Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.12 no.4 Texcoco may./jun. 2021 Epub 21-Feb-2022
https://doi.org/10.29312/remexca.v12i4.2929
Articles
Chitosan nanoparticles improve the nutraceutical quality of triticale sprouts
1Doctorate in Water and Soil-Technological Institute of Torreón. Highway Torreón-San Pedro km 7.5, Ejido Ana, Torreon, Coahuila, Mexico. CP. 27170. (citlaly-rrha@hotmail.com; fortismanuel@hotmail.com; joorvi66@hotmail.com).
2Department of Advanced Materials-Center for Research in Applied Chemistry. Blvd. Enrique Reyna Hermosillo 140, Saltillo, Coahuila, Mexico. CP. 25294. (hortensia.ortega@ciqa.edu.mx).
3Antonio Narro Autonomous Agrarian University-Department of Horticulture. Peripheral and Road to Santa Fe s/n, Torreon, Coahuila, Mexico. CP. 27000. (juan-manuelnava@hotmail.com).
The use of chitosan nanoparticles (NPs CS) has become a promising alternative in modern agriculture as an inducer in the biosynthesis of secondary metabolites. The objective of this research was to evaluate the effect of NPs CS on the nutraceutical quality of triticale sprouts (x Triticosecale Wittmack). Increasing doses of NPs Cs: 0, 0.1, 0.2, 0.4 and 0.8 mg ml-1 were applied only once at the imbibition stage, then they were left in Petri dishes for 7 days at 25 ±2 °C temperature. NPs CS did not affect germination or fresh root weight at the tested concentrations, and the concentration of 0.1 mg ml-1 increased the fresh weight of the shoots up to 83.3%. In the presence of 0.8 mg ml-1 of NPs CS phenolic compounds decrease by 7% and flavonoids increase by 29%. The results confirm a promoter effect of NPS CS on sprouts, opening the possibility of being used as inducers in the biosynthesis of bioactive compounds in triticale sprouts.
Keywords x Triticosecale Wittmack; nanotechnology; secondary metabolites
El uso de nanopartículas de quitosán (NPs CS) se ha vuelto una alternativa prometedora en la agricultura moderna como un inductor en la biosíntesis de metabolitos secundarios. El objetivo del presente trabajo fue evaluar el efecto de NPs CS en la calidad nutraceútica de germinados de triticale (x Triticosecale Wittmack). Dosis creciente de NPs CS: 0, 0.1, 0.2, 0.4 y 0.8 mg ml-1 se aplicaron una sola vez en la etapa de imbibición, después se dejaron en cajas Petri durante 7 días a 25 ±2 °C de temperatura. Las NPs CS no afectaron la germinación ni el peso freso de la raíz a las concentraciones probadas, y a la concentración de 0.1 mg ml-1 aumentó el peso fresco del brote hasta 83.3%. En presencia de 0.8 mg ml-1 de las NPs CS los compuestos fenólicos disminuyen un 7% y aumentan 29% los flavonoides. Los resultados confirman un efecto promotor de las NPS CS en los germinados, abriendo la posibilidad de ser utilizadas como inductores en la biosíntesis de compuestos bioactivos en germinados de triticale.
Palabras clave x Triticosecale Wittmack; metabolitos secundarios; nanotecnología
Introduction
Nanotechnology is an alternative in modern agriculture by producing agro-products such as nanofertilizers, nanopesticides, nanoherbicides and nanosensors, which allow to increase food yield in a sustainable way and reduce the environmental impact (Lira et al., 2018). The use of nanomaterials is of great interest for its study due to its size and the applications that they can have thanks to the physical and chemical properties, which acquire at the nanometric scale compared to the micro-sized material (Hojjat and Hojjat, 2015).
Among these nanomaterials, chitosan nanoparticles (NPs CS) are of great interest as they are obtained thanks to the versatility of the chitosan and availability of functional groups (amino, -NH2) (Salachna and Zawadzińska, 2014; Kumaraswamy et al., 2018), nontoxicity, biocompatibility and biodegradability (Divya et al., 2019).
In this sense NPs CS have become a promising alternative in seed priming, due to their high biological activity since NPs CS interact in conjunction with the living cell (Pedroso, 2017; Divya and Jisha, 2018; Souza et al., 2019), thus causing the synthesis of several biomolecules as inducers that force the sprout to react to them consequently developing a greater synthesis of secondary metabolites (Hidangmayum et al., 2019; Paramo et al., 2020), which has been shown in tomato, rice and wheat sprouts (Colman et al., 2019; Divya et al., 2019; Li et al., 2019).
Sprouts, on the other hand, are a source of carbohydrates, fiber, vitamins, essential nutrients and bioactive compounds, which have been linked to prevention and treatment of diseases. The presence of these compounds, in sprouts, can be increased by production conditions, seed quality and germination conditions (Dziki et al., 2015).
Recent advances in the use of nanotechnology in agriculture allow to try to understand the role of NPs Cs in triticale sprouts (x Triticosecale Wittmack), as it currently presents an interesting increase of 30% in food production worldwide (Aquino and Gómez, 2019). NPs CS are a promising material for seed treatments, so the goal of this work was to evaluate the effect of NPs CS on the synthesis of bioactive compounds in triticale sprouts.
Materials and methods
This study was conducted in a Biotechnology laboratory located at the Technological Institute of Torreón, Mexico in latitude 24° 30’ and 27 north latitude, 102° 00’ and 104° 40’ west longitude.
Synthesis of chitosan nanoparticles (NPs CS)
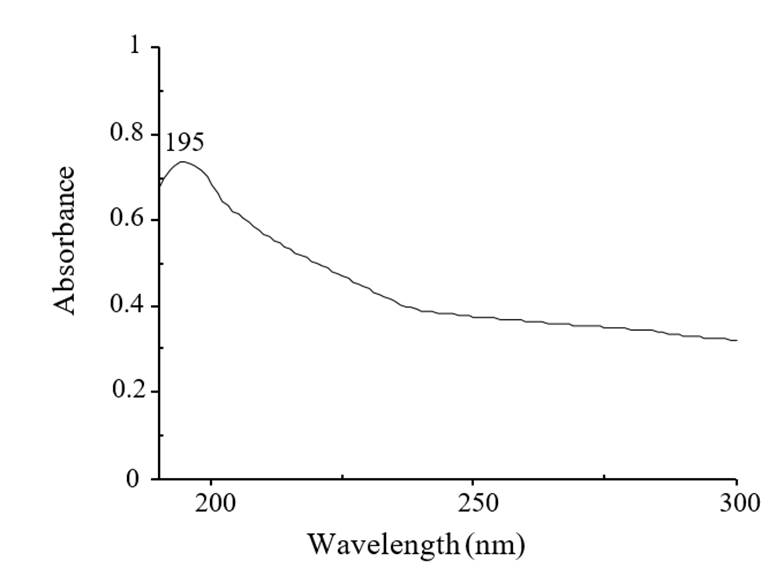
NPs CS were synthesized by the ion gelation method at the Applied Chemistry Research Center (Saltillo, Coahuila), using chitosan (Marine, Hydrocolloids, Kerala, India) and sodium tripolyphosphate (TPP) (Sigma-Aldrich, USA) as crosslinker in a ratio of 10:3 v/v of CS: TPP (Kumaraswamy et al., 2018) with spherical shape and a particle size of 111 ±21 nm, which were characterized by UV-vis, observing an absorption of 195 nm (Figure 1) and by infrared spectrophotometry (FTIR by ATR) where the characteristic bands of the amino group (NH2) at 3 346 cm-1 and carboxyl (C=O) at 1 635 cm-1 (Figure 2) were observed, coinciding with the chemical structure of the compound (Manikandan and Sathiyabama, 2016).
Plant material
Triticale seeds (x Triticosecale Wittmack L) were used, of uniform size, which were disinfected with 75% ethanol for 5 min and washed four times with distilled water (Li et al., 2019).
Germination test and growth measurement
The seeds were divided into five main treatments of ten seeds: (witness) distilled water, 0.1, 0.2, 0.4 and 0.8 mg ml-1 of NPs CS (Colman et al., 2019; Li et al., 2019), the treatments were applied only once to the seed during the imbibition stage, the seeds of triticale were soaked with the corresponding treatment solutions for 8 h in the dark.
Subsequently for germination ten seeds were placed per Petri dish, which had a double layer of Whatman filter paper #1 pre-soaked with 5 ml of distilled water, the Petri dishes were sealed with duct tape and placed in an artificial growth incubator (HGZ-150) with a day/night cycle of 12 h, at 25 ±2 °C respectively, with 60% relative humidity (Li et al., 2019).
The germination of seeds was calculated daily in accordance with the International Seed Testing Association (ISTA) guidelines and growth parameters were recorded for seven days. The germination of seed was verified when the length of the germ reached half the length of the seed (Faraji et al., 2018).
Parameters evaluated during the development of the bioassay
Germination percentage
It was determined seven days after sowing in the second count, for which the total count of germinated seeds was considered, and the result was expressed as shown in equation:
Seed vigor
On the fourth day after sowing, the first count was performed to collect data of the germinated seed (seedlings that have well developed root and plumule, with total development of 2 cm on average). To determine the vigor of the seed, expressing the result in percentage according to the formula:
Fresh weight, shoot and root
The fresh weight of the shoot and root were recorded in an analytical balance (ADN model HR-200®) to determine the value of fresh biomass and it was reported in milligrams per sprout (Martínez et al., 2019).
Photosynthetic pigments
The chlorophyll content (Chl) in triticale sprouts was performed according to the method described by Liu et al. (Liu et al., 2013). For which 0.5 g of sprouts were weighed, which were homogenized in a mortar with 10 ml of 95% ethanol. The homogenized was centrifuged at 1 500 rpm for 20 min and the supernatant was collected, to then measure the absorbance-to-absorbance at 665 and 649 nm, respectively. The Chl content was calculated according to the following formula:
Preparation of extracts for nutraceutical quality
To obtain the extracts, 2 g of fresh sample were mixed in 10 ml of 80% ethanol, with constant orbital shaking for 24 h at 70 rpm at 5 °C. Subsequently the extracts were centrifuged at 3 000 rpm for 5 min and the supernatant was extracted for analysis (Salas et al., 2016).
Total content of phenols
It was determined by a modification of the Folin-Ciocaltea method (Singleton et al., 1999), 50 µl of the ethanolic extract, diluted in 3 ml of distilled water, were taken and 250 µl of the Folin-Ciocalteau reagent (1N) were added, stirred and left to react for 3 min. Subsequently 750 µl of Na2CO3 (20%) and 950 µl of distilled water were added, the solution was left to rest for 2 h, to later be quantified in a UV-Vis spectrophotometer at 760 nm.
Solutions of gallic acid were used to build the calibration curve. The results were expressed as mg equivalents of gallic acid (AGE) 100 g-1 fresh weight.
Total flavonoids
They were determined by colorimetric method (Colina, 2016), 250 µl of ethanolic extract were taken, they were mixed with 1.25 ml of distilled water and 75 µl of NaNO2 (5%). After 5 min of rest, 150 µl of AlCl3 (1-ethyl-3-methylimidazolium chloride-aluminum chloride) (Sigma-Aldrich, St. Louis, MO, USA) were added.
Subsequently 500 µl of NaOH (1M) and 275 µl of distilled water were added, the samples were stirred vigorously, to then be quantified in a UV-Vis spectrophotometer at 510 nm. The standard was prepared with quercetin dissolved in absolute ethanol to obtain the calibration curve. The results were expressed in mg QE 100 g-1 fresh weight.
Statistical analysis
The experiment was conducted by a completely random design with five treatments and ten repetitions. The results obtained were analyzed by analysis of variance and comparison of means with the Tukey test (p≤ 0.05) using the Statistical Analysis System Institute (SAS) version 9.3 statistical package. The normality of the data for each response variable was verified with the Kolmogorov-Smirnov test, the data of the germination percentage and the variables of antioxidant capacity (both expressed as a percentage) were normalized by applying arcsine and square root transformation.
Results and discussion
Germination of seeds
Germination percentage and fresh weight are some of the main properties involved in the physiological quality of the seed (Morales et al., 2017). The results of this work show that the germination percentage and fresh root weight variables showed no significant difference (p> 0.05) at the different concentrations that were applied of NPs Cs; however, they caused a significant difference in the vigor and fresh weight of the shoot (Table 1), with the seed vigor showing a decrease of 9.75% (0.1 mg ml-1), contrary to what Colman et al. (2019) reported in germinated tomato seeds treated with 0.1 mg ml-1, with positive effects on the rate of vigor with respect to the control.
Table 1 Comparison of means for germination and vigor percentage, fresh weight of shoot and root of triticale seeds treated with NP CS.
| NPs CS (mg ml-1) | Germination | Vigor | Shoot fresh weight | Root fresh weight | |
|---|---|---|---|---|---|
| (%) | (mg) | ||||
| Control | 86 ±0.89 a | 82 ±0.81 a | 38.7 ±0.32 b | 74.2 ±0.28 a | |
| 0.1 | 82 ±0.85 a | 74 ±0.86 c | 70.4 ±0.15 a | 79.6 ±0.98 a | |
| 0.2 | 96 ±1.02 a | 80 ±0.8 ab | 55.2 ±0.25 ab | 53.2 ±0.31 a | |
| 0.4 | 88 ±1.05 a | 76 ±0.84 c | 64.8 ±0.58 a | 73.8 ±0.42 a | |
| 0.8 | 88 ±0.98 a | 80 ±0.82 ab | 56.0 ±0.35 b | 56.8 ±0.45 a | |
Values with equal letters in each column are the same according to the Tukey test (p≤ 0.05). The values are the average of five repetitions. Means (n= 5) ± standard deviation.
As for the accumulation of biomass, the fresh weight of the shoot increased up to 81% with the dose of 0.1 mg ml-1, which confirms the positive effect of NPs CS at low concentrations on the germination of triticale seeds, this effect could be attributed to the stimulating capacity of the metabolic activity of NPs CS, achieving an increase in intrinsic potential in the development of the seed with the absorption of NPs CS (Divya et al., 2019; López et al., 2019), either by imbibition, coating or priming of seeds (Costales et al., 2020).
Several studies with high concentrations of NPs Cs or CS have described an inhibition of root growth and alternatively, a promotion in the appearance of a greater number of seminal roots (Colman et al., 2019; López et al., 2019), as shown in Figure 3, attributing it to the stimulation of phytohormone synthesis (López et al., 2019) and the activation of defense genes (Rodríguez et al., 2019) as has been observed in various crops such as tomato (Colman et al., 2019; Solórzano, 2019), salicornia bigelovii (López et al., 2020) (Lanchimba, 2019), sorghum (Holguin et al., 2020), rice (Divya et al., 2019) and wheat (Li et al., 2019), among others.
Photosynthetic pigments
The Chl content of the sprouts showed that the dose of 0.2 mg ml-1 of CS NPs has a significant effect, since it increased 59% over the control (Figure 4). According to the work reported by Acharya et al. (2020), they mention that the use of nanoparticles in seed priming would cause toxicity in sprouts at high concentrations, a decrease in photosynthetic pigments may be observed (Acharya et al., 2020) due to the rupture of the chlorophyll and inhibition of the enzyme p-hydroxyphenylpyruvate in the biosynthesis of chlorophyll (Miras, 2018), produced by the stress caused by nanoparticles.
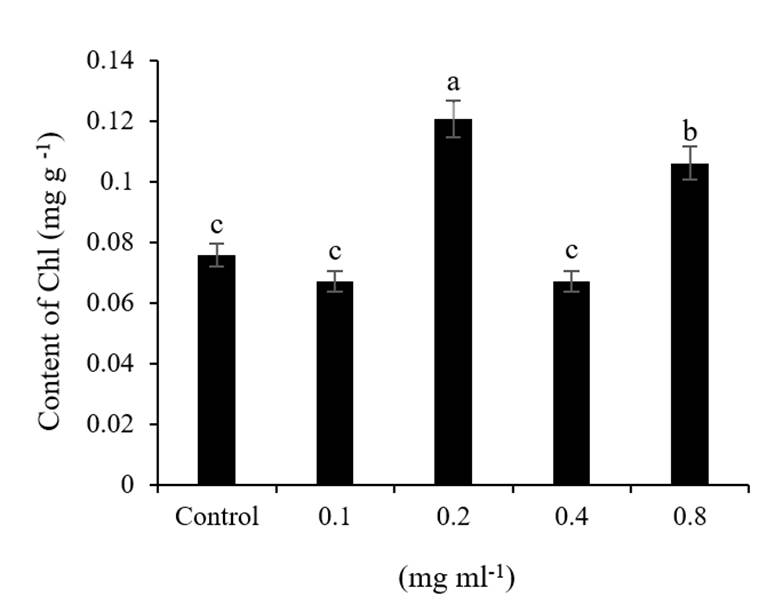
Figure 4 Effect of NPs CS on chlorophyll content on wheat sprouts. Average values in columns with different literals differ statistically between them (Tukey p≤ 0.05).
On the contrary, this work showed that priming with NPs CS increases photosynthetic pigments at low concentrations compared to the control. However, it is difficult to establish a response model of the effects of NPs on the sprouts as the effect depends on the species, concentration and type of NPs (Arruda et al., 2015).
Nutraceutical quality: total phenols and total flavonoids
The use of NPs CS can act as an inducer of metabolic processes as they may increase the content of bioactive compounds prominent in the development and stimulating effect of the production of secondary metabolites (Xoca et al., 2017; Xoca et al., 2019; Montalvo et al., 2020).
The results of this research show that variables related to nutraceutical quality in triticale sprout: total phenols and total flavonoids are affected by the high applied dose of NPs CS (0.8 mg ml-1), phenolic compounds decreased compared to the control up to 7% (Figure 5a), and flavonoids increased by 29% over the control (Figure 5b), corroborating the positive effect of NPs CS on triticale sprouts.
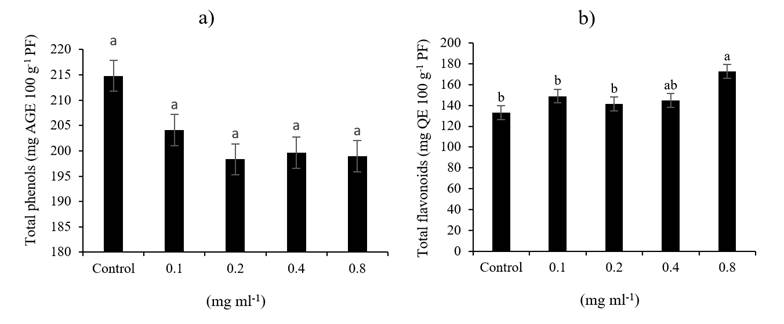
Figure 5 Effect of NPs CS on the content of total phenolic compounds (a), total flavonoids (b) in triticale sprouts.
These results can be attributed to the fact that high concentrations of NPs CS lead to an initial oxidative burst with hydrogen peroxide accumulation (H2O2) (Martínez et al., 2015), and it is believed that this can lead to induction of plant defense enzymes and synthesis of secondary metabolites, such as polyphenols, lignin, flavonoids and phytoalexins (Malerba and Cerana, 2016) improving defense responses to biotic and abiotic stress (Hidangmayum et al., 2019).
It may be a viable alternative to improve the functional and biological properties of the sprout (Rodríguez et al., 2019); however, it is difficult to establish the effects of NPs CS on the sprouts as they vary according to the plant species, growth stages, dose and the exposure of NPs (Medina, 2017).
Conclusions
The application of NPs CS in triticale sprouts at a dose of 0.1 mg ml-1 affects the fresh weight of the shoot, but not the fresh weight of the root, while the dose of 0.2 mg ml-1 showed the best results in percentage of vigor and content of photosynthetic pigments, confirming that seed priming with NPs CS does not cause negative effects, besides high doses of 0.8 mg ml-1 of NPs CS affect nutraceutical quality increasing the content of flavonoids in triticale sprouts.
NPs CS could be a good alternative to improve the quality of sprouts; however, more research is needed to clarify the effects of Ns CS as there are factors that depend on species and concentration.
Literatura citada
Acharya, P.; Jayaprakasha, G. K.; Crosby, K.; Jifon, J. L. and Patil, B. S. 2020. Nanoparticle-mediated seed priming improves germination, growth, yield, and quality of watermelons (Citrullus lanatus) at multi-locations in Texas. Scientific Reports. 10(1):1-16. [ Links ]
Aquino, V. C. y Gómez, N. I. 2019. Triticale (x Triticosecale Wittmack): bioestimulantes orgánicos y fertilización nitrogenada sobre los componentes de rendimiento forrajero en campaña chica-valle del mantaro. Scientia Agropecuaria. 10(4):469-477. [ Links ]
Arruda, S. C.; Diniz, A. L.; Moreto, R.; Antunes, R. and Zezzi, A. 2015. Nanoparticles applied to plant science: a review. Talanta. 131(1):693-705. [ Links ]
Colina, A. C. 2016. Análisis fitoquímico, determinación cualitativa y cuantitativa de flavonoides y taninos, actividad antioxidante, antimicrobiana de las hojas de “Muehlenbeckia hastulata (JE Sm) IM Johnst” de la zona de Yucay (Cusco). Tesis Profesional de Químico. Universidad Nacional Mayor de San Marcos. Lima, Perú. [ Links ]
Colman, S. L.; Salcedo, M. F.; Mansilla, A. Y.; Iglesias, M. J.; Fiol, D. F.; Saldaña, S. M.; Alvarez, V. A.; Chevalier, A. A. and Casalongué, C. A. 2019. Chitosan microparticles improve tomato seedling biomass and modulate hormonal, redox and defense pathways. Plant Physiol. Biochem. 143(1):203-211. [ Links ]
Costales, D.; Falcón, A. B. y Travieso, L. 2020. Efecto de la masa molecular de quitosanos en la germinación y el crecimiento in vitro de soya. Cultivos Tropicales. 41(1):1-10. [ Links ]
Divya, K. and Jisha, M. 2018. Chitosan nanoparticles preparation and applications. Environ. Chem. Letters 16(1):101-112. [ Links ]
Divya, K.; Vijayan, S.; Nair, S. J. and Jisha, M. 2019. Optimization of chitosan nanoparticle synthesis and its potential application as germination elicitor of Oryza sativa L. Inter. J. Biol. Macromol. 124(1):1053-1059. [ Links ]
Dziki, D.; Gawlik, U.; Kordowska, M. and Domań, M. 2015. Influence of elicitation and germination conditions on biological activity of wheat sprouts. J. Chem. 7:1-8. [ Links ]
Faraji, J.; Sepehri, A. and Salcedo, J. C. 2018. Titanium dioxide nanoparticles and sodium nitroprusside alleviate the adverse effects of cadmium stress on germination and seedling growth of wheat (Triticum aestivum L.). Universitas Scientiarum. 23(1):61-87. [ Links ]
Hidangmayum, A.; Dwivedi, P.; Katiyar, D. and Hemantaranjan, A. 2019. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants. 25(2):313-326. [ Links ]
Hojjat, S. S. and Hojjat, H. 2015. Effect of nano silver on seed germination and seedling growth in fenugreek seed. Inter. J. Food Eng. 1(2):106-110. [ Links ]
Holguin, R. J.; Vargas, J. M.; López, G. A.; Rodríguez, F.; Borbón, C. G. y Rueda, E. O. 2020. Efecto de quitosano y consorcio simbiótico benéfico en el rendimiento de sorgo en la zona indígena “Mayos” en Sonora. Terra Latinoam. 38(3):705-714. [ Links ]
Kumaraswamy, R.; Kumari, S.; Choudhary, R. C.; Pal, A.; Raliya, R.; Biswas, P. and Saharan, V. 2018. Engineered chitosan based nanomaterials: Bioactivities, mechanisms and perspectives in plant protection and growth. Int. J. Food Eng. 113(1):494-506. [ Links ]
Lanchimba, W. I. 2019. Evaluación de quitosano en el crecimiento y desarrollo de tomate (Solanum lycopersicum, L.) en condiciones de casa de cultivo. Cuba: bayamo: Universidad Técnica de Cotopaxi (UTC). Tesis de Ingeniero Agrónomo. Universidad Técnica de Cotopaxi. Bayamo, Cuba. [ Links ]
Li, R.; He, J.; Xie, H.; Wang, W.; Bose, S. K.; Sun, Y.; Hu, J. and Yin, H. 2019. Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.). Int. J. Biol. Macromol. 126(1):91-100. [ Links ]
Lira, R. H.; Méndez, B.; De-Santos, G. y Vera, R. I. 2018. Potencial de la nanotecnología en la agricultura. Acta Universitaria. 28(2):9-24. [ Links ]
Liu, H.; Zhang, Y. H.; Yin, H.; Wang, W. X.; Zhao, X. M. and Du, Y. G. 2013. Alginate oligosaccharides enhanced Triticum aestivum L. tolerance to drought stress. Plant Physiology and Biochemistry. 62(1):33-40. [ Links ]
López, B.; Mondaca, I.; Gortáres, P.; Meza, M.; Balderas, J. d. J.; Ruíz, C. and Rueda, E. O. J. 2020. Ecofisiología y bioquímica de salicornia bigelovii (Torr.) por efecto de quitosano-aib bajo condiciones del desierto de sonora. Polibotánica. 49(5):75-92. [ Links ]
López, B. E.; Mondaca, I.; Gortáres, P.; Meza, M. M.; Balderas, J. d. J.; Ruiz, C. y Rueda, E. O. J. 2019. Enraizamiento de esquejes de Salicornia bigelovii (Torr.) por quitosano como un bioproducto de origen marino. Terra Latinoam . 37(4):361-369. [ Links ]
Malerba, M. and Cerana, R. 2016. Chitosan effects on plant systems. Int. J. Mol. Sci. 17(7):996-1011. [ Links ]
Manikandan, A. and Sathiyabama, M. 2016. Preparation of chitosan nanoparticles and its effect on detached rice leaves infected with Pyricularia Grisea. Inter. J. Biol. Macromolecules. 84(1):58-61. [ Links ]
Martinez, B. M. E.; Brown, A. y Coria, C. 2019. Contenido de hierro, calcio y magnesio durante el proceso de producción de germinados de lentejas (lens culinaris) bajo cultivo aeropónico. Universidad Nacional de Cuyo. Facultad de Ciencias Agrarias. Tesis de Licenciatura en Bromatología. Universidad Nacional de Cuyo. Mendoza, Argentina. [ Links ]
Martínez, L.; Reyes, Y.; Falcón, A. y Núñez, M. 2015. Efecto del tratamiento a las semillas con quitosana en el crecimiento de plántulas de arroz (Oryza sativa L.) cultivar INCA LP-5 en medio salino. Cultivos Tropicales . 36(1):143-150. [ Links ]
Medina, E. 2017. Diseño y evaluación de recubrimientos en base a proteínas de quínoa y quitosano que contienen agentes naturales nanoparticulados para su aplicación en frutillas. Tesis de Doctorado en Nutrición y Alimentos. Facultad de Ciencias Químicas y Farmacéuticas. Universidad de Chile. Santiago, Chile. [ Links ]
Miras, M. B. 2018. Estudio metabolómico y genómico de cultivos celulares de zanahoria. Tesis de Doctorado. Facultad de Biología Universidad de Murcia. Murcia, España. [ Links ]
Montalvo, E.; Romero, R.; Sánchez, J. A.; Ruvalcaba, J. M.; Pérez, A. y Anaya, L. M. 2020. Functionalization of edible coating chitosan-based for fruits and vegetables postharvest preservation. TIP Rev. Especializada en Ciencias Químico-Biológicas. 23(1):1-14. [ Links ]
Morales, M. E.; Peña, C. B.; García, A.; Aguilar, G. y Kohashi, J. 2017. Características físicas y de germinación en semillas y plántulas de frijol (Phaseolus vulgaris L.) silvestre, domesticado y su progenie. Agrociencia. 51(1):43-62. [ Links ]
Paramo, L. A.; Feregrino, A. A.; Guevara, R.; Mendoza, S. and Esquivel, K. 2020. Nanoparticles in agroindustry: applications, toxicity, challenges, and trends. Nanomaterials. 10(9):1654-1687. [ Links ]
Pedroso, S. 2017. Modificaciones físico-químicas de nanopartículas de diamante y oro en la formación de nanoestructuras híbridas para uso en la biomedicina. Tesis de Doctorado. Universidad de Sonora. Sonora, México. [ Links ]
Rodríguez, C. A.; González, R. R.; Bautista, S. y Gutiérrez, P. 2019. Efecto del quitosano en el control de Alternaria sp. en plantas de jitomate en invernadero. TIP. Revista especializada en ciencias químico-biológicas. 22(1):1-7. [ Links ]
Salachna, P. and Zawadzińska, A. 2014. Effect of chitosan on plant growth, flowering and corms yield of potted freesia. J. Ecol. Eng. 15(3):97-102. [ Links ]
Salas, L.; Gaucín, J. M.; Preciado, P. M.; Fortis, M.; Valenzuela, J. R y Ayala, A. V. 2016. Efecto del ácido benzoico en la capacidad antioxidante de germinados de trigo. Rev. Mex. Cienc. Agríc. 17(3):3397-3404. [ Links ]
Singleton, V. L.; Orthofer, R. and Lamuela, R. M. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 299(7):152-178. [ Links ]
Solórzano, A. E. 2019. Efecto de quitosano, hongos micorrízicos y ácidos húmicos sobre el crecimiento y desarrollo en variedades de pimiento (Capcicum annuum L) bajo condiciones protegidas. Tesis de Ingeniero Agrónomo. Departamento y Universidad Quevedo-UTEQ. Quevedo, Ecuador. [ Links ]
Souza, J. P.; Cayaso, R.; Martín, P.; Pereira, R.; Fernandes, J. and Martín, J. 2019. Efectos inmediatos y tras almacenaje del recubrimiento con boro de semillas de algodón. In: X Congreso Ibérico de Agroingeniería. 1199-1208 pp. [ Links ]
Xoca, L. Á.; Cuellar, E. A.; González, S.; Gutiérrez, P.; López, U.; Herrera, L.; Vega, J. and Chacón, A. 2017. Transcriptomic analysis of avocado hass (Persea americana Mill) in the interaction system fruit-chitosan-Colletotrichum. Frontiers Plant Sci. 8(1):956-969. [ Links ]
Xoca, L. Á.; Aguilera, S.; Vega, J.; Acevedo, G.; Tovar, E.; Stoll, A.; Herrera, L. and Chacón, A. 2019. Activation of the phenylpropanoid biosynthesis pathway reveals a novel action mechanism of the elicitor effect of chitosan on avocado fruit epicarp. Food Res. Inter. 121(1):586-592. [ Links ]
Received: March 2021; Accepted: May 2021











 texto en
texto en