]
90. Cheuquemán C, Arias ME, Risopatrón J, Felmer R, Álvarez J, Mogas T, et al. Supplementation of IVF medium with melatonin: Effect on sperm functionality and in vitro produced bovine embryos. Andrologia 2015;47(6):604-615. [ Links ]
91. Succu S, Pasciu V, Manca ME, Chelucci S, Torres-Rovira L, Leoni GG, et al. Dose-dependent effect of melatonin on postwarming development of vitrified ovine embryos. Theriogenology 2014;81(8):1058-1066. [ Links ]
92. Mehaisen GMK, Saeed AM, Gad A, Abass AO, Arafa M, El-Sayed A. Antioxidant Capacity of melatonin on preimplantation development of fresh and vitrified rabbit embryos: Morphological and molecular aspects. Fraidenraich D, editor. PLoS One. 2015;10(10):e0139814. [ Links ]
93. Dehghani-Mohammadabadi M, Salehi M, Farifteh F, Nematollahi S, Arefian E, Hajjarizadeh A, et al. Melatonin modulates the expression of BCL-xl and improve the development of vitrified embryos obtained by IVF in mice. J Assist Reprod Genet 2014;31(4):453-461. [ Links ]
94. Dimitrios B. Sources of natural phenolic antioxidants. Trends Food Sci Technol 2006;17(9):505-512. [ Links ]
95. Wright JS, Johnson ER, DiLabio GA. Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc 2001;123(6):1173-1183. [ Links ]
96. Negi G, Kumar A, Kaundal RK, Gulati A, Sharma SS. Functional and biochemical evidence indicating beneficial effect of Melatonin and Nicotinamide alone and in combination in experimental diabetic neuropathy. Neuropharmacology 2010;58(3):585-592. [ Links ]
97. Kumar A, Sharma SS. NF-kB inhibitory action of resveratrol: A probable mechanism of neuroprotection in experimental diabetic neuropathy. Biochem Biophys Res Commun 2010;394(2):360-365. [ Links ]
98. Rojano B, Gaviria CA, Gil MA, Saez JA, Schinella G, Tournier H. Actividad antioxidante del isoespintanol en diferentes medios. Vitae, Rev La Fac Quim Farm 2008;15(63):173-181. [ Links ]
99. Wang ZG, Yu SD, Xu ZR. Effect of supplementation of green tea polyphenols on the developmental competence of bovine oocytes in vitro. Braz J Med Biol Res 2007;40(8):1079-1085. [ Links ]
100. You J, Kim J, Lim J, Lee E. Anthocyanin stimulates in vitro development of cloned pig embryos by increasing the intracellular glutathione level and inhibiting reactive oxygen species. Theriogenology 2010;74(5):777-785. [ Links ]
101. Sakatani M, Suda I, Oki T, Kobayashi S, Kobayashi S, Takahashi M. Effects of purple sweet potato anthocyanins on development and intracellular redox status of bovine preimplantation embryos exposed to heat shock. J Reprod Dev 2007;53(3):605-614. [ Links ]
102. Gambini J, Inglés M, Olaso G, Lopez-Grueso R, Bonet-Costa V, Gimeno-Mallench L, et al. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid Med Cell Longev 2015;2015:837042. [ Links ]
103. Rimando AM, Kalt W, Magee JB, Dewey J, Ballington JR. Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J Agric Food Chem 2004;52(15):4713-4719. [ Links ]
104. McCormack D, McFadden D. A review of pterostilbene antioxidant activity and disease modification. Oxid Med Cell Longev 2013;2013:575482. [ Links ]
105. Sosa F, Fernando de la Torre J, Alvarez H, Perez S, Kjelland ME, Romo S. 84 Pterostilbene can reduce the percentage of lipids and reactive oxygen species in in vitro-produced bovine embryos [resumen]. Reprod Fertil Dev 2017;29(1):149. [ Links ]
106. Kwak S-S, Cheong S-AA, Jeon Y, Lee E, Choi K-CC, Jeung E-BB, et al. The effects of resveratrol on porcine oocyte in vitro maturation and subsequent embryonic development after parthenogenetic activation and in vitro fertilization. Theriogenology 2012;78(1):86-101. [ Links ]
107. Wang F, Tian X, Zhang L, He C, Ji P, Li Y, et al. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil Steril 2014;101(2):577-586. [ Links ]
108. Torres V, Urrego R, Echeverry JJ, Lopez A. 181 Resveratrol during in vitro maturation improves the quality of bovine oocyte and enhances embryonic. Reprod Fertil Dev 2016;29:199-209. [ Links ]
109. Kordowitzki P, Bernal SM, Herrmann D, Aldag P, Niemann H. Resveratrol supplementation during in vitro maturation and fertilisation enhances developmental competence of bovine oocytes [resumen]. Reprod Fertil Dev 2016;28(2):230. [ Links ]
110. Mukherjee A, Malik H, Saha AP, Dubey A, Singhal DK, Boateng S, et al. Resveratrol treatment during goat oocytes maturation enhances developmental competence of parthenogenetic and hand-made cloned blastocysts by modulating intracellular glutathione level and embryonic gene expression. J Assist Reprod Genet 2014;31(2):229-239. [ Links ]
111. Lee K, Wang C, Chaille JM, Machaty Z. Effect of resveratrol on the development of porcine embryos produced in vitro. J Reprod Dev 2010;56(3):330-335. [ Links ]
112. Salzano A, Albero G, Zullo G, Neglia G, Abdel-Wahab A, Bifulco G, et al. Effect of resveratrol supplementation during culture on the quality and cryotolerance of bovine in vitro produced embryos. Anim Reprod Sci 2014;151(3-4):91-96. [ Links ]
113. Giaretta E, Spinaci M, Bucci D, Tamanini C, Galeati G. Effects of resveratrol on vitrified porcine oocytes. Oxid Med Cell Longev 2013;2013. [ Links ]
114. Pocar P, Augustin R, Fischer B. Constitutive expression of CYP1A1 in bovine cumulus oocyte-complexes in vitro: Mechanisms and biological implications. Endocrinology 2004;145(4):1594-1601. [ Links ]
115. Price NL, Gomes AP, Ling AJY, Duarte FV, Martin-Montalvo A, North BJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 2012;15(5):675-690. [ Links ]
116. Kulkarni SS, Cantó C. The molecular targets of resveratrol. Biochim Biophys Acta 2015;1852:1114-1123. [ Links ]
117. Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 2014;25(3):138-145. [ Links ]
118. Park S, Ahmad F, Philp A, Baar K, Williams T, Ke H, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012;148(3):421-433. [ Links ]
119. Mayes MA, Sirard M-A. Effect of type 3 and type 4 phosphodiesterase inhibitors on the maintenance of bovine oocytes in meiotic arrest. Biol Reprod 2002;66(1):180-184. [ Links ]
120. Thomas RE, Thompson JG, Armstrong DT, Gilchrist RB. Effect of specific phosphodiesterase isoenzyme inhibitors during in vitro maturation of bovine oocytes on meiotic and developmental capacity. Biol Reprod 2004;71(4):1142-1149. [ Links ]
121. Sirard MA, Richard F, Blondin P, Robert C. Contribution of the oocyte to embryo quality. Theriogenology 2006;65(1):126-136. [ Links ]
122. Torner H, Ghanem N, Ambros C, Hölker M, Tomek W, Phatsara C, et al. Molecular and subcellular characterisation of oocytes screened for their developmental competence based on glucose-6-phosphate dehydrogenase activity. Reproduction 2008;135(2):197-212. [ Links ]
123. Lequarre AS, Traverso JM, Marchandise J, Donnay I. Poly(A) RNA is reduced by half during bovine oocyte maturation but increases when meiotic arrest is maintained with CDK inhibitors. Biol Reprod 2004;71(2):425-431. [ Links ]
124. Bilodeau-Goeseels S, Panich P. Effects of oocyte quality on development and transcriptional activity in early bovine embryos. Anim Reprod Sci 2002;71(3-4):143-155. [ Links ]
125. Tong Z-B, Bondy CA, Zhou J, Nelson LM. A human homologue of mouse mater, a maternal effect gene essential for early embryonic development. Hum Reprod 2002;17(4):903-911. [ Links ]
126. Pennetier S, Perreau C, Uzbekova S, Thélie A, Delaleu B, Mermillod P, et al. MATER protein expression and intracellular localization throughout folliculogenesis and preimplantation embryo development in the bovine. BMC Dev Biol 2006;6:26. [ Links ]
127. Urrego R, Herrera-Puerta E, Chavarria NA, Camargo O, Wrenzycki C, Rodriguez-Osorio N. Follicular progesterone concentrations and messenger RNA expression of MATER and OCT-4 in immature bovine oocytes as predictors of developmental competence. Theriogenology 2015;83(7):1179-1187. [ Links ]
128. Habermann FA, Wuensch A, Sinowatz F, Wolf E. Reporter genes for embryogenesis research in livestock species. Theriogenology 2007;68(Supl. 1):116-124. [ Links ]
129. Huang Y, Tang X, Xie W, Zhou Y, Li D, Zhou Y, et al. Vitamin C enhances in vitro and in vivo development of porcine somatic cell nuclear transfer embryos. Biochem Biophys Res Commun 2011;411(2):397-401. [ Links ]
130. McKenzie LJ, Pangas SA, Carson SA, Kovanci E, Cisneros P, Buster JE, et al. Human cumulus granulosa cell gene expression: A predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod 2004;19(12):2869-2874. [ Links ]
131. Lopes AS, Wrenzycki C, Ramsing NB, Herrmann D, Niemann H, Løvendahl P, et al. Respiration rates correlate with mRNA expression of G6PD and GLUT1 genes in individual bovine in vitro-produced blastocysts. Theriogenology 2007;68(2):223-236. [ Links ]
132. Wrenzycki C, Wells D, Herrmann D, Miller A, Oliver J, Tervit R, et al. Nuclear transfer protocol affects messenger RNA expression patterns in cloned bovine blastocysts. Biol Reprod 2001;65(1):309-317. [ Links ]
133. Rizos D, Lonergan P, Boland MP, Arroyo-García R, Pintado B, de la Fuente J, et al. Analysis of differential messenger RNA expression between bovine blastocysts produced in different culture systems: Implications for blastocyst quality. Biol Reprod 2002;66(3):589-595. [ Links ]
134. Urrego R, Rodriguez-Osorio N, Niemann H. Epigenetic disorders and altered gene expression after use of assisted reproductive technologies in domestic cattle. Epigenetics 2014;9(6):803-815. [ Links ]
135. Lee S, Park EJ, Moon JH, Kim SJ, Song K, Lee BC. Sequential treatment with resveratrol-trolox improves development of porcine embryos derived from parthenogenetic activation and somatic cell nuclear transfer. Theriogenology 2016;84(1):145-154. [ Links ]
136. Castillo-Martín M, Bonet S, Morató R, Yeste M. Supplementing culture and vitrification-warming media with l-ascorbic acid enhances survival rates and redox status of IVP porcine blastocysts via induction of GPX1 and SOD1 expression. Cryobiology 2014;68(3):451-458. [ Links ]
137. Cebrian-Serrano A, Salvador I, García-Roselló E, Pericuesta E, Pérez-Cerezales S, Gutierrez-Adán A, et al. Effect of the bovine oviductal fluid on in vitro fertilization, development and gene expression of in vitro-produced bovine blastocysts. Reprod Domest Anim 2013;48(2):331-338. [ Links ]
138. Brykczynska U, Hisano M, Erkek S, Ramos L, Oakeley EJ, Roloff TC, et al. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat Struct Mol Biol 2010;17(6):679-687. [ Links ]
139. Heinzmann J, Hansmann T, Herrmann D, Wrenzycki C, Zechner U, Haaf T, et al. Epigenetic profile of developmentally important genes in bovine oocytes. Mol Reprod Dev 2011;78(3):188-201. [ Links ]
140. De Castro LS, De Assis PM, Siqueira AFP, Hamilton TRS, Mendes CM, Losano JDA, et al. Sperm oxidative stress is detrimental to embryo development: A dose-dependent study model and a new and more sensitive oxidative status evaluation. Oxid Med Cell Longev 2016;2016:1-12. [ Links ]
141. Lane M, McPherson NO, Fullston T, Spillane M, Sandeman L, Kang WX, et al. Oxidative stress in mouse sperm impairs embryo development, fetal growth and alters adiposity and glucose regulation in female offspring. PLoS One. 2014;9(7):1-9. [ Links ]
142. Sato D, Itami N, Tasaki H, Takeo S, Kuwayama T, Iwata H, et al. Relationship between mitochondrial DNA copy number and SIRT1 expression in porcine oocytes. Ling F, editor. PLoS One 2014;9(4):e94488. [ Links ]
143. Takeo S, Sato D, Kimura K, Monji Y, Kuwayama T, Kawahara-Miki R, et al. Resveratrol improves the mitochondrial function and fertilization outcome of bovine oocytes. J Reprod Dev 2014;60(2):92-99. [ Links ]
144. Kordowitzki P, Klein S, Hadeler K-G, Aldag P, Nowak-Imialek M, Lucas-Hahn A, et al. SIRT1-A possible marker for reproductive aging of in vivo-derived bovine oocytes? [resumen]. Reprod Fertil Dev 2017;29(1):109. [ Links ]
145. Lee S, Park EJ, Moon JH, Kim SJ, Song K, Lee BC. Sequential treatment with resveratrol-trolox improves development of porcine embryos derived from parthenogenetic activation and somatic cell nuclear transfer. Theriogenology 2015;84(1):145-154. [ Links ]
146. Mojica-Villegas MA, Izquierdo-Vega JA, Chamorro-Cevallos G, Sánchez-Gutiérrez M. Protective effect of resveratrol on biomarkers of oxidative stress induced by iron/ascorbate in mouse spermatozoa. Nutrients 2014;6(2):489-503. [ Links ]
147. Branco CS, Garcez ME, Pasqualotto FF, Erdtman B, Salvador M. Resveratrol and ascorbic acid prevent DNA damage induced by cryopreservation in human semen. Cryobiology 2010;60(2):235-237. [ Links ]
Received: October 03, 2017; Accepted: May 29, 2018











 texto en
texto en