Introduction
The most common approach to intra-aortic balloon pumps (IAPB) percutaneous placement is the transfemoral artery inducing physical deconditioning in prolonged support conditions1. We previously described the percutaneous technique of installation of an IABP through the left axillary or subclavian artery as a feasible and relatively well-tolerated approach to bridge patients with end-stage heart failure (HF) to heart transplantation1. We present – to the best of our knowledge – the first case of axillary IABP percutaneous placement in Mexico. This case reproduces the feasibility and tolerability of the axillary approach in a patient with acute severe HF as a bridge to the left ventricular assist device (LVAD) therapy.
Case report
A 72-year-old male was admitted to ER with a new episode of progressive dyspnea. He had a history of ischemic cardiomyopathy, surgical revascularization, HF with reduced ejection fraction (HFrEF) (35% ejection fraction [EF) with multiple exacerbations, paroxysmal atrial fibrillation (AF), diabetes, and chronic kidney disease (CKD). The previous treatment including bumetanide, spironolactone, aspirin, ivabradine, amiodarone, apixaban, and rosuvastatin. Physical examination with HR 102 bpm, respiratory rate 24 bpm, blood pressure 98/66 mmHg, oxygen saturation of 89%, and venous distension. The chest auscultation revealed S3, bilateral rales, right pleural effusion, and anasarca. The electrocardiogram and chest X-ray showed ischemic findings and congestive lungs with the right pleural effusion, respectively. Laboratories demonstrate hemoglobin 10.7 g/dL, B-type natriuretic peptide 1672 pg/mL, and creatinine 4.4 mg/dL. Transthoracic echocardiography showed severe global ventricular hypokinesis, moderate mitral regurgitation, left atrium enlargement with a volume of 34 ml/m2, diastolic dysfunction, pulmonary artery systolic pressure 59 mmHg, and 21% EF. We established the diagnosis of acute HF and started 40 mg of intravenous furosemide and milrinone 0.25 mcg/kg/min. We performed thoracentesis of 1800 cc, and by medical treatment failure, we increased the milrinone dose to 0.5 mcg/kg/min and start 2 mg of bumetanide every 6 h without success. We started hemodialysis and amiodarone for paroxysmal AF and an episode of self-limiting ventricular monomorphic tachycardia, reverting to sinus rhythm. In the following hours, we started norepinephrine by worsening clinical instability and severe hypotension. Because of persistent amine dependency and poor inotropic response, we perform a right cardiac catheterization as the protocol for LVAD showing pressures of the right atrium (20 mmHg), right ventricle (70/10 mmHg), pulmonary artery (70/30 mmHg), pulmonary capillary wedge pressure (PCWP) of 29 mmHg, a right atrial pressure/PCWP 0.9 and a pulmonary artery pulsatility index of 4, and a cardiac index of 1.7 L/min, with persistent clinical instability and treatment failure. The patient was classified as a D-stage shock, according to the Society for Cardiovascular Angiography and Interventions cardiogenic shock consensus2. During follow-up, the patient continued with inotropic and chronotropic agents with an impending need for the left ventricular support and hemodialysis with total ultrafiltration of 6 L.
In addition, a femoral Doppler ultrasound showed critical stenosis 90% in the right and left proximal femoral common artery, distal occlusion of the common femoral artery (calcified plaques). Considering these findings, we decided the percutaneous axillary approach. A left brachial and axillary Doppler ultrasound showed adequate blood flow without critical stenosis. The patient was considered as bridge therapy for LVAD or cardiac transplantation, with an expected extended in-hospital stay and under medical treatment waiting to transfer to a specialized HF center.
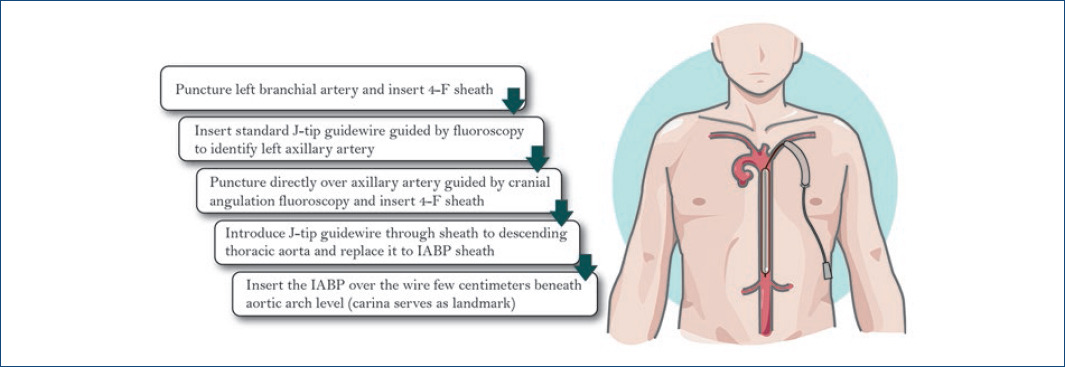
Technique description
We previously described the most extensive series and the technique of percutaneous placement of IABP in the axillary/subclavian1 (Fig. 1).
In-hospital outcome
After the placement of the axillary IABP, heart and renal function improved. Physical therapy interventions were focused on preventing weakness, early ambulation, and stand-up position exercises (Fig. 2). Despite the patient, PCWP (24 mmHg), and cardiac index (2.7 L/min) improvement, he was rejected for an LVAD or a heart transplant.
The clinical outcome after IABP removal was that of a terminal HF patient rejected to advanced treatment options. The patient eventually passed away despite optimal medical treatment.
Discussion
This case showed that the axillary approach IABP in patients with advanced HFrEF is safe and improves the quality of life, and reproduces previous evidence in under urgent heart transplant3. The left axillary/subclavian IABP1 is an option that reaches the benefits of the conventional IABP femoral access reducing complications secondary to immobility and avoids a surgical cut-down and general anesthesia mandatory in intrathoracic surgical approaches3,4. Previously, we described the safety and efficacy of axillar/subclavian IABP in 50 patients with HFrEF under heart or heart-multiorgan transplantation. Cumulative survival on IABP support was 92%; 8% had thromboembolic or bleeding events. Four patients died during the support of secondary multiorgan failure. Our case was complex by multiple comorbidities, severe peripheral arterial disease, hemodynamic instability, vasopressor and inotrope dependence, and high-risk complications with LVAD insertion. We performed the axillary approach successfully5, achieving clinical stability, renal function, and quality of life improvement without bleeding or procedure-related complications. We recommend the left axillary artery route instead of the right access to avoid the cerebral artery vessels6. The axillar percutaneous approach could be an option in patients with extensive and severe PAD or those with expected long time to destination therapy.











 nueva página del texto (beta)
nueva página del texto (beta)