Introduction
Petroleum diesel or fossil diesel is the most common type of diesel fuel [1,2]. It’s produced from fractional distillation of crude oil between 350 oC (662 oF) and 200 °C (392 oF) at atmospheric pressure [3], resulting in a mixture of carbon chains that typically contain between C9 and C21 per molecules [3,4]. The standard specification for diesel fuel oils is ASTM D 975 (American Society of Testing and Materials) [5]. ASTM D 975 Contains a set of physical, chemical, and performance specifications, established by the society to meet the approval requirements of ASTM procedures and regulations. The ASTM is not a regulatory or enforcement agency, but their standards have been adopted by several government agencies [6-8], it is also one of the largest voluntary standards developments systems in the world [5,9].
The diesel fuels are “straight-run” stocks, derived from simple fraction distillation of crude oil [10,11]. Many commercial fuels contain a proportion of catalytically-cracked material to extend the yield and enhance the cetane number [12,13].
The most important properties of diesel fuels are [14] viscosity, heating value, ignition quality, volatility, cetane number, lubricity, low-temperature flow, component compatibility, storage stability, and sulfur content [15].
Andrade et al. 2014 [16] have described the hydrodesulphurization (HDS), which is currently the most common method used by refineries to remove sulfur compounds from petroleum fractions. However, it is not highly effective for removing thiophene compounds such as benzothiophene additionally; this process generates high costs for the oil industry [17,18].
Regarding the opportunity to improve the uses of radioactive energy, which is introduced in different processes of preparation, analysis, and treatment of several materials, it is required to study the influence of various types of radiation on crude oil and petroleum products, although the radiolysis process is diverse and complicated. The effect of gamma radiation on petroleum products has previously studied [19-31]. They are not fully described the mechanism after exposure petroleum product on the gamma irradiations and is still the published research is not enough. Luana et al. 2015 [32] have used ionizing radiation in order to study the influence on the degradation of diesel and petroleum sulfur compositions. They have used the absorbed doses of 30 kGy and 50 kGy in two rate doses (1.27 and 1.45 kGy/h). They have concluded that the high efficiency of ionizing radiation was observed regarding the degradation of sulfur compounds such as benzothiophene and benzenethiol, besides 1.2-dimethyl benzene and toluene have observed as fragments. Not satisfactory results for reduction of sulfur compounds by gamma radiation in diesel have been observed comparing with the crude oil [32]. That's why in the current research we didn't study the degradation of sulfur compounds and we focused only on the conversion of branched and cyclic compounds to the linear hydrocarbons because these compounds effecting negatively on cetane number [33,34,35]. Jabbarova in 2019 and 2020 [19, 36] has studied the effect of ionizing gamma rate and absorbed doses on the Physico-chemical characteristics of diesel and gasoline, using cobalt-60 γ-radiation source at a dose rate of P = 0.18 kGy/s in temperature with various absorbed doses D = 15-150 kGy. Because she hasn’t studied the full characteristics, she wasn't able to conclude the final effect, and she has concluded that the processes related to radiolysis can develop for a long time after the cessation of irradiation to cause changes in the composition of the fuel.
Diesel fuel improves based on gamma irradiation by degradation of the branched, cyclic, and aromatic compounds to form high linear chain hydrocarbons, which improve the cetane number [15]. In contrast, gasoline needs the opposite reaction because these compounds increase the octane number therefore improve all physicochemical characteristics [37,38].
Ezeldin et al. 2015 [37] also studied the effect of x-ray radiation on diesel fuel at one hour, to improve the physico-chemical properties of diesel fuel, the diesel fuel produced from Khartoum refinery (Sudan). They found that the cetane number of diesels has been improved to 61 after exposure to x-ray radiation, as well as physico-chemical properties of sample after the expos process were improved to the limits assigned by ASTM and Khartoum refinery [37].
Herein, ionizing radiation was used in order to study its effect on the degradation of diesel compounds without any pretreatment using cobalt -60 gamma cell in a batch system at different absorbed doses: 3, 6, 10, and 15 kGy, with dose rates 2.27, 4.5, 7, and 1.15 kGy/h respectively. Also, study the influence of gamma radiation on physico-chemical properties of petro-diesel fuel.
Experimental
Physico-chemical characteristics of used sample were tested according to American Society for Testing and Materials (ASTM) [5].
Physico-chemical parameters of diesel fuel
Density of diesel Fuel
Density of diesel samples before and after exposure at 15 °C was investigated by Anton - paar densitometer. The sample was introduced into a U-shaped borosilicate glass tube that is being excited to vibrate at its characteristic frequency. The characteristic frequency changes depending on the density of the sample. Through a precise determination of the characteristic frequency and a mathematical conversion, the density of the sample was measured [38].
The density was calculated from the quotient of the period of oscillations of the U- Tube and the reference oscillator [38]:
Where, KA, KB are Apparatus constants,
Distillation of diesel fuel (ASTM D86)
The diesel sample (100 ml) was put in the distillation flask when it was heated up to initial boiling point (IBP), the sample was evaporated by further heating and afterwards liquefied by means of running through the condenser unit. Recorded volumes of 10 ml, every subsequent 10ml interval to 90 ml, 97 ml to the end point (3 ml lost by ignition or remain with the tube).
The temperatures associated with each incremental volume percentage recovered were converted to incremental temperatures for each incremental volume percentage evaporated by correcting for any sample loss during the test. At the end of the distillation, the observed vapor temperatures were corrected for barometric pressure and the data were examined for conformance to procedural requirements, Such as distillation rates. Test results were commonly expressed as percent recovered versus corresponding temperature, either in a table or graphically, as a plot of the distillation curve [5].
Flash point of diesel Fuel (ASTM D93)
The flash point of diesel fuel was determined using passkey-martens flash point APP. A brass test cup of specified dimensions, filled to the inside mark with test specimen and fitted with a cover of specified dimensions was heated and the specimen was stirred at specified rates, by either of two defined procedures (A or B). An ignition source was directed into the test cup at regular intervals with simultaneous interruption of the stirring, until a flash was detected [5,39,40].
Calorific value
The calorific value was investigated from the heat of combustion, which is measured from the heat of reaction. It is determined from the value of enthalpy change for the reaction at constant pressure and temperature. At constant pressure system, the enthalpy change is obtained from the equation [41, 42].
Where:
The calorific value of liquid fuel was measured by the bomb calorimeter. The total quantity of heat generated by combustion including the heat needed to vaporize the water is obtained, which is called gross calorific value [43].
These measurements are obtained by burning a representative sample in a high-pressure oxygen atmosphere within a stainless-steel pressure vessel or bomb. The heat released by this combustion was absorbed by water within the calorimeter and the resulting temperature change of water is noted [44].
Kinematic viscosity of diesel fuel (ASTM D445)
The time was measured for a fixed volume of liquid to flow under gravity through the capillary of a calibrated viscometer under a reproducible driving head controlled and known temperature. The kinematic viscosity was measured by flow time and the calibration constant of the viscometer [5].
Cetane number of diesel fuel
Cetane numbers can only be measured by a laboratory engine test. Usual methods, such as using a cetane index (a calculation using API gravity and the distillation mid-point) cannot measure cetane numbers accurately when using cetane improvers. The most commonly used correlation is the ASTM D47374, 5 equations. The D4737 equation correlates the API gravity and the T 10, T 50, and T 90 points on the boiling curve to the CN of the fuel and is summarized in equation (3) [5,45].
Where,
| CN= Calculated Cetane Number. | T50 is the 50 % (v/v) distillation recovery temperature, in degrees Celsius. |
|
T10N = T10 - 215. T50N = T50 - 260 ; T90N = T90 - 310. |
T90 is the 90 % (v/v) distillation recovery temperature, in degrees Celsius. |
| T10 is the 10 % (v/v) distillation recovery temperature, in degrees Celsius |
|
Exposing diesel fuel Sample to gamma radiation
Diesel samples were irradiated using a cobalt-60 gamma cell in a batch system at absorbed doses of 3, 6, 10 and 15 kGy. All the irradiations were performed in a batch system; gamma irradiation was carried out at room temperature using acobalt-60 gamma irradiator, gamma cell type B (U). The diesel samples were placed in completely filled 500ml vials. The delivered doses for B (U) were 3, 6, 10, and15 kGy with dose rates 2.27, 4.5, 7, and 11.15 kGy/h respectively.
Gas Chromatography Mass Spectrometry (GC/MS) analysis of diesel fuel
The samples before and after Irradiation were analyzed using GC/MS from Shimadzu (GC/MS-QP2010Ultra) Japan with serial number (020525101565SA). The sample was injected into fused silica capillary column (Rtx-5MS, Length (30 m), and diameter (0.25), film thickness 0.25 μm). Helium was the gas carrier at a constant velocity of 46.3 cm/s. The temperature program was started at 60 °C programmed to 300 °C at 10 °C/min [46].
Results and discussion
Influence of gamma irradiation on density of diesel fuel
Determination of the density of petroleum and its products is necessary for the conversion of measured volumes to weight at the standard temperature of 15 °C and gives evidence of quality of fuel [47]. The effect of different doses of gamma irradiation on diesel fuel samples is shown in Fig. 1.
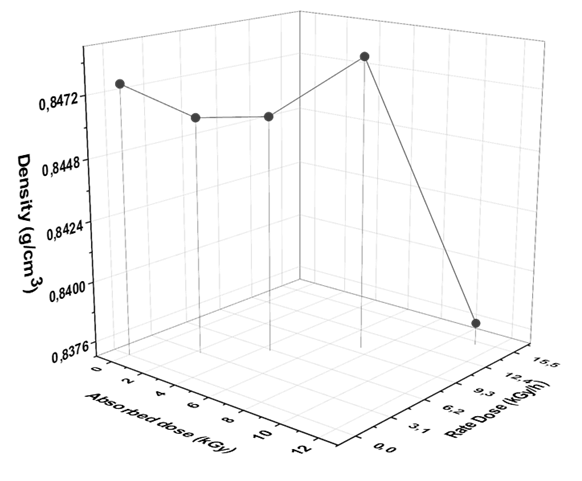
Fig. 1 Dependence of density of diesel fuel on different rate and absorbed doses of gamma irradiation.
Density of fuels is an important quality characteristic, which is used for the description the petroleum products and to calculate other physico-chemical characteristics [34]. The density of diesel has a direct effect on the other physico-chemical characteristics, and it uses in the calculation of cetane number that determines the quality of diesel fuel. Fig. 1 reveals that the density of diesel fuel was decreased after exposure to 3, 6, and 15 kGy of gamma radiation but at 10 kGy it was almost the same. The behavior of decreasing density may attribute to degradation of organic compounds of diesel fuel. Moreover, it improves with increasing of rate dose (2.27, 4.5, 11.15 kGy/h) but the density wasn’t affected when it’s exposure to 10 kGy by dose rate 7.5 kGy/h. This behavior can be attributed to the broken and formed bonds as a result of high energy dose and the formed fragments at (10 kGy; 7.5 kGy/h) have made a new compound that have a physical parameter similar to diesel sample before exposure or the same compounds formed. Although, the density of fuel increased after exposure to 10 kGy but all recorded densities were found to be within the write permissible limits assigned by ASTM (>0.9 g/ml).
Influence of gamma irradiation on distillation points of diesel fuel.
The volatility (distillation) properties of petroleum products have an important influence on their performance and safety, especially in the case of fuels [48]. The presence of high boiling point components in fuels can significantly influence the degree of formation of solid combustion deposits [47]. The distillation characteristics are critically important for both automotive and since it affects starting, warm-up, and tendency to vapor lock at high operating temperature or at high altitude, or both [48]. The effect of different absorbed and rate doses on recovered distillation points (0 % (Initial boiling point), 20, 50, 90, and 95 %) is represented Fig. 2.
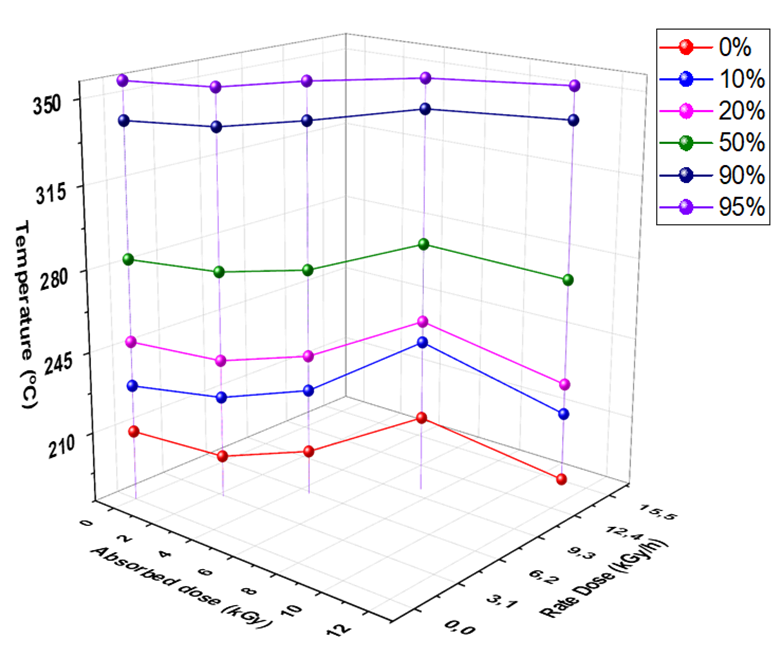
Fig. 2 Dependence of temperature recovered points of diesel fuel on different rate and absorbed doses of gamma irradiation.
Fig. 2 illustrate that the temperature recovered points decreased after exposure to 3 (2.227 kGy/h), 6 (4.5 kGy/h), and 15 kGy (11.15 kGy/h). The sample which exposure to the highest rate (11.15 kGy/h) showed the lowest recovery distillation temperature, whereas the recovered temperature increased at rate dose 7.5 kGy/h, it’s higher than the row sample. Furthermore, this result supports the obtained results for density of diesel at the same rate and absorbed dose because the density of produced fragments influences on the boiling points of diesel sample. The rate dose (11.15 kGy/h) showed the best recovery distillation temperature. All distillation results improved within the right permissible limits assigned by ASTM [5]. It will be interested to use this rate dose and apply it at different time less and more than the time of the current study (1.3 h), with different adsorbed doses.
Influence of gamma irradiation on viscosity of diesel fuel
Viscosity is one of the important physical characteristics of diesel fuel. The fuel which is having higher viscosity can make damage in the fuel pump for example follower and cam wear due to higher pressure. Besides, the lowest viscosity fuel may lead to a lack of lubrication in engines. It also influences the fuel delivery rate and the atomization of the fuel during injection. That's why the ASTM put a limts for diesel fuel (2.2 - 8.8 mm2/s) [49]. The effect gamma irradiation rate and absorbed doses on kinematic viscosity of diesel fuel is shown in Fig. 3.
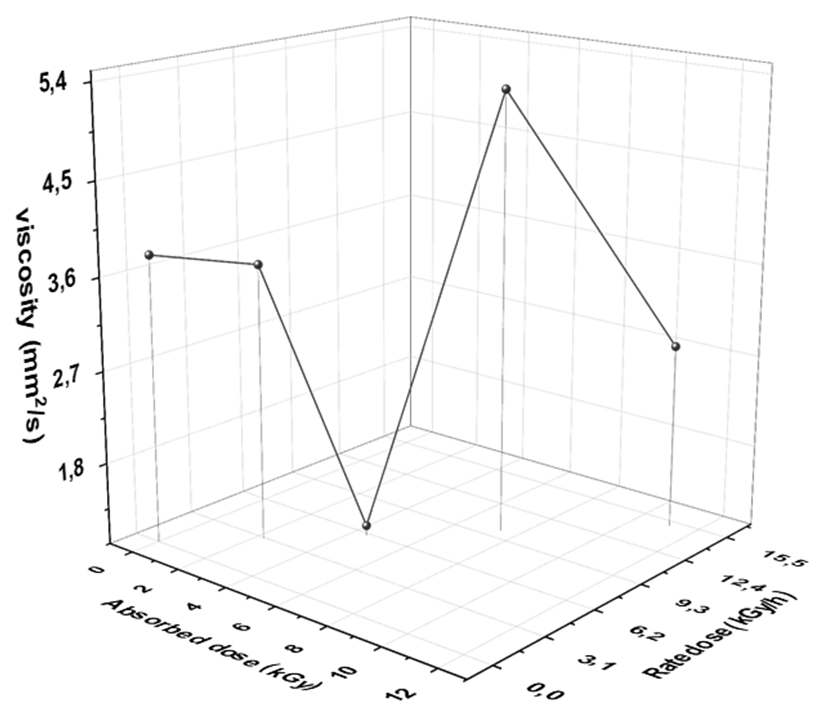
Fig. 3 Dependence of kinematics viscosity of diesel fuel on rate and absorbed doses of gamma irradiation.
Fig 3 shows that the viscosity of diesel fuel sample gave a strong indication for improving the quality of diesel after exposure to gamma radiation with doses 3 (2.227 kGy/h), 6 (4.5 kGy/h) and 15 kGy (11.15 KG/h), because the viscosity decreased. The highest result obtained after exposure to 10 kGy (7.5 kGy/h) = 5,269 mm2/S, the highest viscosity of diesel fuel attributed to the formed fragments and corresponding isomers compounds. The results obtained from the kinematic viscosity agree with ASTM specification (see table 1). From the results obtained from the investigation of density, distillation, and viscosity we can expect that the highest quality diesel produced after exposure to the rate doses (2.227, 4.5, and 11.15 kGy/h) but the quality of diesel sample will decrease after exposure to the rate dose (7.5 kGy/h).
Table 1 The ASTM permissible limits for diesel fuel.
| Prosperity | Unit | Value |
|---|---|---|
| Density@15 oC | g/ml | max 0.9 |
| Kinematic viscosity | mm2/ S | 2.2 - 8.8 |
| Flash point | oC | 57-98 |
| Distillation | ||
| 10 % Recovered | oC | max 250 oC |
| 50 % Recovered | oC | max 300 oC |
| 90 % Recovered | oC | max 350 oC |
| 95 % Recovered | oC | max 370 oC |
| Calorific value | J/Kg | 43-50 |
| Cetane number | - | min 45.0 |
Influence of gamma irradiation on flash point of diesel fuel
Flash point temperature is an assessment for the overall flammability hazard of materials [32], it’s the lowest temperature at which it will produce enough vapor to produce a flammable mixture in the air. The lower the flash point temperature, the easier it is to ignite the air if an ignition source is present. The higher the flashpoint, the safer the material is to handle. The flashpoint of any liquid can change as the pressure in the air around it changes. High flash point temperature leads to the delay of flammability. Likewise, the low flash point causes ignite of fuels in the fuel stores, which lead to explosions [50]. Influence of absorbed and rate doses on flash point temperatures is represented in Fig. 4.
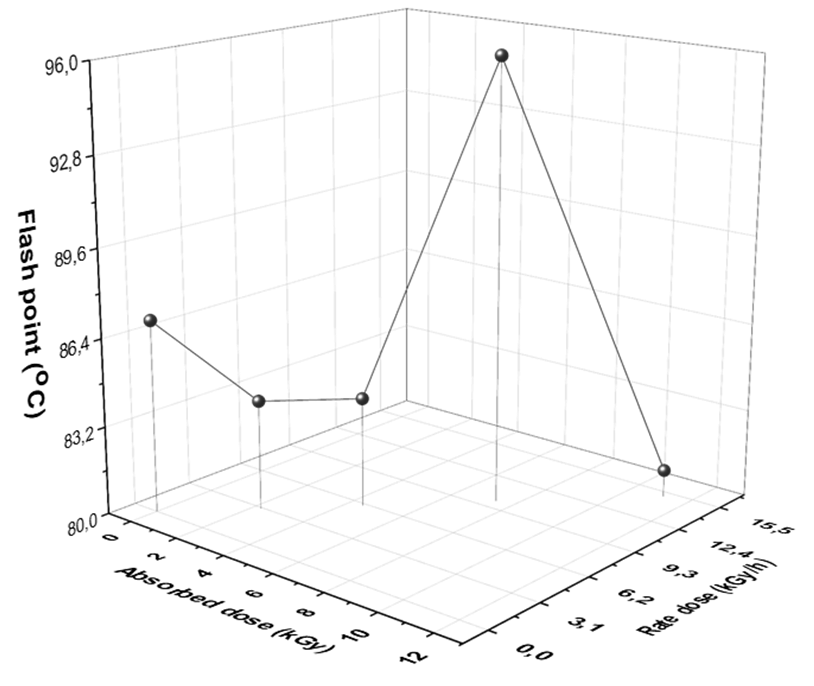
Fig. 4 Dependence of flash point temperature of diesel fuel on different rate and absorbed doses of gamma radiation.
Fig. 4 shows that the flash point of diesel fuel decreased after exposures to 3 (2.227 kGy/h), 6 (kGy/h), and 15 kGy (kGy/h), but it increased at 10 kGy (7.5 kGy/h). Furthermore, the obtained results from density, distillation, and viscosity as well as flash point can support us to believed that the isomerization which is formed from ionization reaction of diesel after exposure is formed more branched or cyclic compounds after exposure to 10 kGy because these compounds effects negatively on physical properties of diesel fuel as well as cetane number. The flash point of samples before and after exposure agrees with ASTM specification (see table 1).
Influence of gamma irradiation on calorific value of diesel fuel
Calorific value (CV) is a determination of heating power and is dependent upon the composition of the gas. The CV represents to the amount of energy released when a known volume of gas is completely combusted under control conditions [35]. The CV of fuel depends on the heat of reaction and the type of exothermic reaction. Influence of gamma irradiation rate and absorbed doses on calorific value is represented in Fig. 5.
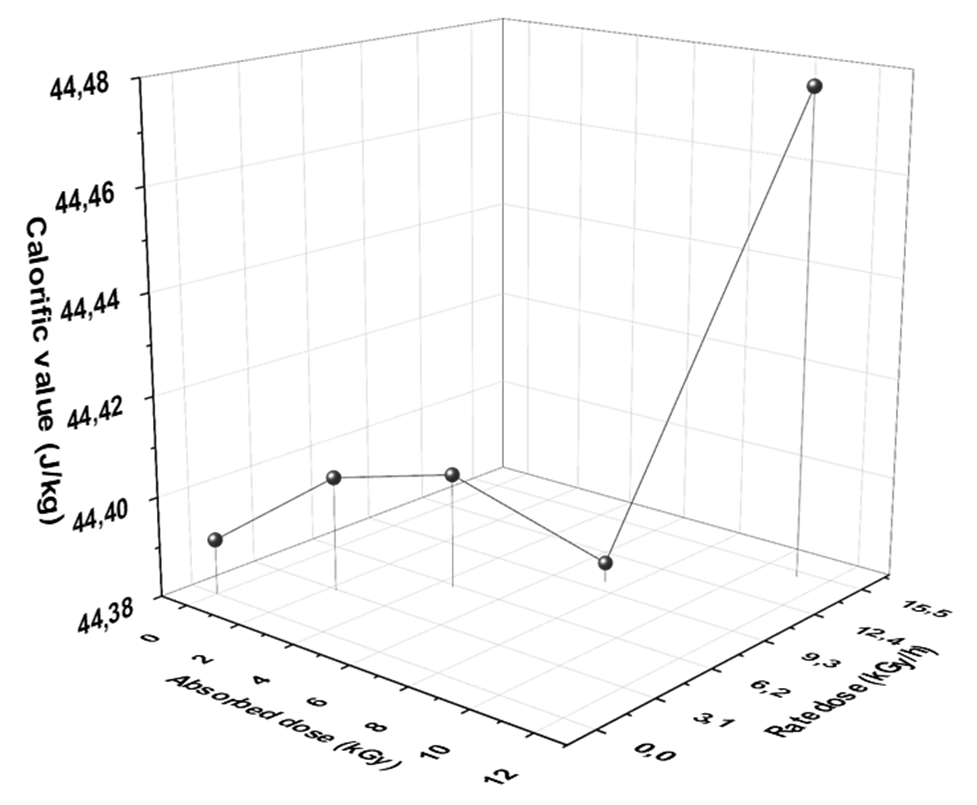
Fig. 5 Dependence of calorific value (CV) of diesel fuel on different rate and absorbed doses of gamma radiation.
From Fig. 5, the CV increased after exposure to 3, 6, and 15 kGy. The highest CV observed at 15 kGy (48.8 J/Kg). Likewise, the CV decreased at 10 kGy. The disadvantage of 10 kGy dose can be attributed to the same reasons that explained on paragraph (3.4). However, the CV of diesel fuel under study agrees with right permissible limits assigned by ASTM.
Influence gamma irradiation on cetane number of diesel fuel
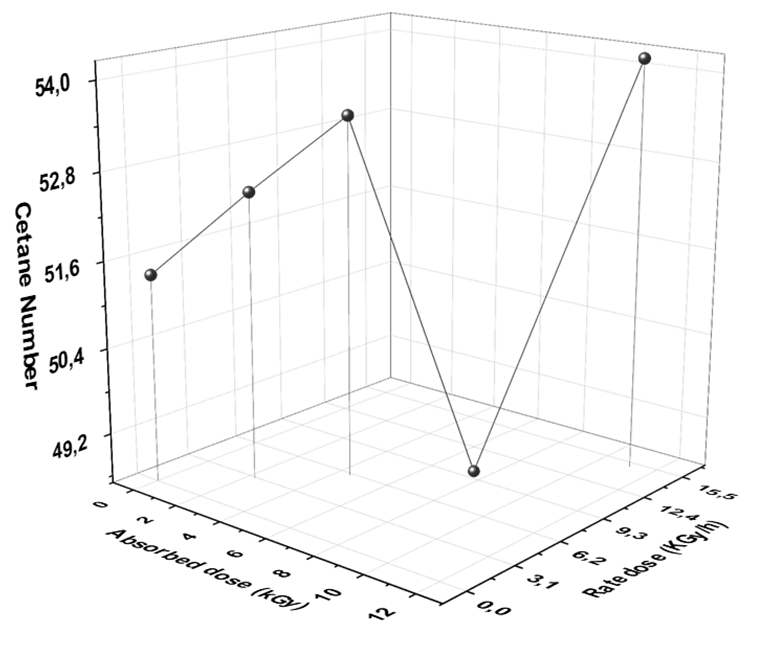
Cetane number (CN) is a measurement of performance or quality of petro- or bio-diesels fuel. The higher CN, the better in fuel burns within the engine of a vehicle. The difference between CN and octane number (ON) is that ON rates gasoline whereas cetane rates diesel [14]. Moreover, just as higher performance gasoline vehicles require fuels with a higher ON, high performance diesel vehicles require fuel with a higher CN. However, the cetane number measures the delay in the ignition time of the fuel. In other words, it is how minimized the delay is between when the fuel is injected into the chamber and when the combustion begins [17]. Unlike gasoline engines which attempt to resist any ignition due to compression, diesel engines rely on this compression ignition and thus no spark is involved. A higher CN means the time between when the fuel is injected into the combustion chamber and when the fuel ignites is minimized. This means the fuel has the ability to ignite more easily and readily due to compression. This shorter delay time results in more complete fuel combustion [42]. The Cetane number of diesel fuel before and after exposure to different doses of gamma radiation is shown in Fig. 6.
From Fig. 6, the cetane number of diesel sample increased from 51.4 to 52.7, 53.7, and 54.2 after exposing to 3, 6, and 15 kGy (2.27, 4.5, and 11.15 kGy/h), respectively, whereas CN of diesel fuel decreased after exposure to 10kGy, this results is expected from the physical characteristics because the CN depends on density, kinematic viscosity, flash point, distillation, and CV, besides the samples which is exposure to 10 kGy (7.5 kGy/h) recorded negative results. Moreover, we can interpret the cetane number as well as the other physico-chemical results by analysis the formed organic compounds after absorbed doses. If the amounts of branched, cyclic, and aromatic compounds increasing, the quality of diesel fuel will decrease because the cetane number improves with inhibition of isomerization and conversion of cyclic and aromatic compounds into linear chain hydrocarbons [15, 34, 50-53].
Effect of Gamma Irradiation on Organic compounds of diesel fuel
As we mentioned above the liner chain organic compounds increase CN. In contrast, branched, cyclic, and aromatic organic compounds lead to decrease the CN, consequently the quality of diesel decreases. The organic compounds of biodiesel which were determined by GC/MS technique plotted against the irradiation rate and absorbed doses are shown in Fig. 7 (a), (b).
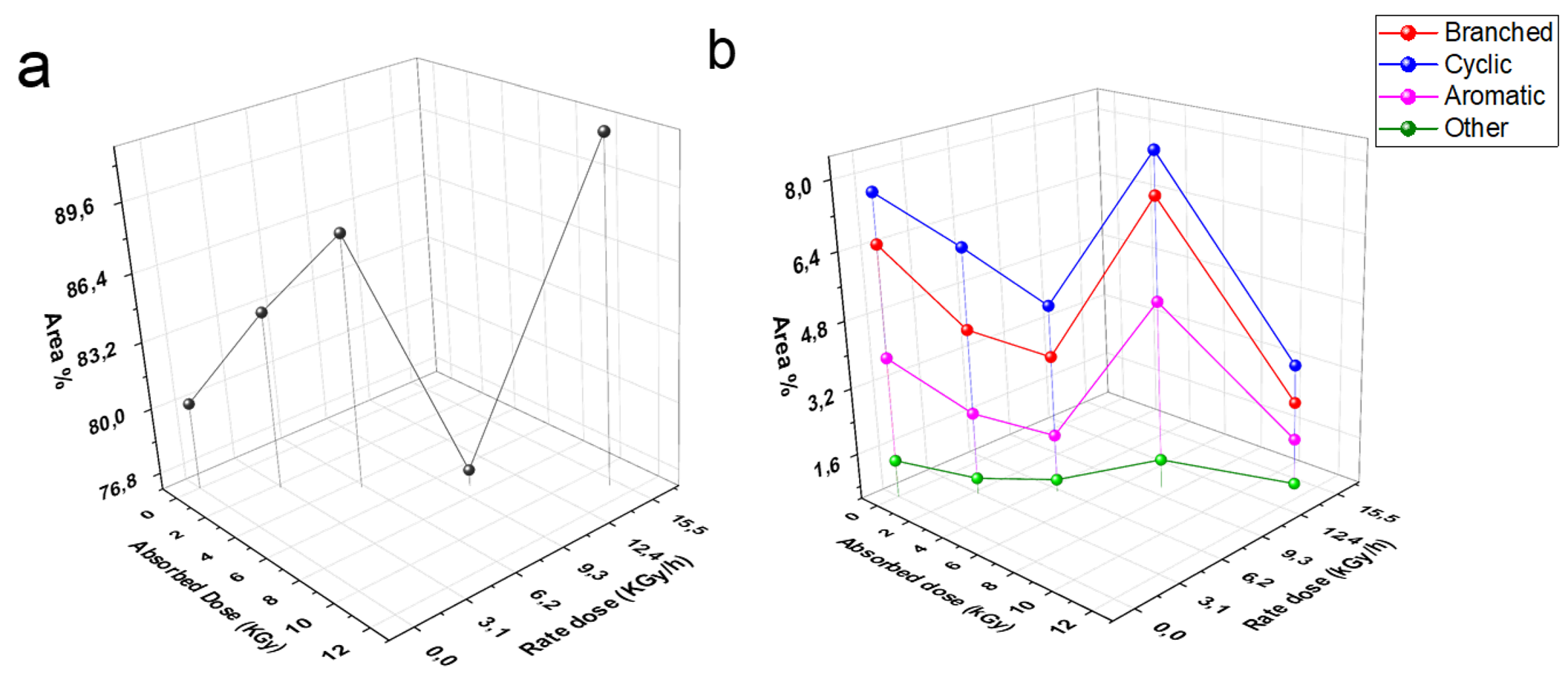
Fig. 7 Effect of Irradiation doses on linear chain organic compounds (a); branched, cyclic, anaromatic, and other organic compounds (b).
The linear chain organic compounds of diesel fuel under studying increased after exposure to 3, 6, 10, and 15 kGy (2.27, 4.5, and 11.15 kGy/h), whereas decreased at 10 kGy (7.5 kGy/h) (Fig. 7(a)). In contrast, branched, cyclic, aromatic, and other organic compounds decreased at 3, 6, and 15 kGy, but they were increased at 10 kGy (Fig. 7(b)). However, we believe that the formed fragments and isomerization reaction in diesel fuel after exposure to 3, 6, and 15 kGy (2.27, 4.5, and 11.15 kGy/h) produced high linear chain organic compounds (Fig. 7(a)), also the gamma radiation in these doses played a role in conversion of some cyclic, and branched organic compounds into linear hydrocarbons, which support increasing of cetane number (Fig. 7(b)). In contrast, the same reactions produce branched, cyclic, or aromatic compounds in the case of 10 kGy (7.5 kGy/h) and thus compounds affect negatively on CN [34, 51].
Conclusion
The effect of rate and absorbed doses of gamma irradiations on the physico-chemical characteristics and organic compounds of petro-diesel fuel using constant time of exposure (1.3 h) has been studied. The cetane number of diesel fuel increased after exposure to absorbed doses 3, 6, and 15 kGy (corresponding rate doses: 2.27, 4.5, and 11.15 kGy/h, respectively) but decreased at 10 kGy (7.5 kGy/h). These results can be attributed to the broken and formed bonds as a result of the high energy doses. Improvement accomplished by 3, 6, 15 kGy (2.27, 4.5, and 11.15 kGy/h) has converted some of branched, cyclic, and aromatic compounds to linear chain hydrocarbons. Such compounds exhibit a negative effect on the cetane number of diesel fuel. The negative results of (10 kGy; 7.5 kGy/h) can be attributed to the formed fragments and corresponding formed isomers which has observed as branched, cyclic, and aromatics compounds. Although these results are very promising, some more studies and improvements are needed by using constant rate dose to study the radio mechanism and overall effect.











 nueva página del texto (beta)
nueva página del texto (beta)