1 Introduction
Selenium (Se) is an essential micronutrient to the human organism. It has a mineral nature and has been shown to have an antioxidant effect (Steinbrennera and Siesa, 2013; Zeng et al., 2013). Additionally, it can prevent heart disease and help in the treatment of some other diseases such as cancer (Hatfield et al., 2014; Mashmouli and Abdollah, 2013; Hurst et al., 2012). Selenium deficiency may cause neuromuscular disorders. Therefore, a recommended daily intake of selenium has been proposed for humans: 60 μg/day for men and 53 μg/day for women (Rayman, 2012). Although this concentration is very low, it is not always met through diet since Se is not available for absorption by the human body. In humans, this mineral can be found in some proteins. These have been called selenoproteins. The family of selenoproteins includes glutation-peroxidases, which are involved in redox reactions. Within this family, there are eight different types and five of them contain selenocysteine in their active core (Castets et al., 2012; Álvarez-Fernández et al., 2010). It has been observed that several species of Lactobacillus have the ability to biotransform inorganic selenium to selenocysteine and selenomethionine, intracellularly (Calomme et al., 1995), being that selenocysteine is the main form in which the Se is incorporated (Lamberti et al., 2011). There are studies in which Se-accumulating lactic acid bacteria are used for the production of fermented dairy products (Alzate et al., 2010; Palomo et al., 2014; Deng et al., 2015). Thus, these products have an added value as functional food due to the beneficial properties of selenium as an antioxidant, anti-pathogenic, anti-mutagenic, anti- carcinogenic and anti-inflammatory agent (Noguera-Velasco et al., 2005). Owing to the importance of selenium in human health, the purpose of this study was to quantify the concentration of inorganic selenium absorbed by lactic acid bacteria during a fermentation process.
2. Materials and methods
2.1 Isolation and adaptation of microorganisms
Four strains of lactobacilli were used: Lactobacillus delbrueckii subsp. bulgaricus NCFB-2772 (LbD), Lactobacillus rhamnosus GG (LbR) and Lactobacillus helveticus IUAMI-70129 (LbH) and Lactobacillus jhonsonii (LbJ). The first three strains were provided by the Laboratory of Food Biotechnogy at the Autonomous Metropolitan University, Iztapalapa (UAMI). Lactobacillus jhonsonii was isolated from a commercial product whose label states the presence of this probiotic. The isolation was conducted in MRS agar, which contained NaCl (40 g/L). It was incubated for 24 h at 37 °C in anaerobiosis. The lactobacillus was isolated according to the colony morphology following the technique described by Cruz-Guerrero et al. (2014). All the microorganisms were stored in refrigeration in MRS broth. Before each fermentation, 1 mL of the culture broth of each lactobacillus was seeded in 9 mL of MRS broth and then, were incubated at 37 °C for 24h.
2.2 Determination of critical inhibitory concentration
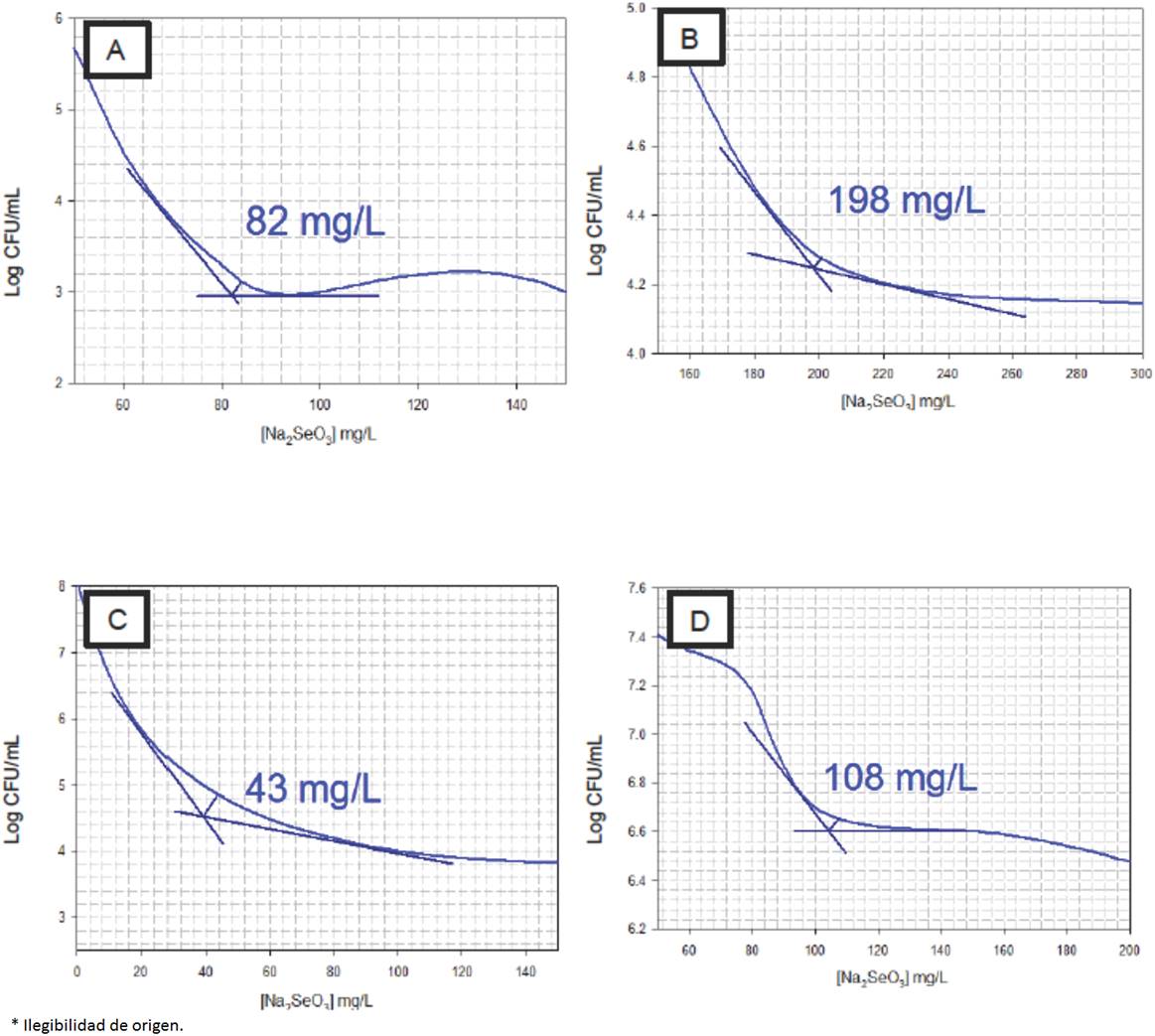
In order to determine Se (IV)-tolerance, 1 mL of the conditioned culture was inoculated in test tubes with 10 mL of MRS supplemented with sodium selenite (Na2SeO3). Selenite concentrations assayed were 0, 20, 40, 60, 80, 100, 150, 200, 250, 300 and 500 mg/L. The cultures were incubated at 37 °C or 42 °C for 48 h, depending on the optimum growing temperature of each microorganism. The viable count on MRS agar plate culturing was conducted. The data obtained were placed in a graph in order to calculate the concentration of Na2SeO3 necessary to inhibit lactobacilli growth using the Talmadge and Fitch graphic method (Peña and Circo, 2007). Tangent lines were drawn on the two lines of the graph where the change of order was found. Afterwards, a line was inserted forming a 45° angle in the tangents interjection, creating a bisector that intersected with the graph. From the intersection point, a vertical line was traced towards the abscissas axis, where the junction represented the critical inhibitory concentration.
2.3 Fermentation in media enriched with Na2SeO3
A concentration of 106 colony-forming units (CFU) was inoculated in test tubes containing 10 mL of MRS broth enriched with Na2SeO3. Sodium selenite concentrations used corresponded to the critical inhibitory concentration for each lactic acid bacteria under study. The fermentation was carried out for 36 h at 37 °C for LbJ and LbD and 42°C for LbH and LbR.
2.4 Determination of biomass
After fermentation, the test tubes containing broth were centrifuged at 39,000 x g for 15 min at 4 °C. The centrifuged cells were resuspended in 20 mL of 0.3% dithiothreitol (w/v) for 20 min. The resuspended cells were centrifuged again under the same conditions (Andreoni et al., 2000). The supernatants of the first and second centrifugation steps were mixed to determine the residual selenium by ICP, as described below.
The recovered cells were put in glass vials previously heated to constant weight. The vials containing those cells were heated at 105 °C for 4 h. They were weighed and returned back to heat for 30 additional min until the constant weight of the vials was obtained.
2.5 Determination of selenium
The concentration of Se was determined through inductively coupled plasma (ICP) as described by Alzate et al. (2010), with some modifications. Supernatant (10 mL) was digested using 10 mL of concentrated HNO3. A microwave digestion was conducted, with a temperature ramp from room temperature to 175 °C for 5.5 min and then from 175 to 180 °C for 4.5 min. The pressure limit was 110 psi. After the digestion, the supernatants were made up to 25 mL for LbD and LbH and 50 mL for LbR and LbJ, using deionized water (18μS/cm) in volumetric flasks. A calibration curve of selenium standard solutions was carried out from 2 to 10 mg/L. Selenium estimation was made at a wavelength of 196 nm.
3 Results and discussion
3.1 Effect of the inorganic selenium concentration in lactobacillus growth
Table 1 shows the changes in the viable count for each of the lactobacilli in terms of concentration of Na2SeO3. LbH had the highest decrease of viability with the least concentration of this salt, which corresponded to two logarithmic cycles in a concentration of 20 mg/L of Na2SeO3. LbD growth was totally inhibited. It has been observed that lactobacilli growth is influenced by Se concentration as well as the presence of other metals such as zinc, in the culture medium (Ren et al., 2011). Also, the resistance to the presence of selenium can be increased with the encapsulation of the microorganisms in matrixes like chitosan (Vodnar and Socaciu, 2014).
Table 1 Viable count of lactobacilli with different concentration of Na2SeO3. Results are expressed as average of log CFU ± standard deviation (n=3 independent fermentation processes)
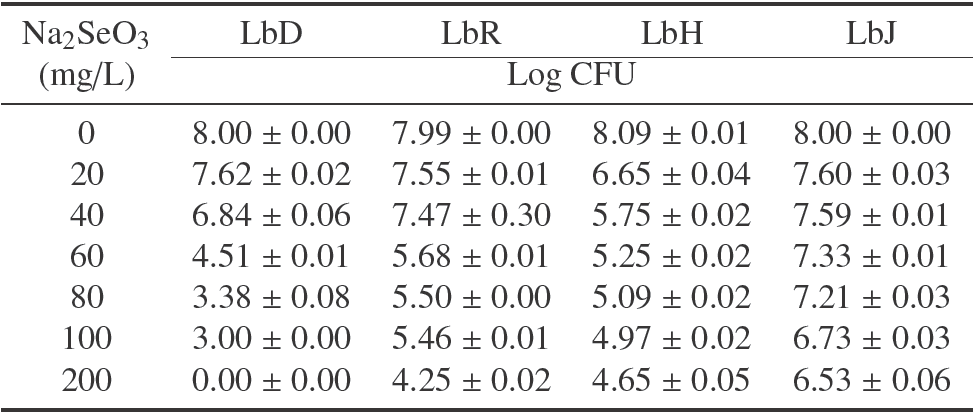
With respect to LbR, this strain presented a tolerance range of 0 to 300 mg of Na2SeO3/L. These results are different from those reported by Andreoni et al. (2000), who determined that species of this microorganism are not capable of metabolizing inorganic selenium and some others do not show tolerance to the presence of this salt in growth media.
On the other hand, it has been observed that the lactic acid bacteria are capable of surviving in concentrations over 200 mg/L of Na2SeO3 (Calomme et al., 1995). Actually, all the lactic acid bacteria studied survived higher or similar concentrations than 200 mg/L of sodium selenite, excepting LbD, which exhibited a complete growth inhibition at this value.
3.2 Determination of the critical inhibitory concentration
Figure 1 features the critical inhibitory points of the graph of each microorganism under study, following the modified method of Talmadge and Fitch cited by Peña and Circo (2007). The data revealed that from the four microorganisms analyzed, LbR presented the highest tolerance with a critical concentration of 198 mg/L of Na2SeO3 (Fig. 1B), whereas LbH (Fig. 1C) experienced the least tolerance (43 mg/L). LbD (Fig. 1A) and LbJ (Fig. 1D) presented tolerance at critical concentrations of 82 mg/L and 108 mg/L, respectively. In all cases, the tolerance results were higher than those reported for other species of lactobacilli (Andreoni et al., 2000). Kai-Xia et al. (2007) proved that MRS medium containing over 16 mg/L of Na2SeO3 had an inhibitory effect in L. delbrueckii growth. Nonetheless, the presence of other metals like manganese, increases the tolerance of lactic acid bacteria for these type of chemical species, mainly in lactobacilli and streptococcus (Calomme et al., 1995).
3.3 Determination of absorption and accumulation of selenium
Table 2 shows the capacity of inorganic selenium absorption of the lactic bacteria studied. LbH showed the highest absorption (76.5%) despite being the bacteria with the lowest tolerance to inorganic selenium. In contrast, LbD showed the least absorption (9.14%).
Table 2 . Relation between selenium absorption and generation of biomass a
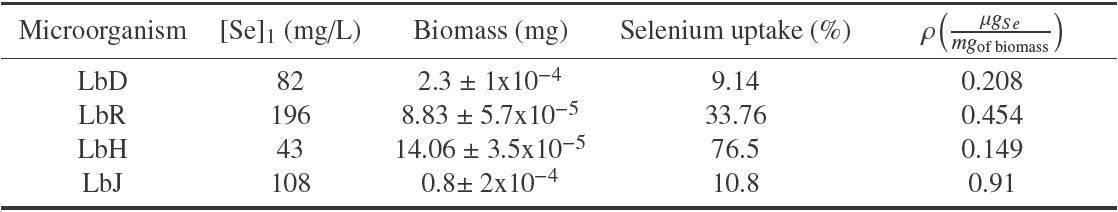
aResults are expressed as average of log CFU ± standard deviation (n=3 independent fermentation processes)
Two of the strains assayed (LbH and LbR) presented higher absorption values than the 13% reported by Andreoni et al. (2000) for some Lactobacillus species. Most lactobacilli transform inorganic selenium in selenocysteine (SeC) through a biochemical mechanism integrated in the cytoplasm (Andreoni et al., 2000). Zhi-Qiang et al. (2009) obtained values up to 28% of selenium transformation by some probiotic bifidobacteria. It has been proven than bioconversion of inorganic selenium to colloidal or organic selenium occurs more efficiently in systems with specific nutrients for lactic acid bacteria, such as fermented milk. As a matter of fact, up to 73% of inorganic selenium can be bio-transformed during milk fermentation (Alzate et al., 2008). Kai-Xia et al. (2007) proposed to add some species of lactobacilli as functional ingredients due to their capacity of converting inorganic selenium to organic selenium.
Considering the biomass generated by each bacteria, its individual capacity for the use of selenium was estimated, expressed as Se micrograms/mg biomass (ρ) (Table 2). LbJ showed a larger use of selenium absorbed per milligram of biomass generated (0.91μg/mg) while LbH had the lowest rate of use with 0.15 μg of selenium per milligram of biomass. In this context, it has been reported that when inorganic selenium is found in the medium, the lactobacilli and bifidobacteria are capable of inhibiting cysteine production to start producing SeC. The higher the requirements of cysteine for microbial development, the higher the percentage of selenium absorbed during growth (Sarang et al., 2014; Kai-Xia et al., 2007).
It has been determined that the concentration of selenium absorbed by the lactobacilli can reach up to 407 μg/g of dry biomass (Calomme et al., 1995). However, the latest data show out that in the case of Lactobacillus reuteri NCDC77, the capacity of absorbing up to 800 μg/g of dry biomass has been described (Saini et al., 2014; Galano et al., 2013).
Conclusions
Although the critical inhibitory concentration of Na2SeO3 is specific for each microorganism, there is a direct proportional relation between this concentration and the percentage of accumulation of selenium by the lactic acid bacteria under study. Nonetheless, this rule does not apply when comparing the absorbed percentage with the concentration of selenium accumulated since, regardless of the absorbed quantity of inorganic selenium, the concentration accumulated inside the cell varies. Thus, L. jhonsonii and L. rhamnosus GG can be considered for their application as functional food ingredients due to their ability of biotransforming selenium.











 nueva página del texto (beta)
nueva página del texto (beta)