Introduction
Chlorophenols originated from pulp and paper industries, petrochemicals, agrochemicals, plastic industries, preservatives industries as industrial waste being used as pesticide, herbicides, insecticides etc., are a group of chemicals in which chlorines (between one and five) have been added to phenol. The chlorophenols are toxic to aquatic organisms with possible long-term adverse effects. Dichlorophenol (DCP) and its solutions are toxic in contact with skin and causes severe skin burns and eye damage. So, it is necessary to treat effulents containing chlorophenols prior to its discharge into water.
Liquid )molten) 2,4-DCP is readily absorbed through the skin and contact with large amounts may be fatal [1] Solid 2,4-DCP is not readily absorbed through skin. This is primarily caused by instantaneous renal failure, liver damage and various other organ failures. Repeated exposure may affect the nervous system causing headache, dizziness, nausea, vomiting, weakness and even coma.
Different types of physicochemical techniques such as chemical oxidation, coagulation, chlorination, solvent extraction, liquid membrane permeation and adsorption [2] are being executed in removing chlorophenol from aqueous solutions. Among these techniques adsorption is a well-established and powerful technique for treating domestic and industrial effluents by using adsorbents such as activated carbon, peat, chitin, clay and others.
As it was mentioned earlier that the chlorophenols are found in the industrial effluents causing serious detrimental effect on the living organism as well as the human being several types of adsorbents have been investigated to remove these organic pollutants from water. However, the bioadsorbents have been proved to be the effective materials to remove chlorophenols from the wastewater.
Activated carbon has been the most widely used adsorbent because of its high capacity for the adsorption of organic species. However, due to the difficulty and expense involved in regeneration, used black tea leaves (UBTLs) are considered as alternative low-cost absorbent. Since UBTLs can easily be obtained from the local market and because of its easy processing, this is now being executed in the removal of chlorophenols from water by implementing adsorption technique.
Sameer Al-Asheh et al. [3] investigated the removal of 2,4-dichlorophenol from aqueous solution by using physically and chemically activated oil shale. These authors not only found that the sorption of 2,4-DCP had been negatively influenced by an increase in initial pH but also observed that the rate of 2,4-DCP sorption onto ZnCl2-OS (zinc chloride -oil shale) was faster than that by Pyr-OS (pyrolyzed oil shale). The adsorption of phenol and dichlorophenols(2,5 DCP and 3,4-DCP) from water by a porous clay heterostructure (PCH) were studied [4].The adsorption capacity showed by the PCH for both phenol and DCPs from water (48.7 mg/g for 3,4-DCP,45.5 mg/g for 2,5-DCP and 14.5mg/g for phenol), suggests that the PCH have both hydrophobic and hydrophilic characteristics, due to the presence of silanol and siloxane groups formed during the pillaringand preparation of the PCH. Adsorption equilibrium, kinetics and thermodynamics of 2,4-dichlorophenol (2,4-DCP) onto bituminous coal based Filtrasorb-400 grade granular activated carbon, were studied in aqueous solution in a batch system with respect to temperature [5]. The obtained values of thermodynamics parameters showed that adsorption of 2,4 DCP is an endothermic process. Synthetic resin (XAD) used for comparative studies was not found efficient to adsorb 2,4-DCP compared to activated carbon. Xuequan Zou et. al. [6] investigated the adsorption of 2,4-dichlorophenol(2,4-DCP) onto microwave modified carbon (AC) at three different temperatures (303K,313K and 323K). The thermodynamic parameters of the adsorption process were estimated, showing that the adsorption of 2,4-DCP was exothermic and spontaneous and the adsorption studied in this paper can be assigned to a physisorption mechanism. F.W. Shaarani and B. H. Hameed [7] modified an oil palm empty fruit bunch-based activated carbon using ammonia solution for the adsorption of 2,4-DCP. The monolayer adsorption capacity of modified activated carbon µmAC) was found to be 285.71 mg/g at 30ºC. The surface modification of the activated carbon using ammonia was shown to be able to increase its adsorption capacity for 2,4-DCP. Hanzhong Jia and Chuanyi Wang [8] utilized a multi-functional organo -smectite template zero-valent iron composite for the adsorption and dechlorination of 2,4-DCP. The results demonstrate that hydrophobic conditions in clay interlayer facilitate the 2,4-DCP adsorption. Furthermore, organo-smectite -ZVI provides strong adsorptive affinity to 2,4-DCP and its reaction products. This is beneficial for the long -term stability to remove contaminants. The adsorption characteristics of 2,4-DCP on ACMB (activated carbon derived from moso bamboo processing waste) were evaluated from various perspectives [9]. The ACMB with particle size of 10-16 mesh was found to be adsorbing 2,4-DCP, and the adsorption capacity decreased considerably with increasing solution pH. T. S. A. Islam et. al. [10] carried out the adsorption of 2,4-dichlorophenol (2,4-DCP) from aqueous solution on ferric oxide in a batch process. The adsorption of 2,4-DCP on ferric oxide was found to increased with increasing pH of the solution from 3.0-5.0 but further increase of solution pH from 5.0-8.0 decreased the adsorption. Electrolytic effect showed that the adsorption was favorable in presence of NO3 - ion as compared with Cl- ion. Li Wang, Jian Zhang et.al. [11] studied Mn-modified activated carbon (PLAC-Mn) which was prepared by impregnating Polygonumorientale Linn activated carbon (PLAC) with a MnNO3 solution. 2,4-DCP adsorption capacity of PLAC-Mn was higher as compared to plain PLAC. The adsorption kinetics followed the pseudo-second-order equation for both adsorbents. The adsorption of 2,4-DCP on both adsorbents decreased with an increase in pH. Jian-Wei Ma et. al. [12] studied that acidic pH was favorable for the adsorption of 2,4-DCP onto activated bamboo charcoal. The initial concentrations of 2,4-DCP and adsorbent dose were found to influence the removal of 2,4-DCP. Adsorption kinetics which was independent of adsorbent dosages could be best described by the pseudo-second-order model.
There is tremendous paucity of the study of UBTLs to remove 2,4-DCP from the aqueous solution. In this research work the adsorptive removal of 2,4-DCP on unmodified used black tea leaves (UM-UBTLs) and sodium chlorite modified used black tea leaves (SCM-UBTLs) was studied in mitigating its menace from industrial effluents
Experimental
Materials and methods
Chemical and instrumentation
Analytical grade 2,4-Dichlorophenol (LobaChemie, India) was used as adsorbate in this research work. Tea leaves collected from the local market were treated by sodium chlorite (80%NaClO2) (BDH Laboratory Supplier, England) for using as adsorbent.
UV-visible Recording Spectrometer (Double Beam) (model: UV-1800, Shimadzu, Japan) was used to analyze 2,4-DCP. FTIR spectra of unmodified and modified UBTLs were recorded before and after adsorption using a FTIR Spectrometer [Model: IRTracer-100: FTIR-ATR] Shimadzu, Japan).
Preparation of adsorbents
The tea leaves (Camellia sinensis(L)) were bought from the local market. After boiling with distilled water tea leaves were sieved and batch portion of the boiled tea leaves were washed three times by hot distilled water followed by cold distilled water several times until the tea colour completely disappeared. Then it was dried at room temperature after washing and eventually was dried in oven at 110°C for 10-12 hours. The dried and stored tea leaves were then sieved through the metallic sieve of mesh size. It is considered as used black tea leaves (UBTLs). The surface thus prepared for the adsorption study is considered as unmodified UBTLs (UM-UBTLs).
According to the matrix designed by Box-Behnken experimental design [13], an appropriate amount of NaClO2 was dissolved in 100 mL of distilled water in a three-neck flask, while a required amount of acetic acid was added and stirred. Afterwards, 1.50 g of UBTLs was added and stirred for 60 min at 700 rpm with designed reaction temperature. The treated UBTLs were then washed with distilled water until the filtrate reached pH 6-7, then dried at 70(C to a constant weight, screened through a 20-mesh sieve and put in a desiccator for further use. This type of UBTLs is termed as sodium chlorite modified UBTLs (SCM-UBTLs).
Preparation of 2,4-Dichlorophenol solution
Analytical grade solvents and chemicals was used in this experiment. 2,4-Dichloropenol solution was prepared by using 0.05 M sodium nitrate solution. The concentration of 2,4-Dichloropenol solution was kept within molarity level. The batch experiment was carried out with the solution of concentration not more than 5.0 × 10-3 M.
Concentration of stock solution was 5.0 × 10-3 M and that of standard solution was 5.0 × 10-4 M.
Batch adsorption studies
About 0.2 g of UM-UBTLs (particle size of 50-100 (m measured by using metallic sieve) was charged in reagent bottles containing 2,4-DCP solution of concentration about 5.0 × 10-4 M at pH 2±0.02. These bottles were shaken in the mechanical shaker at room temperature (≈28.0±0.5ºC). After a particular interval of time the reagent bottles were withdrawn from the shaker. The supernatant was centrifuged to obtain a clear solution. The experiment was repeated for the UM-UBTLs of particle size 100-200, 200-300 µm and SCM-UBTLs of particle size 50-100 µm.
Analysis and characterization of surfaces
DCP concentration in the solution was determined by using UV-visible spectrophotometer. The µmax of the solution was found to be within 290 nm. Adsorption kinetics was investigated using three different particle sizes, 50-100, 100-200 and 200-300 µm of UM-UBTLs and 50-100 µm of SCM-UBTLs at room temperature ((28.0±0.5ºC).
The surface of the UM-UBTLs and SCM-UBTLs before adsorption and UBTLs after adsorption of 2,4-DCP were characterized by FTIR Spectrometer (model: IRTracer-100: FTIR-ATR; Shimadzu, Japan) in order to find out whether any shifting of IR peak due to the interaction of DCP with UM-UBTLs occurred or not.
Results and discussion
Estimation of equilibrium
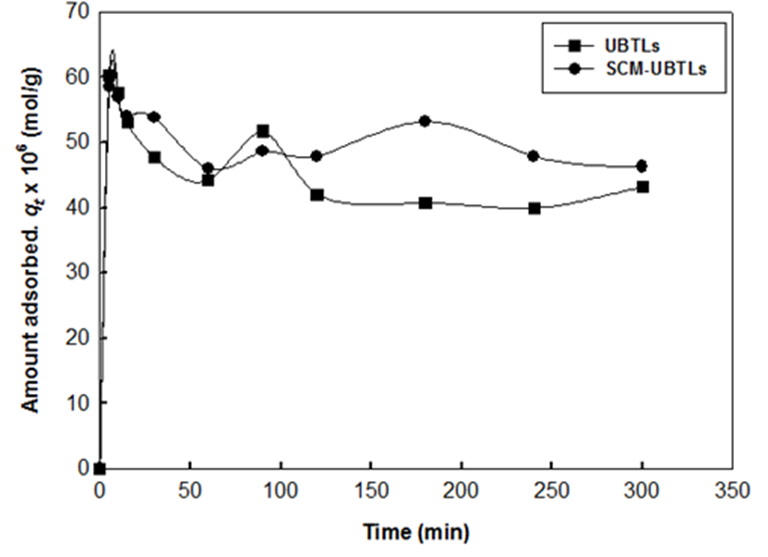
The adsorption of 2,4-DCP through establishing equilibrium time was studied on UM-UBTLs and SCM-UBTLs in different conditions in order to search for the maximum removal of DCP. The adsorption experiment was optimized at the pH of 2. It was observed that in the case of both UM-UBTLs the extent of the removal of 2,4-DCPhad been influenced by the particle size of UBTLs.The equilibrium established within three hours although there is an initial hike in the amount adsorbed (Fig. 1).
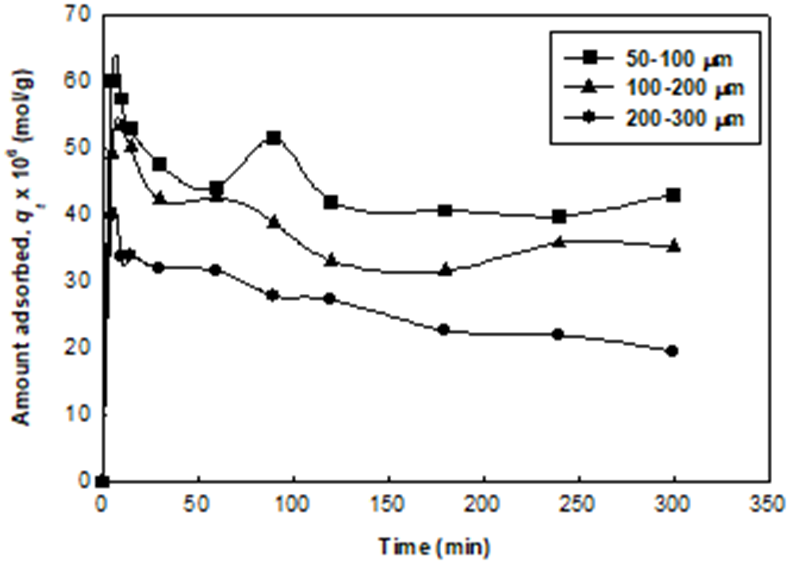
Fig. 1 Variation of the amount of 2,4-DCP adsorbed per gram of UM-UBTLs of different particle sizes with time, at pH 2 at the temperature 28±0.5°C.
The comparison of equilibrium amount of DCP adsorbed per gram of UM-UBTLs and SCM-UBTLs was depicted in Fig. 2. From this plot it was observed that equilibrium amount of DCP adsorbed on UM-UBTLs and SCM-UBTLs was comparable at pH 2. Though the amount adsorbed of DCP was found to be similar for both the adsorbentsat initial stage SCM-UBTLs shown relatively better adsorbent than UM-UBTLs at the equilibrium stage which was also attained within three hours (Fig. 2).
Effect of particle size
The particle size of UM-UBTLs was observed to have profound effect on the adsorption of 2,4-DCP. The amount of DCP adsorbed onto UM-UBTLs decreased with the increase in the particle size of the adsorbent. This usually happens due to the decrease in the surface area at larger particle size (Fig. 3). The similar trend in the variation of the amount adsorbed with particle size was observed in the previous research work [14].
Effect of pH
The adsorption of 2,4-DCP on UM-UBTLs was found to be regulated by the pH of the adsorbate solution. The adsorbed amount of DCP was observed to be higher (Fig. 4) at extremely low pH values. At acidic medium phenolic compound exists as hydroxide form. However, at low pH surface became positive and it might cause an interaction with 2,4-DCP through hydrogen bonding. On the other hand, phenolic compound remains as phenonate ion at higher pH value or at the basic medium. As the surface became negative at higher pH value, the interaction of 2,4-DCP with UBTLs was found to be weak. So, the amount adsorbed of 2,4-DCP onto UM-UBTLs was found to be maximum at lower pH. This result is quite contrary to the previous research findings [15] where the removal of 2,4-DCP was found to be almost constant from the pH 2 to 3 and decrease significantly with the further increase in pH.
Effect of initial concentration
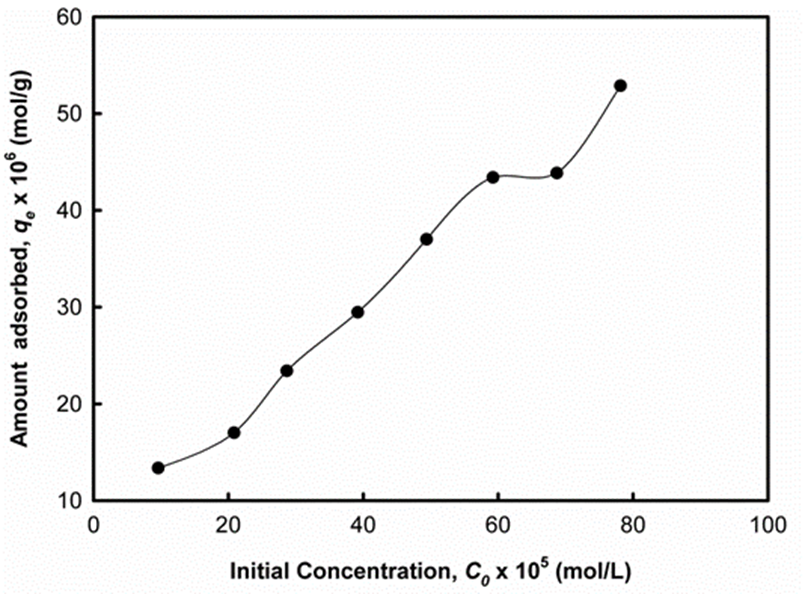
The equilibrium amount of DCP adsorbed on UM-UBTLs was investigated with varying initial concentration of DCP. Experiment for DCP was carried out by selecting a concentration range of 1.0 x 10-4 to 8.0 x 10-4 M with an adsorbent dosage of 0.2 g forUM-UBTLs at 28±0.2°C. The experimental result showed that an increase in the concentration led to an increase in the amount of DCP adsorbed. This increase in adsorption of DCP with concentration is attributed to the retardation of resistance toward DCP uptake, which increases the diffusion of DCP (Fig. 5). From the Fig. 5 it was observed that the maximum adsorption of DCP on UM-UBTLs had been achieved at 8.0 x 10-4 M.
Effect of dose
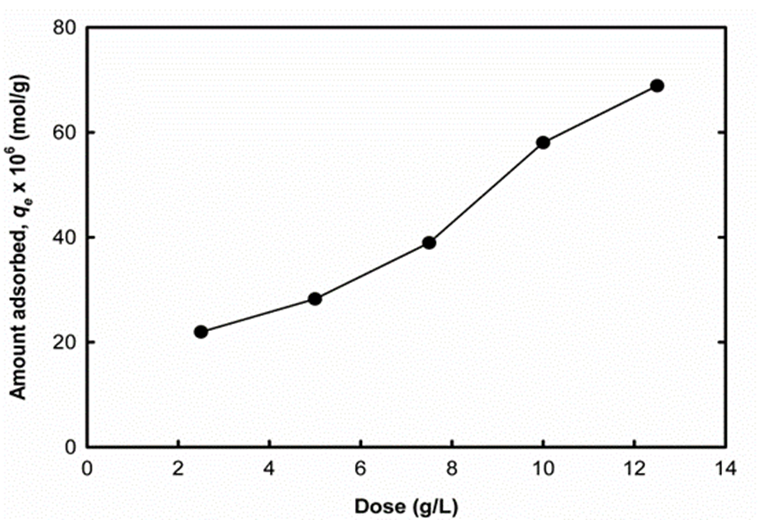
The adsorbent dose is an important parameter in adsorption studies because it determines the capacity of adsorbent for a given initial concentration of dye solution. The effect of dosages of UM-UBTLs on the amount of DCP adsorbed was investigated by contacting 40.0 mL of DCP solution with an initial concentration of 5.0 x 10-4M for the adsorbent, for a contact time of 180 min at a temperature of 28.0± 0.5(C, a shaking speed of 75 rpm and optimum pH of 2.0. Different dosages of adsorbent (2.5, 5.0, 7.50, 10.0 and 12.50 g/L) were used. After equilibrium, the samples were allowed to settle for some time after which the supernatant solutions were collected andanalysed. Fig. 6 shows the effect of adsorbent dosages on the removal 2,4-DCP by UM-UBTLs. The increase in adsorption with adsorbent dosage can be attributed to an increase in the adsorption surfaceand availability of more adsorption sites [16].
Adsorption kinetics
The data obtained from the estimation of equilibrium time for the adsorption of 2,4-DCP on UM-UBTs as well as SCM-UBTLs at pH 2 was only fitted in Ho’s pseudo-second-order kinetic equation rather than Lagergren pseudo-first-order kinetic equation. In this case the values of regression coefficient, R2, were found to be 0.994, 0.996 and 0.984 (Fig. 7) at pH 2for the particle size of 50-100 µm, 100-200 µm and 200-300 μm respectively and 0.995 for SCM-UBTLs (Fig. 8).

Fig. 7 A plot of t/q t vstfor fitting with Ho’s equation of pseudo-second-order kinetics for adsorption of DCP on UM-UBTLs at different particle sizes.
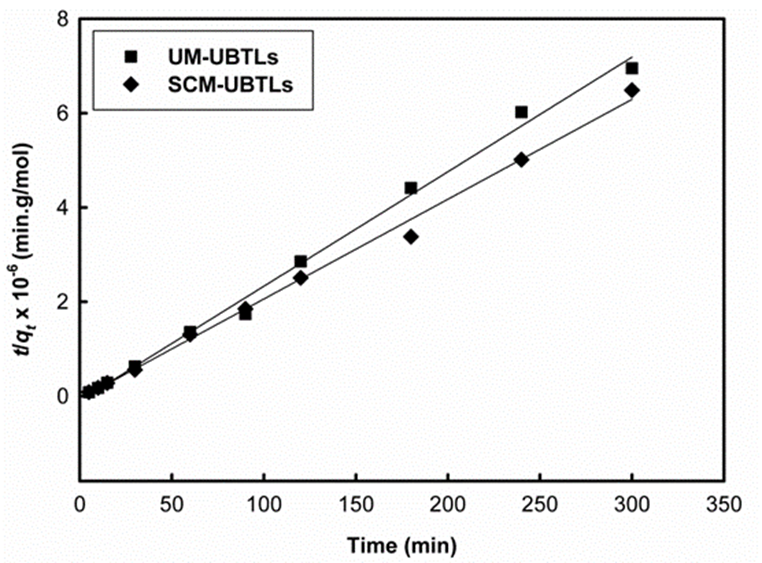
Fig. 8 A plot of t/q t vstfor fitting with Ho’s equation of pseudo-second-order kinetics for adsorption of DCP on UM-UBTLs and SCM-UBTLs at particle size of 50-100 µm.
The kinetic model fitted with Legergren pseudo-first-order,
and Ho’s pseudo-second-order kinetic equation as follows:
In the above equation q e and q t denote the amounts adsorbed at equilibrium and at any time t, respectively, and k 1 and k 2 are the pseudo-first-order and pseudo-second-order rate constants respectively. The kinetic parameters are enlisted in Table 1.
Table 1 Pseudo second order kinetic parameters for the adsorption of 2,4-DCP onto UM-UBTLs of different particle sizes and on SCM-UBTLs of 50-100µm.
| Adsorbents | Particle size (µm) | q e µmol/g) | Pseudo-Second-order kinetic model | |
|---|---|---|---|---|
| k 2 (g /µmol min)) | R2 | |||
| UM-UBTLs | 50-100 | 4.12 (10-5 | 5.99(103 | 0.994 |
| 100-200 | 3.61(10-5 | 3.15(103 | 0.996 | |
| 200-300 | 2.00 (10-5 | 3.62(103 | 0.984 | |
| SCM-UBTLs | 50-100 | 4.72 (10-5 | 7.84 (103 | 0.995 |
Percent removal of 2,4-DCP
In this research work, it was found that percent removal of 2,4-DCP from aqueous solution was predominantly influenced by the particle size of the adsorbent and surface modification has small influence on percent removal. In the case of UM-UBTLs percent removal decreased with increasing the particle size of UBTLs (Fig. 9(a), 9(b)). In the case of UM-UBTLs above 40% of DCP was removed at equilibrium stage whereas SCM-UBTLs exhibited above 54% removal of DCP at equilibrium.
Characterization of FTIR spectra
The FTIR spectra for bare UM-UBTLs and SCM-UBTLs and 2,4-DCP, bare UM-UBTLs and DCP adsorbed UM-UBTLs were depicted in Fig. 10 and in Fig. 11(a-c) respectively. In Fig. 10 not too much difference was found between the FTIR spectra of UM-UBTLs and SCM-UBTLs except the spectrum for -OH band of SCM-UBTLs which was found to be little broader compared with that of the UM-UBTLs. The FTIR spectrum for the bare UM-UBTLs (Fig. 11(b)) confirms the presence of the-OH, C-H (stretch), -CHO and N-H (bend) groups in tea leaves. The FTIR spectrum for -OH in the case of 2,4-DCP (Fig. 11(a)) was found to be broader (3267.47 cm-1) because of the hydrogen bonding between the hydrogen of phenolic -OH and the chlorine atom residing at the ortho- position in 2,4-DCP. On the other hand, the frequency band for -OH existing in the cellulose of UM-UBTLs was observed to be at 3417.92 cm-1 (Fig. 11(b)). After the adsorption of 2,4-DCP onto UM-UBTLs the -OH band was found to be relatively broader than the bare UM-UBTLs (Fig. 11(c)). It indicates the interaction between DCP and UBTLs through hydrogen bonding at low pH value even though the interaction is weak.
SEM analysis
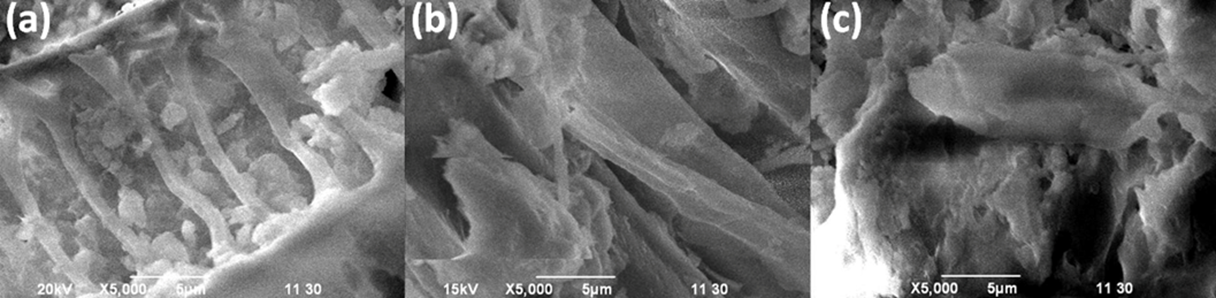
Surface morphologies of UM-UBTLs and SCM-UBTLs before and after adsorption were studied by using scanning electron microscope (SEM) (model: JSM-6490, JEOL, USA). The SEM images of the bare UM-UBTLs, SCM-UBTLs and DCP adsorbed SCM-UBTLs were depicted in Fig. 12. Macroporous structure was observed in bare UM-UBTLs whereas bare SCM-UBTLs exhibited inhomogeneous or cracking morphology. Though macroporous structure influences the adsorption of DCP onto UBTLs, inhomogeneity of SCM-UBTLs matrix is also conducive to adsorbing DCP molecules. Both the adsorbents thereby showed comparable adsorption capacity against DCP (Fig. 2).
Conclusion
In this research several physic-chemical conditions were optimized to remove DCP from the aqueous solution by using low cost UM-UBTLs and SCM-UBTLs. As used black tea leaves are easily available and non-toxic rather than any other chemically synthesized materials, it can serve both the purpose of financial and experimental. As a low cost and environmentally benign bioadsorbent used black tea leaves can be proved to be an excellent candidate to remove phenolic compounds such as 2,4-DCP from water. A comparable scenario was observed for both the adsorbents. The equilibrium time was found to be similar for both the adsorbents although the amount of DCP adsorbed onto SCM-UBTLs at equilibrium was little bit higher than that on the UM-UBTLs. Lower pH and smaller particle size were observed to have significant impact on the maximum removal of DCP compared to higher pH value and larger particle size. This research widened the scope for setting up optimized conditions in removing DCP from water using low cost and environmentally benign used black tea leaves.











 nueva página del texto (beta)
nueva página del texto (beta)