Introduction
The industry of power generation and hydrocarbons extraction is expecting that pressures and temperature operations will rise above 20000 psi and 200°C in environments with high levels of H2S and CO2 (DNV, 2015) (Chail, 2016). These drastic conditions will force to the use of special alloys with high corrosion resistance and excellent mechanical properties such as the duplex stainless steels, which were developed taking advantage of both phases austenite and ferrite coexisting in a 50/50 proportion (Chail, 2016). The continuous engineering challenge has powered the evolution of different duplex alloys like the superduplex and lately, the hyperduplex stainless steels. In order to improve the new generations of duplex alloys, the content of chromium, molybdenum, nickel and nitrogen was increased to maintain the balance of ferrite and austenite at room temperature. It is well known that the alloy elements improve the corrosion resistance and mechanical properties of the hyperduplex stainless steels. However, they also compromise the ferrite/austenite balance since they increase the occurrence of intermetallic phases such as sigma phase, chi phase, or chromium nitrides (Sathirachinda, 2011). The precipitation of these intermetallic phases usually occurs in the temperature range 475°C-900°C (Nilsson, 1992; Lo, 2015) and is normally associated with the loss of ductility, toughness and corrosion resistance as they consume a portion of the alloying elements in the ferrite matrix, specially chromium and molybdenum (Padilha, 2012). From all the intermetallic phases, the sigma phase is considered the most detrimental for all duplex alloys because its formation involves the decomposition of ferrite through the eutectoid reaction of ferrite → austenite + sigma, which modifies the final phase balance. Therefore, it is important to identify the adequate as-cast processing of the hyperduplex stainless steels like i.e. the annealing heat treatment to avoid the occurrence of intermetallic phases in order to produce alloys with the microstructural balance or to dissolve any of them that could have formed during fabrication. For duplex alloys, the annealing heat treatment is generally performed in the temperature range 1040°C-1100°C, where the formation of intermetallic phases is not possible (Sieurin, 2006) (Magnabosco, 2005). Nevertheless, the occurrence of intermetallic phases may be influenced by the high content of chromium, molybdenum, nickel and nitrogen since they can expand the temperature range where ferrite becomes unstable. The objective of this work is to study the effect of the conventional annealing heat treatment in the microstructural balance of the experimental hyperduplex alloys.
Materials and methods
Fabrication of experimental samples
Samples of experimental compositions equivalents to UNS S33207 were produced in the laboratory by the button melting system. All samples were fabricated with 99.999% pure elements and melted under Ar+4%N atmosphere to add and avoid the loss of nitrogen. The chemical analysis of the resulting as-cast samples is shown in Table 1, which complies with the ASTM A789 chemical composition standard (ASTM).
Table 1 Chemical composition of experimental sample
| Sample | Cr | Ni | Mo | Mn | Si | N | Fe |
| UNS S33207 | 31.5 | 9.71 | 4.8 | 1.33 | .342 | .29 | Bal. |
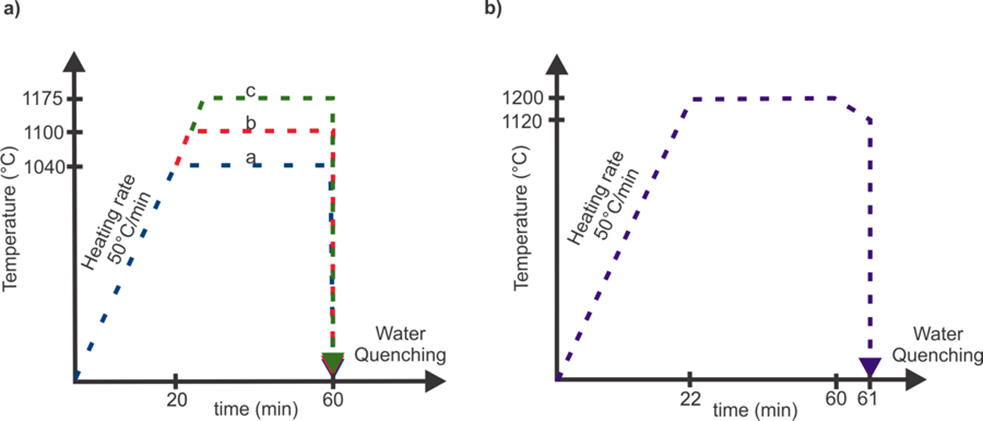
In order to achieve the microstructural balance of ferrite and austenite free of intermetallic phases, the as-cast samples were submitted to different annealing heat treatments at 1040°C, 1100°C and 1175°C held for one hour and quenched in water which are recommended by the literature for all duplex grades (Figure 1a). All heat treatments were performed in a horizontal furnace with a heating rate of 50°C/min and argon atmosphere to avoid the oxidation of the samples.
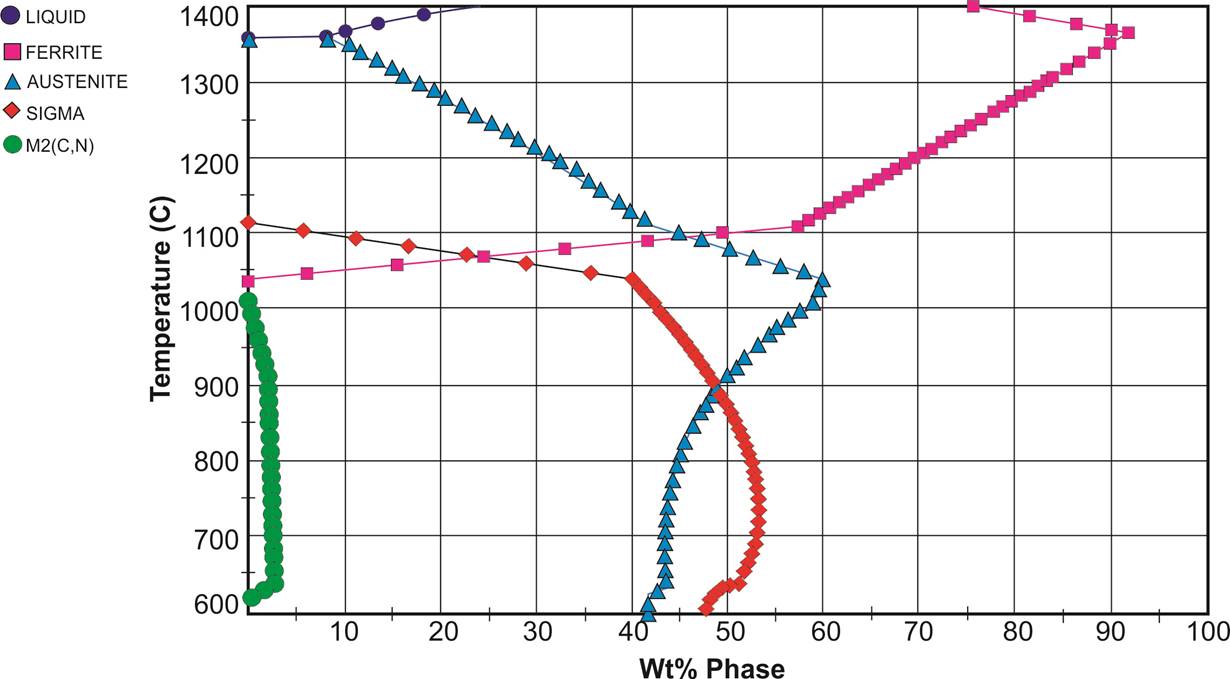
Based on the resulting microstructure of the recommended heat treatments and with the aid of a simulated phase diagram, one specific heat treatment was designed and performed as an alternative to achieve the microstructural balance. This heat treatment consisted in heating the samples to 1200°C for one hour, furnace cooled to 1120°C, held for one minute and then water quenching (Figure 1b). The simulated phase diagram in Figure 2 was based on the equilibrium calculations of phases and accomplished by using the Fe-thermodynamic database with JMatPro® software and according to the chemical composition of the experimental samples.
For optical and scanning electron microscopy, the samples were prepared by standard procedure of hand-ground with successive grade abrasive paper (240-800) and then hand polishing with 9, 6, 3 and 1 microns diamond paste. Final polishing was performed using colloidal silica. The microstructure was revealed by electrolytic etching in 20% NaOH solution to differentiate the ferrite from the austenite and to reveal the presence of intermetallic phases and quantitative metallography was used in order to measure the amount of phases.
Results and discussion
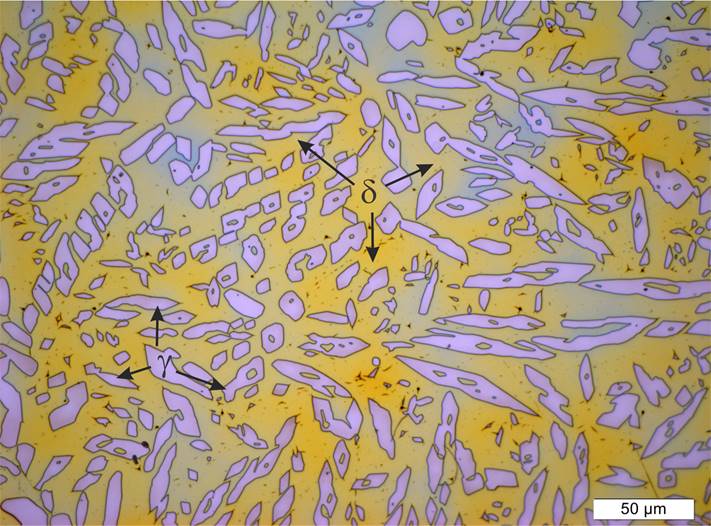
Samples of the as-cast experimental hyperduplex stainless steel showed the typical ferrite-austenite microstructure, free of secondary phases (Figure 3). In the case of duplex alloys, the solidification mode is in function of the alloy ́s chemical composition. Since they have high amount of Cr, they solidify as ≈100% ferrite and transform to austenite on cooling, below the solidus temperature and after the solidification is complete (Ogawa, 1989). Ferrite to austenite transformation is observed only in the solid state, following the sequence L → -L+F-F-F+A (Lippold, 2005). The as-cast microstructure in Figure 3 displayed the direction of solidification showing columnar grains depicting a ferrite matrix with precipitates of austenite. Some ferrite grain boundaries are delineated with allotriomorphic austenite. It can also be seen the growth of Widmanstätten austenite in the ferrite grains and some particles of austenite well developed intragranularly. The amount of ferrite is 75% and for austenite 25%. According to the content of elements that promotes the ferrite such as chromium and molybdenum (Table 1), it is estimated to have more ferrite than austenite in the as-cast microstructure. The final microstructural balance is given by the subsequent solution annealing heat treatment in order to promote the ferrite to austenite transformation. After solution annealing, a range of between 45% and 60% of austenite is expected (Gunn, 1997).

Figure. 3 As-cast microstructure showing a ferrite matrix with particles of austenite of sample UNS S33207
The experimental samples experience a microstructural evolution as the temperature increases being the ferrite phase the most affected at the end of the heat treatment as seen in Figure 4. After annealing at 1040°C, all the ferrite has transformed to sigma phase and secondary austenite. Sigma phase is present as continuous colonies of small and round particles, suggesting that sigma formed in a high Cr-concentrated region in the ferrite, taking all the chromium in order to grow and eventually, consuming all the ferrite grain. The fact that is located all over the ferrite grain, indicates several points with a high Cr-concentration where sigma could nucleate and grow from. The absence of ferrite phase is noticeable since the microstructure consists in 33% sigma and 57% austenite and 10% secondary austenite.
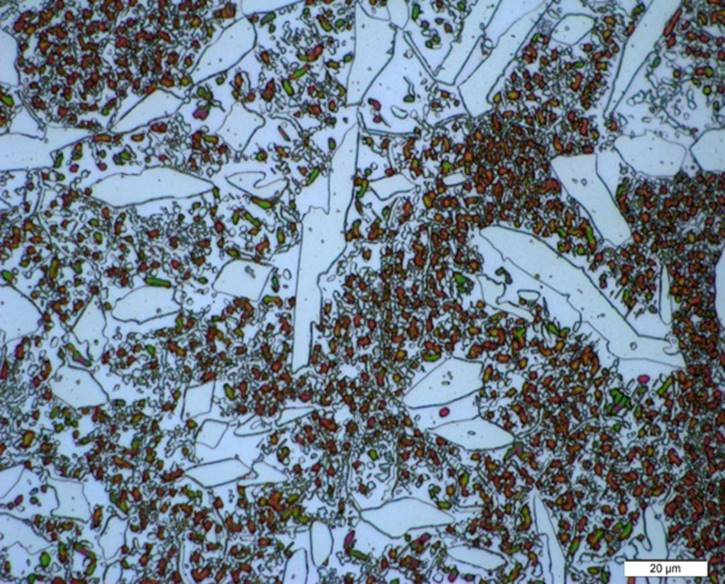
Figure 4 Microstructure of UNS S33207 after annealing heat treatment at 1040 ºC, showing particles of sigma phase inside the ferrite grains
The growth of the primary austenite grains is evident, showing a smooth appearance and free of precipitates. It is well known that solution annealing is given to duplex stainless steels in order to restore the mechanical properties and corrosion resistance after hot forming or welding processes through the austenite/ferrite balance. However, the occurrence of sigma phase at 1040 °C points out that is still thermodynamically stable at that temperature, promoting the decomposition of ferrite into a mixture of austenite and sigma in order to reach the equilibrium instead of obtaining the desired microstructure. The fact that the prior ferrite grains are still distinguished, suggest that the decomposition of ferrite into sigma and secondary austenite took place before the transformation from primary austenite to ferrite. This behavior can be related to the diffusion of chromium and molybdenum in the ferrite phase, which are concentrated in the grain center, promoting the nucleation of sigma phase particles. Sigma phase then depletes the adjacent ferrite in these elements and induces the formation of yet more secondary austenite and a eutectoid structure forms (Karlsson, 1999). Since the sigma phase is driven by the high contents of chromium and molybdenum, the kinetics of formation is faster in the experimental samples of UNS S33207 than in any other duplex alloys.
After solution annealing at 1100 ºC, the experimental samples in Figure 5 showed the presence of sigma (10%), ferrite (50%) and austenite (40%), indicating that the temperature range of sigma phase is extended to a high temperature compared to another grade of duplex alloys. Nevertheless, it is well known that intermetallic precipitation occurs in the temperature range 600 ºC-1000 ºC for all duplex grades (Chih, 2012). It can be observed mainly the original ferrite grain, suggesting that sigma phase formed intragranularly, nucleating within the ferrite grains and grow to the center of the grain. Since all the chromium and molybdenum from the ferrite goes directly to form sigma phase, the previous ferrite remains with no changes in the original morphology.

Figure 5 Microstructure of UNS S33207 after annealing heat treatment at 1100°C, showing particles of sigma phase inside the ferrite grains
The microstructure after heat treatment at 1175 °C for one hour and water quenching in Figure 6, shows the ferrite matrix (70%) with small islands of austenite (30%) and free of secondary phases. It can be noticed that the most predominant phase is ferrite since the ferrite/austenite transformation was not enough during cooling, which is in a good agreement with the simulated phase equilibrium diagrams from Figure 2. However, the austenite grains are elongated, result of the partial transformation of austenite from the ferrite occurring during cooling (Ghosh, 2008). The previous ferrite grain can be identified by the austenite contouring the grain boundary, where Widmanstätten side plates of austenite and particles of austenite that grew intragranularly are visible.
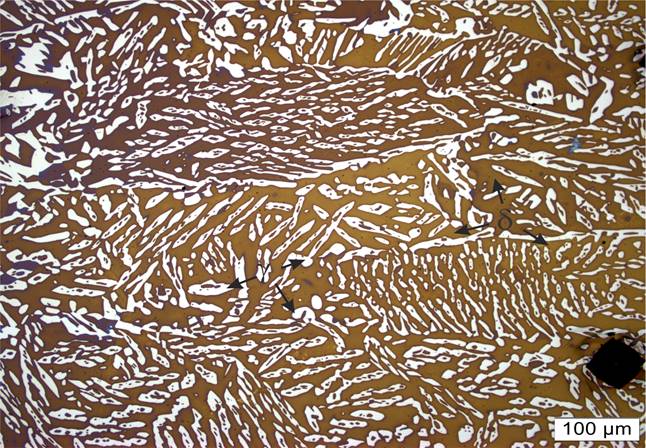
Figure 6 Microstructure of UNS S33207 after solution annealed at 1175°C for one hour and water quenching
After solution annealing at 1200 °C for one hour, cooling down to 1120 °C for 1 minute and then water quenching, the experimental samples showed a balanced microstructure free of secondary phases (Figure 7) with islands of austenite (40%) uniformly distributed all over the ferrite matrix (60%). According to the simulated phase equilibrium diagrams from Figure 2, at 1200 °C the most predominant phase is ferrite. Then, at 1120 °C there is a concurrence of ferrite and austenite. It has been reported that nitrogen is an effective austenite stabilizer at elevated temperatures and can retard the precipitation of intermetallic phases by raising the ferrite to the austenite transformation temperature (Igual, 2005). Therefore, it can be assumed that the balanced microstructure through the ferrite to austenite transformation is governed by the amount of nitrogen present in the alloy.
Conclusions
Solution annealed in the temperature range 1040 ̊C-1100 ̊C for experimental samples of hyperduplex stainless steels is not high enough to avoid sigma phase precipitation, which is assumed to have direct relation with the high content of chromium and molybdenum compared to other duplex grades. At this temperature range, basically all the ferrite transformed to sigma phase and secondary austenite through a eutectoid reaction. After annealing at 1175 °C, the microstructure showed no intermetallic phases. However, the balance of ferrite/austenite was not adequate since the amount of ferrite was high indicating that there was not enough time for the ferrite to transform in austenite during cooling. Nevertheless, the annealing at 1200 °C, cooling down to 1120 °C for one minute and water quenching allowed the time needed for the ferrite/austenite in order to obtain a more balanced microstructure consisting in austenite islands embedded in a ferrite matrix free of intermetallic phases.











 nueva página del texto (beta)
nueva página del texto (beta)