Introduction
As a result of the increase in the human population, agriculture techniques must be improved to face the challenge of feeding humanity with healthy and nutritious food, exchanging ancient methods for the use of chemical substances that enhance growth and reduce losses due to pests (Hahn, 2014). Moreover, the trend of eating fresh products for an appropriated diet has consequently led to food being processed faster, which increases the risk of contamination by pesticides, toxins, or microorganisms (Jung, Jang & Matthews, 2014).
In the context of agricultural production, there are risks associated with the loss of crops at all stages of the process. However, post-harvest losses are one of the most significant since they occur immediately prior to the product reaching the consumer (Kasso & Bekele, 2018). Post-harvest activities represent 34% of global crop losses, with the most frequent causes being pests, natural ripening processes, environmental conditions and microbial infection mainly with fungi (Abass et al., 2014; Kasso & Bekele, 2018; Kumar & Kalita, 2017).
The infection of harvested crops with phytopathogenic fungi represents one of the most important challenges in current agricultural techniques, not only from an economic point of view but for the risk to human health. Several factors are related to fungal infection under post-harvest conditions, such as pH, temperature, UV and oxidative stress, among others (Liu et al., 2018). In recent decades, the use of organic fungicides (such as site-specific fungicides, thiabendazole, pyrimethanyl, o-phenylphenol and imazalil), has been the main force in avoiding crop decay due to their effectiveness and spectrum of action. However, the use of these substances has a serious environmental impact and causes human health disorders and antimicrobial resistance (Hahn, 2014; Peris-Vicente, Marzo-Mas, Roca-Genovés, Carda-Broch & Esteve-Romero, 2016).
The current trends in crop protection involve a range of actions that mitigate the environmental impact, have a broad spectrum of action and are of natural origin, biodegradable and easy to apply (Seiber, Coats, Duke & Gross, 2014). One alternative is the use of natural materials such as chitosan and Opuntia mucilage as edible film presentation.
Chitosan (CS) is one of the most promising resources for the generation of bioactive, biodegradable materials against infections that affect crops of economic importance. The main chain of this semi-natural polymer displays a polycationic behavior in acidic solution due to the free amino groups in its structure (Olicón-Hernández, Uribe-Álvarez, Uribe-Carvajal, Pardo & Guerra-Sánchez, 2017). This polymer is obtained by the deacetylation of chitin, the second most abundant structural polymer related to fungi, crustaceans and insects (Olicón-Hernández, Zepeda Giraud & Guerra-Sánchez, 2017). Due to its properties, such as resistance, flexibility, polymerization, and polycationic charge, chitosan has been used as a raw material for the formation of gels, fibers and films and has been shown to have extensive antimicrobial activity against bacteria and fungi (Anitha et al., 2014; Olicón-Hernández et al., 2015; Olicón-Hernández, Zepeda Giraud, et al., 2017; Younes & Rinaudo, 2015).
In the context of the edible films formulation, a typical edible film has three major components; film forming material, plasticizer, and additives. From this point of view Nopal (Opuntia ficus-indica) is a potential alternative as element of edible film. The nopal is a traditional element of mexican food and culture but is considered an exotic ingredient in an international context (de Albuquerque et al., 2018). Extracts of this plant have shown benefits to human health, such as hypolipidemic, hypocholesterolemic, antidiabetic, hypoglycemic, antioxidantand anti-inflammatory efficacies (Otálora, Carriazo, Iturriaga, Nazareno & Osorio, 2015). Opuntia mucilage (OM) is a complex material formed from carbohydrates including L-arabinose, D-galactose, D-xylose, L-rhamnose and D-galacturonic acid in variable proportions, which has been cataloged as a promising natural resource for use in the food, pharmaceutical and construction industries (León-Martínez, Cano-Barrita, Lagunez-Rivera & Medina-Torres, 2014; Otálora et al., 2015).
In the present study, we designed a CS-OM film for the protection of crops, which has the characteristic of being edible, allowing safe and practical handling for its application. Physicochemical characterization of the film was performed and the in vitro and in situ antifungal activity against Rhizopus stolonifer, one of the most important phytopathogenic fungi, was evaluated.
Materials and Methods
Regents and Solutions
Low-molecular weight chitosan (50,000−190,000 Da) was provided by Sigma−Aldrich (St. Louis, MO, USA). A stock solution of 10 mg/mL was made in 1% acetic acid, according to our previously reported protocol, followed by sterilization via autoclaving (Olicón-Hernández et al., 2015).
The Opuntia (nopal) cladodes for OM extraction were obtained from a local market in México City. The cladodes were thoroughly washed, cut into uniform pieces (0.5 cm x 0.5 cm), placed in water (5:1 w/v) and incubated overnight at room temperature. Subsequently, the mixture was filtered and concentrated by evaporation.
Glycerol and gelatin were of commercial food grade and purchased from a local supermarket.
CS-OM film formulation
The film formulation was made by mixing four main elements: 1) CS, 2) OM, 3) glycerol and 4) gelatin. The proportions of OM and gelatin kept constant (20 and 10%, respectively) to maintain the integrity of the membrane. Eight different preparations were made, with two concentrations of glycerol (1 and 3%), to evaluated the flexibility and four concentrations of chitosan (0.05, 0.1, 0.15 and 0.20%), to test the homogeneity and antifungal effect of the resulting film (Table I). Each mixture was homogenized using a vortex and placed into Petri dishes (8.7 cm), at room temperature until dry. The film was manually separated from the plate. Selection was made based on the integrity, flexibility and handling of the resulting film, in addition to the homogeneity, as confirmed by conventional microscopy.
Scanning electron microscopy (SEM)
The selected film was cut into 1cm x 1cm pieces and placed on metallic bases for structural evaluation by SEM. After the samples were dried, a gold layer was directly applied under a vacuum for 10 min and observed using a JEOL 5800LV scanning electron microscope (Tokyo, Japan) at 15 kV (Olicón-Hernández et al., 2015).
Viscosity
The viscosity of the selected formulation was determined as a characterization element. Likewise, the viscosity of each of the elements of the mixture was determined separately to evaluate its rheological behavior. A viscometer Brookfield model RVT Spring Torque (Dyne-cm), 7,187.0 (Middleboro, MA, USA), was used. Six spindles and eight velocities were tested to standardize the determination of each component (manual instructions). A volume of 600 mL sample was placed in a beaker and the spindle of the viscometer was submerged and centered in the sample. Rotation of the viscometer was maintained until the reading stabilized and the determination of viscosity was carried out according to the specifications described in the manual (Viscometer, 2016). The experiments were performed in triplicate and the viscosity is expressed in centipoises (cp).
Color
The color determination of the film was carried out using a CR-10 colorimeter (Konica Minolta, Japan). The determination was randomly performed on the surface of three different samples and the L*, a*and b* values were measured based on the CIE Lab* color space (Valadez-Carmona et al., 2016).
Percentage humidity and film solubility in water
The humidity was measured by gravimetric standard methods and is expressed as a percentage (%). The experiments were performed in triplicate. Percentage solubility in water was measured according to García et al., 2004, based on the comparison of the weight of the dry sample prior to and following exhaustive agitation in water, as calculated using the following equation (Garcı́a, Pinotti, Martino & Zaritzky, 2004):
Qualitative evaluation of the protection of stored fruits
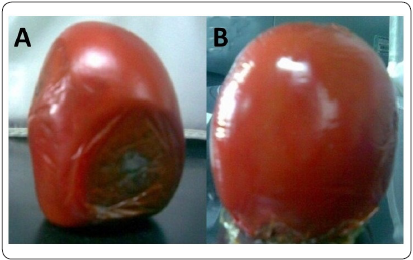
Ten Saladette tomatoes (Lycopersicon esculentum) were selected according to color, state of health, size and firmness. These fruits were divided into two equal groups: one as a control without protection and the other protected by adhesion of the CS-OM on the surface. The two groups were stored at room temperature and humidity for 30 days. No phytopathogenic agent was intentionally added. After this time, a visual evaluation was made to determine the protective effect of the film as compared with the control.
Antifungal activity
Rhizopus stolonifer R3 was isolated from post-harvest contaminated crops and also provided by CEPROBI-IPN, Yautepec, Morelos, Mexico (Hernández-Lauzardo et al., 2008).
Determination of in vitro antifungal activity
Rhizopus stolonifer R3 was grown on commercial potato dextrose agar (PDA) plates for 72 h. After this time, approximately 1-cm2 pieces of mycelia-agar were excised to inoculate three PDA plates without CS-OM film (control system, Db) and three plates coated with the film (positive system, Da). The plates were incubated at 28 °C until the fungus in the control system covered the plate. In both systems, the diameter of the mycelia was measured to determine the antifungal index according to the following equation (Guo et al., 2006):
where Da is the diameter of the mycelia in the positive system and Db is the diameter in the control system.
Determination of in situ antifungal activity
R. stolonifer R3 was grown on PDA plates for 72 h at 28 °C. After this time, a spore suspension was made by mechanical extraction in water. The number of spores was calculated using the Neubauer chamber method and adjusted to 1x106 spores/mL in sterile water (Olicón-Hernández, Camacho-Morales, Pozo, González-López & Aranda, 2019).
A total of 90 Saladette tomatoes (Lycopersicon esculentum) were selected using the same parameters as described in section 2.5. These tomatoes were extensively washed, disinfected with 1% sodium hypochlorite and divided into six groups of 15 samples. Three of these groups were protected with the CS-OM film (test groups) and three groups were the control. The spore solution was sprinkled on all tomatoes, which were subsequently placed in humid chambers at room temperature for 72 h. After this time, the severity index (damage) was calculated according to the protocol-scale-equation described by Mayek−Perez et al., 1995, based on the ratio of samples with visible symptoms of infection to the total samples in the group (Mayek-Pérez, Pedroza-Flores, Villarreal-García & Valdés-Lozano, 1995).
Results
Selection of the CS-OM film
Evaluation of the different formulations is displayed in Table I. In general, a low proportion of glycerol resulted in lumpy films with a lack of continuity, which did not allow complete drying. On the other hand, micrographs of the films (Figure 1) revealed that by increasing the concentration of chitosan, the surface was more homogeneous and the flexibility was improved. According to these results, the formulation containing 3% glycerol and 2% chitosan was selected, with the proportions of gelatin and mucilage as specified in section 2.2.
Table I Evaluation of the physical characteristics of the different formulations of CS-OM film.
| Formulation | Chitosan (%) | Glycerol (%) | Integrity | Flexibility | Homogeneity |
|---|---|---|---|---|---|
| 1 | 0.05 | 1.0 | + | + | + |
| 2 | 0.10 | + | ++ | + | |
| 3 | 0.15 | ++ | ++ | + | |
| 4 | 0.20 | +++ | +++ | + | |
| 5 | 0.05 | 3.0 | ++ | ++ | ++ |
| 6 | 0.10 | ++ | ++ | +++ | |
| 7 | 0.15 | +++ | ++ | ++++ | |
| 8 | 0.20 | ++++ | ++++ | ++++ |
+ = Qualitative evaluation scale. A greater number of symbols represents an increase in the characteristic evaluated.
Characterization of the CS-OM film
According to SEM analysis, the CS-OM film had a fibrous structure with the absence of pores and a homogenous appearance throughout its surface (Figure 2), which is in accordance with the simple light microscopy data.

Figure 2 SEM analysis of the CS-OM film. No damage or irregularity was observed in the structure of the film following the drying process. The fibrous structure originated from the interaction of the different components, with chitosan conferring that property. (A) 500x; (B) 1500x; (C) 4000x.
The data obtained from the individual elements and the aqueous phase of the CS-OM film (Figure 3), demonstrated that the viscosity was cumulative, with glycerol as the most important element affecting this property.
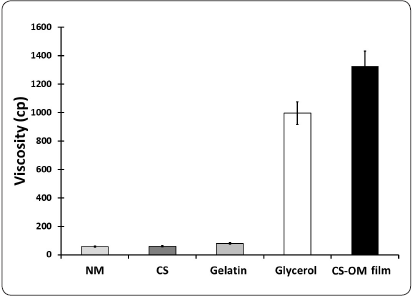
Figure 3 Viscosity determination of the CS-OM film elements. The viscosity is expressed in centipoises. OM = Opuntia mucilage; CS = Chitosan; CS-OM film = aqueous phase of the designed film.
The data corresponding to humidity, solubility and color are summarized in Table II. Following drying, the resulting film had an average of 12.5% and 43% humidity and solubility, respectively. According to the scales of the L*, a* and b* parameters, the CS-OM film was slightly dark with a light green-blue coloration.
Effect on the protection of stored fruits
Changes in tomatoes with and without (control) CS-OM film after 30 days of storage are shown in Figure 4. In the control, it was observed that the fruits without protection lost firmness and had slight changes in color, in addition to there being clear evidence of fungal disease. It should be noted that for this determination, no phytopathogenic agent was intentionally added. In contrast, the protected fruits maintained uniform coloration and firmness comparable to the tomatoes under initial storage conditions and no symptoms of the microbial disease were observed.
In vitro and in situ antifungal activity
Changes in the growth of R. stolonifer with and without CS-OM film on the PDA plates and determination of the antifungal index (%) are shown in Figure 5. The presence of the CS-OM film inhibited the growth of the phytopathogenic agent by approximately 50%. This difference was statistically validated using Tukey’s test (P < 0.001).

Figure 5 In vitro antifungal effect of CS-OM film against R. stolonifer. Mycelial growth without film (A, Control) and with the protection of film (B). The fungus took 36 hours to invade the control plates. There was a statistically significant difference validated by Tukey’s test (P < 0.001).
In the case of in situ antifungal activity, the tomatoes in the control group (Figure 6A), showed an abundant growth of fungus, which in some cases covered more than 50% of the total surface. During this stage of infection, symptoms of the disease or important changes in coloration or form were not appreciated. However, the severity index in this group reached the maximum value on the scale, indicating that more than 75% of the total fruits were affected.

Figure 6 In situ antifungal effect of CS-OM film against R. stolonifer. Fruits without film (A, Control), had massive mycelial growth on the surface. On the tomatoes protected by CS-OM film (B), a lower and limited fungal growth was observed.
On the other hand, the fruits protected by the CS-OM film (Figure 6B), obtained a severity index of 2, indicating that less than 25% of the fruits were affected to some degree. Growth in the area of affected fruits was lower and limited to specific areas.
Discussion
The use of edible films for the protection of crops, fruits and/or meat is a current trend with a view to increasing shelf-life, maximizing productivity and maintaining the quality and flavors of food (Zambrano-Zaragoza et al., 2018). In general, the composition of edible biofilms requires a protein component, a plasticizing agent and in some cases a structural component and/or bioactive agent (Galus & Kadzińska, 2015). In the present work, gelatin and glycerol were selected as the protein and plasticizing agent, respectively, since it has been reported that the combination of these components improves the quality of film and increases the shelf-life of fruits such as avocado and strawberries (Aguilar-Méndez, Martín-Martínez, Tomás, Cruz-Orea, & Jaime-Fonseca, 2008; Del-Valle, Hernández-Muñoz, Guarda & Galotto, 2005).
Several components have been used as elements in film formulation to improve the flexibility and integrity of the coating. Casein, collagen, lipids, soy, corn, alginate, cellulose and essential oils are substances that have been proven to be efficient structural, gelling and active agents (Galus & Kadzińska, 2015). In the present work, the use of Opuntia mucilage, an unconventional cheap raw material that contributes carbohydrates, as well as structural and functional elements that make the film edible and complement its biological activity, were explored. In this context, the use of Opuntia mucilage as an element in edible films increases water vapor permeability (Domínguez-Martínez et al., 2017), maintains the quality of fruits and vegetables (Allegra et al., 2016; Del-Valle et al., 2005; Treviño-Garza, García, Heredia, Alanís-Guzmán & Arévalo-Niño, 2017)and improves thermal stability and flexibility (Guadarrama-Lezama et al., 2018). Moreover, the combination of Opuntia mucilage and chitosan as a bioactive material improves the structure, stability and homogeneity of the film, as observed by SEM analysis and fundamental parameters (Maqbool, Ali, Alderson, Zahid & Siddiqui, 2011). The physicochemical and rheological characteristics of the CS-OM film in the present study are in accordance with those previously reported, where there is no standard trend in these parameters and they depend on the composition and proportions in each particular case (Razavi, Mohammad Amini & Zahedi, 2015; Saberi & Golding, 2018). However, maintaining the stability and the physicochemical characteristics of the film is important prospective work, since these can modify the initial sensory characteristics of the product and affect the perception of potential buyers
The use of chitosan as an antifungal element is a widely explored field. However, the underlying mechanisms have not yet been fully elucidated. We propose a global mechanism that includes electrostatic interactions with the plasma membrane (the most accepted model) and a series of stress conditioning elements (such as the formation of reactive oxygen species) and a complex metabolic imbalance (Olicón-Hernández et al., 2015; Olicón-Hernández, Uribe-Álvarez et al., 2017). With respect to the effect of chitosan on R. stolonifer, it was observed that the polymer modified the germination of spores, affected fungal morphology, increased glucose consumption, inhibited H+-ATPase activity in the plasma membrane and caused a high potassium efflux (Dos Santos et al., 2012; García-Rincón et al., 2010; Guerra-Sánchez, Vega-Pérez, Velázquez-del Valle & Hernández-Lauzardo, 2009; Hernández-Lauzardo et al., 2008; Hernández-Lauzardo et al., 2011). An increase in the shelf-life of different fruits using edible CS-OM films has been observed in recent years, which is in agreement with the results of the present study (Ávila-Sosa et al., 2012; Treviño-Garza et al., 2017). However, in most of the cases, the films were mixed with essential oils or adjuvants to increase the antifungal effect (Yuan, Chen & Li, 2016) and few recent studies evaluate the effectiveness of these films under in situ or in vitro conditions.
As the results indicate, the presence of the polymer mixed with the Opuntia mucilage inhibited the proliferation of the fungus under in vitro and in situ conditions for the protection of tomatoes. These results are consistent with those reported by Martínez-Camacho et al., who determined similar antifungal index values against Aspergillus niger. Nevertheless, in situ protection was not tested by them (Martínez-Camacho et al., 2010). Recently, Robledo et al., 2018, described thymol nanoemulsions incorporated into quinoa protein/chitosan edible films for the protection of cherry tomatoes, which significantly reduced fungal infection by Botrytis cinerea following incubation at 5 °C for seven days (Robledo et al., 2018). In summary, the results obtained in the present study describe an attractive potential alternative that can be used under real conditions for the protection of fruits against infection with phytopathogenic fungi.
Conclusion
An edible film was designed based on chitosan and Opuntia mucilage that presented a uniform and homogenous surface/structure. The best integrity was observed in the film containing the highest proportions of glycerol and chitosan, which affected its stability and antifungal effect. The CS-OM film significantly reduced the growth of R. stolonifer under in vitro and in situ conditions for the protection of tomatoes and increased their shelf-life, making it a potential product for possible massive application in agriculture.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.











 nueva página del texto (beta)
nueva página del texto (beta)