Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 no.5 Texcoco jun./ago. 2018
https://doi.org/10.29312/remexca.v9i5.1509
Investigation note
Isolation, characterization and pathogenicity of fungi causing descending death of American Black Walnut
1University Agrarian University Antonio Narro. Calzada Antonio Narro 1923, Buenavista, Saltillo, Coahuila, Mexico. CP. 25315. Tel. 01 (844) 4158138.
2Terán Experimental Field-INIFAP. Road Montemorelos-China km 31, Nuevo León, Mexico. CP. 67400.
American black walnut (Juglans nigra L.) is affected by phytosanitary problems, highlighting fungi that cause cankers in branches, reducing fruit production up to 60% of the average yield that is 1.9 t ha-1. The objectives of this investigation were to identify the fungi associated with cankers present in branches of trees of J. nigra with symptoms of descending death and to evaluate their pathogenicity. Canker fungi were isolated from branches with symptoms of descending death collected in two orchards of the state of Coahuila. Four fungal morphotypes were obtained and one strain of each was used for its morphological identification at the genus level, and by sequencing the ITS1 region to ITS2 of the ribosomal genes (rDNA) for its identification at the species level. Pathogenicity tests were performed on four-month-old J. nigra seedlings, making a 3 mm long stem fissure and inoculating with each identified species. The fungi associated with the cankers of J. nigra were morphologically identified as members of the genera Trichothecium, Pestalotiopsis, Alternaria and Rhizoctonia. By means of the BLAST analysis in the GenBank of the sequences of the ITS1 to ITS2 region it was possible to determine that the strains were related to the species Trichothecium roseum, Pestalotiopsis steyaertii, Alternaria alternata and to an undetermined binucleate species of Rhizoctonia. The inoculation of the four strains to seedlings showed that they are pathogenic to J. nigra.
Keywords: Alternaria alternata; Pestalotiopsis steyaertii; Rhizoctonia binucleada; Trichothecium roseum; fungi in cankers
El nogal negro americano (Juglans nigra L.) es afectado por problemas fitosanitarios, destacando hongos causantes de cancros en ramas, reduciendo la producción de fruto hasta 60% del rendimiento promedio que es 1.9 t ha-1. Los objetivos de esta investigación fueron identificar los hongos asociados a cancros presentes en ramas de árboles de J. nigra con síntomas de muerte descendente y evaluar su patogenicidad. Se aislaron hongos de cancros de ramas con síntomas de muerte descendente colectadas en dos huertas del estado de Coahuila. Cuatro morfotipos de hongos fueron obtenidos y una cepa de cada uno se utilizó para su identificación morfológica a nivel de género, y mediante la secuenciación de la región ITS1 a ITS2 de los genes ribosomales (rDNA) para su identificación a nivel de especie. Las pruebas de patogenicidad se realizaron en plántulas de J. nigra de cuatro meses de edad, realizando una fisura en el tallo de 3 mm de largo e inoculando con cada especie identificada. Los hongos asociados a los cancros de J. nigra fueron identificados morfológicamente como miembros de los géneros Trichothecium, Pestalotiopsis, Alternaria y Rhizoctonia. Mediante el análisis BLAST en el GenBank de las secuencias de la región ITS1 a ITS2 fue posible determinar que las cepas se encontraron relacionadas a las especies Trichothecium roseum, Pestalotiopsis steyaertii, Alternaria alternata y a una especie binucleada no determinada de Rhizoctonia. La inoculación de las cuatro cepas a plántulas demostró que éstas son patogénicas a J. nigra.
Palabras clave: Alternaria alternata; Pestalotiopsis steyaertii; Rhizoctonia binucleada; Trichothecium roseum; hongos en cancros
American black walnut (Juglans nigra L.; Juglandaceae), native to North America (Michler et al., 2007) is widely grown in Europe (Šalek and Hejcmanova, 2011), South America and East Asia (Fisher et al., 2013), and it is valued for the nutritional characteristics and antioxidant content of its nut, and for its wood (Gilman and Watson, 1993, Shifley, 2004). Between 1990 and 2001, walnut mortality was observed in several entities of the United States of America (USA) and the death of trees was associated to the presence of the beetle Pityophthorus juglandis (Coleoptera: Scolitidae) on branches and trunks and the subsequent development of cankers. Around the galleries caused by an association between this and the fungus Geosmithia morbida Blackman (Fisher et al., 2013). From 2008 onwards, the disease got the common name of thousands of cankers disease (TCD), Due to the enormous amount of cankers that form in the bark of the affected trees. By 2013, TCD was found widely distributed in the USA (Fisher et al., 2013; Wiggins et al., 2014), and came to cause with a severity of up to 60% of the total population and a removal due to the downward death of 300 individuals in the states of Washington and Idaho (Tiserrat et al., 2011).
In Mexico, J. nigra is grown in the states of Puebla, Tlaxcala, State of Mexico, Oaxaca and Querétaro. In the state of Coahuila of Zaragoza, the main producing municipalities of this crop are Parras, Zaragoza, Muzquiz, Ramos Arizpe and Saltillo, with an area of 1 446 ha and an average yield of 1.9 t ha-1. It has been estimated that the losses of the fruit in this state reach up to 60%, where even the loss of trees occurs due to the descending death possibly caused to cankers present in branches. The objective of this investigation was to isolate, identify and determine the pathogenicity of the fungi present in cankers associated with the descending death of J. nigra in two Municipalities of Coahuila of Zaragoza, Mexico.
In April and August 2015, branches of 16 trees of J. nigra with symptoms of descending death were collected (Figure 1) in two 15-year-old orchards located in the municipalities of Arteaga and Saltillo. The fungal isolation was based on previously described procedures (Alvidrez-Villarreal et al., 2012; Fisher et al., 2013) with some modifications. Cankers were extracted from the internal tissue of branches, disinfected with 3% sodium hypochlorite and placed in Petri dishes with potato dextrose agar (PDA) culture medium, placing five pieces per box. After 24 h, fungal growths were transferred to new boxes and from the colonies obtained, monosporic strains were developed or from hyphal tips. The strains were kept active in PDA and were conserved in water. Four morphotypes were obtained and one strain of each was selected and used in subsequent analyzes. The strains were identified morphologically at the genus level as Trichothecium, Pestalotia and Alternaria and Rhizoctonia using keys for imperfect fungi (Barnett and Hunter, 2006).

Figure 1 Symptoms of descending death in Juglans nigra; a) tree with partial branch death; b) view of superficial lesion on green branch; and c) internal cankers.
Identity at the species level was based on the analysis of the sequence of the ITS-1 to ITS-2 region of the ribosomal DNA using the ITS1 and ITS4 primers (White et al., 1990). A consensus sequence of each fungus was obtained to perform a BLAST analysis (Basic Local Alignment Search Tool) in the database of the National Biotechnology Information Center (NCBI). The Pestalotia strain showed a relationship with six species and to determine its identity, a phylogenetic analysis was performed with the Neighbor-Joining method based on Kimura’s 2-parameter model and the Bootstrap test with 1 000 repetitions.
The pathogenicity tests were carried out using four-month-old healthy seedlings, developed from seed sown in sterile soil contained in half-liter unicel cups. Each strain was inoculated to five seedlings that previously had a wound in the stem of 3 mm in length with a knife for sterile scalpel. On the wound 1 ml of the suspension of 1 × 108 spores of Trichothecium, Pestalotia and Alternaria was inoculated, while for Rhizoctonia the inoculum consisted of an explant of 5 mm in diameter placed directly on the wound on the stem.
The control seedlings were inoculated with sterile distilled water over the wound. After the inoculation, the wound was covered with plastic to prevent the rapid drying of the inoculum and the wilting of the seedlings. The plastic was removed after a week. The treatments were distributed under a completely random design in a greenhouse. Every seven days during the 45 days after the inoculation (ddi) the seedlings were observed and the symptoms they showed were described. The incidence was registered as the percentage of plants (considering the plant established in each repetition) with some type of symptom in relation to the control. The severity was determined based on a visual scale of five values (1= 0-20%, 2= 21-30%, 3= 31-50%, 4= 51-80% and 5= more than 80% of the stem with lesion) and the data were analyzed by the Kruskal-Wallis test.
The height of the plant at 28 ddi was also analyzed because in the evaluation of the 35 ddi the plants of some treatments inoculated with fungi were already dead. The plants were extracted after 45 days and they were determined the length, fresh weight and dry weight of the root, as well as isolating fungi from tissue around the inoculated wounds to confirm the postulates of Koch. The data were subjected to a completely random analysis of variance and the comparisons of means were carried out using the Tukey test (p≤ 0.05). The analyzes, were performed with the statistical package Rdata, version 3.2.3 (Ihaka and Gentleman, 1996).
Strains of morphotypes isolated from cankers were morphologically identified as members of the genera Trichothecium, Pestalotia, Alternaria and Rhizoctonia; whereas, based on the BLAST analysis, the identity of the Trichothecium and Alternaria strains correspond to the species T. roseum and A. alternata, respectively. The Rhizoctonia strain showed a high relationship with R. solani; however, when verifying the number of nuclei in the analyzed strain, it was determined that it corresponds to an unidentified binucleated species of the genus Rhizoctonia (Sneh et al., 1991). The phylogenetic analysis of the Pestalotia strain showed more relationship with Pestalotiopsis steyaertii (=Neopestalotiopsis steyaertii according to Maharachchikumbura et al., 2014).
The first symptoms appeared at 7 ddi in seedlings inoculated with T. roseum, P. steyaertii and Rhizoctonia sp., and caused symptoms in 100% of the plants at 7, 14 and 21 ddi, respectively. While A. alternata caused the first symptoms at 14 ddi, and at 28 ddi it only caused symptoms at 60% of plants. The plants inoculated with water appeared apparently healthy (Figure 2A) during the experiment, while the inoculated fungi caused chlorosis (Figure 2B), stunting (Figure 2C) and defoliation (Figure 2D) in the first 35 ddi. Seedlings inoculated with T. roseum, P. steyaertii and Rhizoctonia sp. showed a reddish pigmentation at the apex (Figure 2E). After 45 days, all the plants inoculated with fungi died (Figure 2F), being T. roseum, P. steyaertii and Rhizoctonia sp., which they caused the death of all plants at 35 ddi and only survived the witness until the end of the experiment.
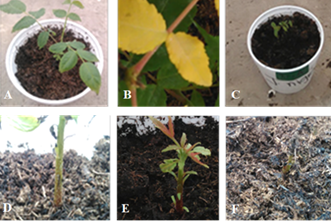
Figure 2 Symptoms in seedlings inoculated with strains of fungi isolated from cankers of branches of J. nigra. A) healthy plant (control) 45 days after inoculation; B) yellowing at 7 ddi in a seedling inoculated with T. roseum; C) reduction in the growth of the inoculated seedling; D) defoliation in inoculated seedling; E) seed apical part with reddish coloration; and F) inoculated dead seedling.
In the treatments inoculated with fungi the severity was greater while the height, length, fresh weight and dry weight of the root were lower, compared to the control (Table 1). The fungi isolated from plants inoculated with fungi corresponded to those inoculated, while it was not possible to isolate any of them from the control plants.
Table 1 Averages obtained from five variables evaluated after inoculation of J. nigra plants with four fungal species.
| Treatment | Severity | Height p= 0.001 | Root length p= 0.001 | Fresh weight of root | Dry weight of root |
|---|---|---|---|---|---|
| Control | 18 a | 6.18 a | 5.14 a | 1.1 a | 0.44 a |
| Alternaria alternata | 103.35 b | 2.84 b | 3.12 b | 0.66 bc | 0.21 ab |
| Pestalotiopsis steyaertii | 106.21 b | 3 b | 2.16 bc | 0.82 ab | 0.21 ab |
| Trichothecium roseum | 106.21 b | 1.82 c | 0.52 d | 0.33 c | 0.06 b |
| Rhizoctonia sp. | 106.21 b | 2.6 b | 1.65 c | 0.4 c | 0.04 b |
It has been reported that A. alternata is capable of causing cankers in the apple tree (Abou Al Fadil et al., 2010), T. roseum in rose (Sweets et al., 1982), and Pestalotiopsis species in various plant species (Espinoza et al., 2008; Patel et al., 2013), but no reports of binucleated Rhizoctonia species associated with cankers in plants were found. The results obtained suggest that these species are associated agents responsible for the downward death of J. nigra, due to the pathogenic capacity that caused the presence of various symptoms.
Conclusions
In none of the samples obtained from J. nigra in Coahuila, Mexico, it was possible to identify the fungus G. morbida, the main cause of descending death in this tree in other countries. The identification and pathogenicity tests carried out with T. roseum, P. steyaertii, A. alternata and a binucleated species of Rhizoctonia, show that these fungi occur in cankers associated with the descending death of J. nigra, and are pathogenic to this plant. It is considered that this is the first report of T. roseum., P. steyaertii., A. alternata y Rhizoctonia sp. as pathogens of J. nigra.
Literatura citada
Abou Al Fadil, T.; Naffaa, W.; Abou Fakher, T.; Muzher, B. and Amer, H. 2010. Apple stem canker disease in Sweida: the causal fungus and the susceptibility of some apple varieties to the disease. Arab J. Plant Protec. 28:16-19. [ Links ]
Alvidrez-Villarreal, R.; Hernández-Castillo, F. D.; García-Martínez, O.; Mendoza-Villarreal, R.; Rodríguez-Herrera, R. and Aguilar, C. N. 2012. Isolation and pathogenicity of fungi associated to ambrosia borer (Euplatypus segnis) found injuring pecan (Carya illinoensis) wood. Agric. Sci. 3:405-416. DOI: 10.4236/as.2012.33048. [ Links ]
Barnett, H. L. and Hunter, B. B. 2006. Illustrated genera of imperfect fungi. Fourth edition. The Am. Phytopatol. Soc. 218 p. [ Links ]
Espinoza, J. G.; Briceno, E. X.; Keith, L. M. and Latorre, B. A. 2008. Canker and twig dieback of blueberry caused by Pestalotiopsis spp. and a Truncatella in Chile. Plant Dis. 92:1407-1414. [ Links ]
Fisher, J. R.; McCann, D. P. and Taylor, N. J. 2013. Geosmithia morbida, Thousand Cankers Disease of Black Walnut Pathogen, Was Found for the First Time in Southwestern Ohio. Plant Health Progress. 12 p. [ Links ]
Gilman, E. F. and Watson, D. G. 1993. Juglans nigra: Black Walnut. Fact Sheet ST-320. Environmental Horticulture Department, Florida Cooperative Extension Service. Institute of Food and Agricultural Sciences. University of Florida. 85 p. [ Links ]
Ihaka, R. and Gentleman, R. 1996. R: a language for data analysis and graphics. J. Comp. Graphical Statistics 5: 299-314. [ Links ]
Maharachchikumbura, S. S. N.; Hyde, K. D.; Groenewald, J. Z.; Xu, J. and Crous, P. W. 2014. Pestalotiopsis revisited. Stud. Mycol. 79:121-186. Doi: 10.1016/j.simyco.2014.09.005. [ Links ]
Michler, C. H.; Woeste, K. E. and Pijut, P. M. 2007. Black walnut (Juglans nigra L.). In: Kole, C. (Ed.). Genome mapping and molecular breeding in plants. Forest trees. Springer Berlin Heidelberg New York. Vol. 7. 189-198 pp. [ Links ]
Patel, J. S.; Norman, D.; Brennan, M. and Ali, G. S. 2013. First report of elm canker caused by Pestalotiopsis mangiferae in the United States. Plant. Dis. 97:426. [ Links ]
Šálek, L. and Hejcmanová, P. 2011. Comparison of the growth pattern of black walnut (Juglans nigra L.) in two riparian forests in the region of South Moravia, Czech Republic. J. For. Sci. 57:107-113. [ Links ]
Shifley, S. R. 2004. Black walnut in a new century. In: Proceedings of the 6th Walnut Council research symposium. St. Paul, Department of Agriculture, Forest Service, North Central Research Station. Lafayette , 25-28. 188 p. [ Links ]
Sneh, B.; Burper, L. and Ogoshi, A. 1991. Identification of Rhizoctonia species. The American Phytopathological Society. St. Paul, Minnesota, USA. 133 p. [ Links ]
Sweets, L. E.; Pfleger, F. L.; Morgan, F. C. and Mizicko, J. R. 1982. Control of fungi associated with cankers of greenhouse roses. Plant Dis. 66:491-494. [ Links ]
Tisserat, N.; Cranshaw, W.; Putnam, M. L.; Pscheidt, J.; Leslie, C. A.; Murray, M.; Hoffman, J.; Barkley, Y.; Alexander, K. and Seybold, S. J. 2011. Thousand cankers disease is widespread in black walnut in the western United States. Plant Health Progress. Doi: 10.1094/PHP-2011-0630-01-BR. [ Links ]
White, T. J.; Bruns, T.; Lee, S. y Taylor, J. 1990. Sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M. A.; Gelfand, D. H.; Sninsky, J. J. and White, T. J. (Eds.). PCR Protocols: a guide to methods and applications: Academic Press, Inc. 315-322 pp. [ Links ]
Wiggins, G. J.; Grant, J. F.; Lambdin, P. L.; Merten, P.; Nix, K. A.; Hadziabdic, D. and Windham, M. T. 2014. Discovery of walnut twig beetle, Pityophthorus juglandis. Associated with forested black walnut, Juglans nigra, in the Eastern USA. Forests. 5:1185-1193. [ Links ]
Received: April 2018; Accepted: July 2018











 texto en
texto en 


