Introduction
Researches were developed in order to increase the productivity of alcoholic fermentation processes. The productivity, expressed as grams of ethanol produced per hour per unit of fermentation volume, can be increased by optimizing the composition of the culture medium, the selection of an appropriate microorganism strain or through the adaptation of the design of reactors (Amin et al., 1983). One challenge is to reduce ethanol production costs and an alternative is to low the cost of the culture media, which can represent about 30 % of the final production costs (Sivers et al., 1994).
Some fermentation technologies were developed to improve the production of ethanol and the concentration in the culture media (Amin and Verachtert, 1982; Demirci et al., 1997; Siripattanakul-Ratpukdi, 2012). Among these, the immobilization technology offers advantages in contrast to free cell cultures, such as increased retention time in bioreactors, high cellular metabolic activity, high cell load and protection of the cells (Tam et al., 1994; Siripattanakul-Ratpukdi, 2012).
The immobilization cell technologies were applied for purposes as the production of hydrogen (Wu et al., 2003) and compounds commercially used in the food industry (Kawaguti et al., 2006). Some studies were developed with immobilized algal cells to remove nutrients (N and P) from wastewater; phenol and hexavalent chromium (Bandhyopadhyay et al., 2001; Park et al., 2002; Humphries et al., 2005; Ruiz-Marin et al., 2010). Other studies for bio-ethanol production from waste materials used strains of Zymomonas and Saccharomyces (Amin and Verachtert, 1982; Amin et al., 1983; Krishnan et al., 2000; Vallejo-Becerra et al., 2008; Yu et al., 2010; Siripattanakul-Ratpukdi, 2012).
The immobilization technology provides the possibility of efficiently symbiotic bacteria incorporation (Travieso et al., 1996; De-Bashan et al., 2002). The interaction between two microorganisms in the same matrix is called coimmobilization and this association can be positive with higher growth and production. However, there are less applications in the ethanol production involving the immobilization of mixed-culture systems and coimmobilized cultures.
In a petroleum deficiency situation, bio-ethanol from yeast and bacterial fermentation has become a promising alternative source for fuel. Agricultural and industrial waste containing sugar, starch, and cellulose, such as cassava peels, fruit bunches, and the effluents from sugar and pineapple cannery productions were successfully applied for the bioethanol production (Nigan 2000; Kassim et al., 2011; Sarkar et al., 2012; Colognesi et al., 2015). According to Liu et al., (2010), high concentration studies showed ethanol production, from corncob residues of 57.2 g L-1 during 141.5 h fermentation, and ethanol yield of 26.1 and 85.2 % conversion of substrate.
The municipality of Ciudad del Carmen, Campeche, Mexico, has an annual production of 2868 ha mango (Mangifera indica). The lack of local market and the poor fruit distribution to other locations causes the product becomes a waste, with significant losses. Hence the need to seek alternatives to use these wastes and generate added value in the economy of the region.
The aim of this study was to determine whether the association between S. cerevisiae coimmobilized with Z. mobilis improves growth and ethanol production, and to create an opportunity for using a regional fruit (M. indica) for ethanol production. In this study, both microorganisms were confined in small alginate beads, a practical means of using microorganisms for environmental applications.
Materials and Methods
Microorganism and medium
The yeast strain S. cerevisiae (ATCC® 2601) and bacteria Z. mobilis (ATCC® 8938) were obtained from the laboratory Microbiologis® and used for fermentation in coimmobilized and immobilized systems. Both microorganisms were cultured in a medium (g L-1), as described by Demirci et al. (1997): 20 g glucose, 6 g yeast extract, 0.23 g CaCl2•2H2O, 4g (NH4)2SO4, 1 g MgSO4•7H2O, and 1.5 g KH2PO4, autoclaved. Strains were maintained in 250 mL of culture at 30 °C and pH 4.5 with manual shaking three times every day. Transfers of fresh medium were made every 24 h for 3 consecutive d prior to use in experiments. The selected culture medium allows favorable growth for both microorganisms, similar to the reported for the culture medium described by Ramasamy and Paramasamy, (2001) for S. cerevisiae and Z. mobilis, allowing adjustment of total sugars content (20 %) relative to the mango juice.
Preparation of immobilized and coimmobilized cells
Fort the preparation of immobilized cells we used the technique described by Tam and Wong (2000). Both microorganisms were harvested by centrifugation at 3500 rpm for 10 min. The bacteria and yeast cells were resuspended in 50 mL of distilled water to get a concentrated cell suspension. The suspension was then mixed with a 4 % sodium alginate solution (1:1 v:v) to obtain a mixture of 2 % microorganism-alginate suspension. The mixture was transferred to a 50 mL burette and drops were formed when titrated into a calcium chloride solution (2 %). This method produced approximately 6500 uniform alginate beads of 2.5 mm in diameter with biomass content for Z. mobilis-alginate beads of 0.0055 g per bead, and for S. cerevisiae of 0.00317 g per bead for every 100 mL of the microorganism-alginate mixture. The beads were kept for hardening in the CaCl2 solution for 4 h at 25±2 °C; then rinsed with sterile saline solution (0.85 % NaCl) and subsequently with distilled water. A concentration of 2.6 beads mL-1 of medium (equivalent to 1:25 bead: medium v/v) were placed in a Chemostat Ommi Culture Plus (Virtis) containing 2 L of culture medium. The reactor was maintained at 120 rpm and 30 °C.
A similar procedure was used for coimmobilization. The difference was that the concentrate of bacteria (25 mL) and yeast (25 mL) was mixed first, and afterwards mixed with 50 mL of alginate. This procedure allowed retaining the same concentration of cells in all experiments.
Experimental setup and procedure
This study evaluated growth in cultures with free cells, immobilized and coimmobilized, as well as ethanol production. It also evaluated the effect of glucose concentration (50-100 g L-1) in the production of ethanol in the system selected in the first experiment, based on the ethanol productivity. A low concentration of substrate was used in order to prevent the inhibition effect of substrate and products.
Fermentation was carried out in a Chemostat Ommi Culture Plus (Virtis) with a volume of 2 L operation, adjusting stirring at 120 rpm and at 30 °C. The medium was similar to that described by Demirci et al. (1997), by adjusting the composition to a concentration of 200 g L-1 glucose (20 % total sugars and 4.5 % reducing sugars), equivalent to that observed in the mango juice.
The experimental design consisted of triplicate cultures in a Chemostat reactor Ommi Culture Plus for S. cerevisiae and Z. mobilis in free cell culture, immobilized and coimmobilized. For each experiment, the biomass was collected, as well as samples of the culture medium to the end of the logarithmic phase every 20 h.
For determination of ash-free dry weights, five beads were dissolved in 5 mL of 0.25 M Na2HPO4.7H2O solution (pH 7.0) in triplicate and filtered through a GF-C glass fiber filter (2.5 cm diameter), previously rinsed with distilled water, and incinerated at 470 °C for 4 h. The samples were dried at 120 °C and put to constant weight for 2 h in a conventional oven and then in a muffle furnace at 450 °C for 3 h. The soluble solids of each fermenting medium was determined every 20 h by taking 1 mL aliquot from each reactor and testing for the °Brix level in an refractometer.
Ethanol content (%, v:v) was obtained using the Anton Paar DMA 4100M instrument, which determines the density of the mixture in relation to the standard OIML-STD-90, which can determine the content of distillate ethanol (v:v, %). According to the ethanol density recorded, it was possible to obtain the ethanol content (g of ethanol per L of culture) produced for each experiment. Prior to the determination of the ethanol content, a distillation of cultures was carried out with a plate column distiller PS-DA-005/PE of four plates, at small-scale. The cooling water flow was 3 L h-1 at 15 °C. An aliquot of 3 L was distilled for 4 h, maintaining the operating conditions at atmospheric pressure, without reflux and with a temperature ramp in the heating jacket of 30 °C up to 80 °C.
Statistical analysis
STATISTICA 7.0 software was used for statistical analysis, and the mean and standard deviation for each treatment were calculated. The covariance analysis (ANCOVA) was used (p≤0.05) to evaluate the grown in free cell cultures, immobilized and coimmobilized; besides, the Tukey test (p≤0.05) was utilized.
Results and Discussion
Growth
In free cell cultures, the growth was observed immediately after being inoculated in the reactor of 2 L. Growth kinetics shows an exponential phase for S. cerevisiae and Z. mobilis of 120 h. After this period of cultivation, both species showed a decline in the production of biomass, finalizing treatment after 200 h of culture. The maximum values of biomass concentration were 14.18 g L-1 and 11.80 g L-1 dry weight for S. cerevisiae and Z. mobilis, respectively. Both microorganisms grew satisfactorily under the culture conditions used in this study (Figure 1A), with a higher specific growth rate (μ) for S. cerevisiae (0.0547 d-1) than Z. mobilis (0.0418 d-1). Specific growth rates in free cell cultures for both microorganisms were not different (p>0.05).

Figure 1: Average increase of biomass for Saccharomyces cerevisiae and Zymomonas mobilis in free culture (A) and immobilized cells (B).
For immobilized cells, both yeast and bacteria presented immediate growth after adding the beads to the culture medium. In both treatments the exponential phase of growth reached a maximum of 80 h. Although both microorganisms were immobilized under the same procedure, the content of biomass per bead at the beginning of treatment was lower for Z. mobilis (0.0031 g) than S. cerevisiae (0.0039 g). Despite these differences, both microorganisms tolerated immobilization (Figure 1B), reaching maximum biomass content values of 0.0055 and 0.0047 g per bead for S. cerevisiae and Z. mobilis; this showed a similar specific growth rate (0.142 d-1) as S. cerevisiae (0.106 d-1).
Glucose-substrate removal
The decrease of substrate showed significant differences (Tukey test; p≤0.0001) between treatments with free and immobilized cells for both species (Table 1). However, the two species in free culture were not different (p>0.05) in 200 h of treatment. For the immobilized and coimmobilized cell cultures, only the immobilized Z. mobilis showed no significant differences (p=0.245) during removal of the substrate with the coimmobilized system during 140 h of culture (Table 1).
Table 1: Uptake rate, productivity (Y) and ethanol mole produced per glucose mole for Saccharomyces cerevisiae and Zymomonas mobilis in free culture, immobilized and coimmobilized.
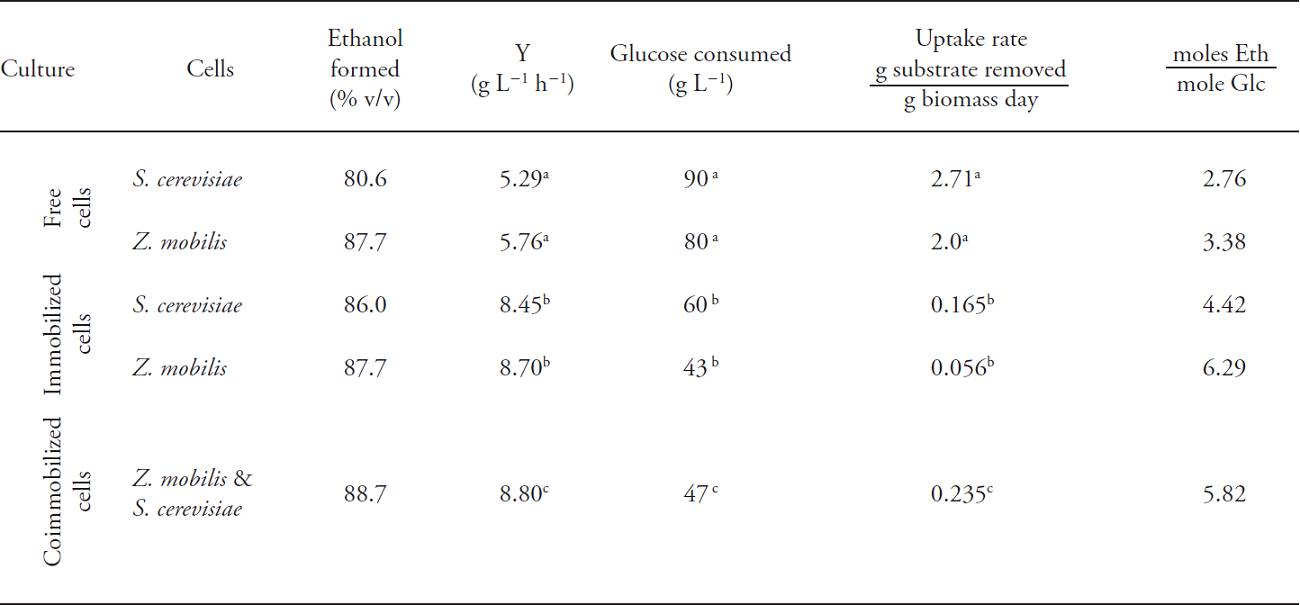
*Means with different letters are statistically different (Tukey; p≤0.05).
The consumed substrate was greater in free culture for S. cerevisiae and Z. mobilis from 200 g L-1 to 80 g L-1 (60 % removal) after the 200 h treatment period (Table 1), compared to the immobilized system with 40 % removal for S. cerevisiae (120 to 200 g L-1) and 30 % removal for Z. mobilis (140 to 200 g L-1). In those cultures of coimmobilized cells consumption ranged from 130 to 200 g L-1 (35 % removal) (Table 1).
The average consumption analysis, based on removal rates determined during the exponential growth for both species, showed that free culture S. cerevisiae and Z. mobilis reached removal rates of 2.0 and 2.7 g substrate g-1 biomass d-1. This suggested greater productivity for the bacteria (5.76 g h-1) than yeast (5.29 g h-1) (Table 1).
In cultures with immobilized cells, the removal rate in the exponential phase (80 h) was greater for S. cerevisiae (0.165 g substrate g-1 biomass d-1) than Z. mobilis (0.056 g substrate g-1 biomass d-1), but in coimmobilized culture it was greater (0.235 g substrate g-1 biomass d-1) since both species contributed to reducing glucose and increasing the removal rate. Similar results were observed in the productivity, where the coimmobilized cell culture showed higher values (8.80 g L-1 h-1) than the immobilized cells of S. cerevisiae and Z. mobilis (Table 1). The highest productivity levels were recorded in coimmobilized and immobilized cultures because the shorter ethanol production time (80 h) than free cultures (120 h).
Effect of initial concentration of glucose on ethanol production
For the coimmobilized of Z. mobilis and S. cerevisiae within alginate beads there was an immediate increase in biomass concentration. Although the biomass concentration for both treatment showed significant differences (p≤0.002), the results suggest that both concentration of substrate allowed favorable bacteria and yeast growth (Figure 2). The maximum biomass concentration in the treatment of glucose to 50 g L-1 (Gl50) was obtained in the first 100 h of culture with 0.0063 g per beads; whereas for the treatment of 200 g L-1 glucose (Gl200) was 0.053 g per beads during 80 h (Figure 2).
The alcohol produced (%, v:v) had no significant differences (p>0.05) with respect to glucose concentration. However, uptake rates exhibit a decline as the glucose content in the reactor decreases (Table 2). The highest uptake rate occurred at a concentration of 200 g L-1 glucose with a 76.5 % removal, compared to 50 g L-1 glucose. Although the production of alcohol was similar in both treatments, the ratio mol-ethanol produced per consumed molglucose was higher in cultures of 50 g L-1 glucose with a value of 6.91, in comparison to 200 g L-1 glucose with a ratio of 5.82 mol ethanol produced per consumed mol glucose (Table 2). Similarly, higher productivity was obtained at a lower glucose concentration compared to a medium with high glucose content.
Table 2: Productivity (Y), uptake rate and ethanol mole produced per glucose with minimum content of glucose for coimmobilized Zymomonas mobilis and Saccharomyces cerevisiase.

C0: Initial concentration of glucose (g L-1). Means with different letters are statistically different (Tukey; p≤0.05).
Immobilized systems have a greater capacity of cell growth and high metabolic activity (Cohen, 2000; Mallick, 2002); this is consistent with the results obtained in our study. The result showed a high specific growth rates for immobilized Z. mobilis and S. cerevisiae with respect to free cell cultures, suggesting that immobilization positively affects the growth for both microorganisms and increase the biomass concentrations. Furthermore, this high metabolic activity in immobilized cell was observed with a decrease of substrate concentration in a shorter time of treatment compared to free cell cultures. The short time of treatment for immobilized cell culture could be attributed to the increase of biomass within the beads and, consequently, an immediate decay of the substrate. However, this indicates that with increasing cell population, within the beads, nutrients can be limited, especially for cells located at the center of the beads, causing a decrease in cellular activity (Uemoto and Saiki, 2000; Mallick, 2002).
The rapid decline in the cell density for immobilized cells culture could be attributed to the production of CO2 due to fermentation activity. Adverse effect of CO2 results because its diffusion is lower than its production, and it will accumulate inside of alginate bead (Kim and Choi, 1984). In our study, the CO2 observed in the reactor as bubbles attached on the surface of the beads suggests that the spread of CO2 in the first 80 h did not inhibit growth and alcohol production. Although CO2 concentration was not determined in our study, after this time the gas saturation in the reactor was probably high, affecting the CO2 diffusion. This is probably due to a limitation in the nutrients transport and subsequent inhibition of growth, which caused lower glucose consumption compared to free cells (Table 1).
For the free cells culture, the low percentage of alcohol obtained by yeast during the fermentation culture period could be attributed to the fact that this is affected (inhibit metabolism and decrease efficiency) by the ethanol concentration in the culture medium, as reported by Nuwamanya et al. (2012); unlike bacteria Z. mobilis as observed by Kim and Choi (1984).
In our study, the lowest biomass produced by the bacteria (0.0047 g L-1) with respect to yeast (0.0055 g L-1) may be practical from the standpoint of waste generation. This result is similar to those reported by Amin and Verachtert (1982) for Z. mobilis and S. bayanus immobilized in carrageenan with 5.6 g L-1 and 9.9 g L-1, respectively.
Ethanol production was not inhibited in immobilized or coimmobilized systems, and even showed higher productivity than to free cells (Table 1), suggesting a high sugar-conversion efficiency for the immobilized cells system. The productivity (Y) obtained for Z. mobilis alginate-immobilized (8.7 g L-1 h-1) was higher than 1.6 g L-1 h-1 reported for Z. mobilis immobilized in carrageenan (Krishnan et al., 2000). This difference may be attributed to the lower glucose concentration in the culture medium (32 g L-1) than the 200 g L-1 used. The result suggest a high conversion of substrate for immobilized systems than free cells of yeast and bacteria. These results were higher than those reported by Amin and Verachtert (1982) for Z. mobilis and S. bayanus immobilized in carrageenan, with 1.8 to 1.9 mole of ethanol produced per mole of consumed glucose. Gunasekaran et al. (1986) and Krishnan et al. (2000) suggest that Z. mobilis is a good candidate to obtain alcohol with 1.9 mole ethanol per mole of glucose. According to Rogers et al. (1979) specific productivity of ethanol (g ethanol g-1 biomass dry weight) is greater for Zymomonas than for S. uvarum.
Immobilization and coimmobilization exhibited a lower uptake rate than free cells; this shows that there was less substrate consumption (Table 1). Nevertheless, the greater productivity indicates that it is possible to obtain high alcohol content with a lower requirement of substrate, but with the disadvantage of residual glucose in the medium. This problem can be solved with sequenced systems, as suggested by Demirci et al. (1997). Another alternative of solving this problem is to increase the cell number or inoculum size within the reactor. This is reasonable because a high number of cells could create a greater sorption of substrate (glucose) into the cell and consumed substrate. However, Siripattanakul-Ratpukdi (2012) suggested that with different cell yeast loads, the same reduction (>90 %) of substrate is obtained at the end of a treatment period of 10 h.
The low glucose reduction in our study in alginate beads can be attributed to the decline in cell density and substrate diffusion within the alginate matrix. The adsorption of substrate by the matrix was observed in the first hours of treatment, with a possible decrease of substrate diffusion within the matrix in a continuous process (Siripattanakul and Khan, 2010).
Robinson et al. (1989) suggested that the diffusion rate within the alginate matrix depends on the concentration gradient between the culture medium and matrix; this is, when the nutrient concentration in the culture medium decreases, the diffusion rate occurs within the matrix and therefore the removal rate. In our study, during the first hours of treatment the substrate decreased and probably the matrix had a partial saturation with glucose; but this had not a negative effect on the both microorganisms growth. This, the immobilized cell system successfully decreased glucose by matrix adsorption (immobilized glucose) and biodegradation (bioconversion of glucose), being the main process the biodegradation. This suggests that the main factor that could limit glucose removal might be the high concentration of CO2 in the reactor. For immobilized cultures, the high concentration of glucose dissolved in the culture medium (200 g L-1) caused an immediate decline in growth, compared to cultures with 50 mg L-1. However, this did not have any growth inhibition and negative effect on productivity and ethanol produced (Table 2).
The high uptake rate for cultures with 200 g L-1 confirms that the high concentration of glucose saturates beads faster, allowing rapid availability of substrate for the microorganisms within the beads (Table 2). However, the cultures with high concentration of substrate does not necessarily suggest a high substrate conversion; at a lower uptake rate (minimum substrate concentration) it is possible to obtain a higher conversion compared to enriched cultures. Therefore, the low concentration of substrate of the culture medium indicates the presence of a soft transport and substrate accumulation within the matrix, allowing a proper consumption and growth of bacteria and yeast. Thus, this may actually increase the production of alcohol with minimal residual glucose, reaching 6.91 moles of ethanol per mole of glucose, in comparison to a high glucose concentration (Table 2).
Conclusions
The association of Z. mobilis and S. cereviase was positive, obtaining a higher ethanol content and high conversion of substrate compared to free and immobilized cells. The immobilization technology offers an alternative by increasing productivity and substrate conversion compared with free cells culture systems. Higher conversion capacity to obtain alcohol was observed with lower substrate.
The possible substrate inhibition was not a factor affecting cell growth in both organisms. Therefore the immobilized cell technology successfully reduced glucose by the matrix adsorption (immobilized glucose) and biodegradation (bioconversion of glucose); the latter was the main process. This suggests that the main factor that could limit further growth was the high concentration of CO2 in the reactor. Furthermore, although no significant differences were detected in the alcohol content in immobilized culture in diluted medium, the conversion from glucose to ethanol is greater in those media with a glucose concentration of 50 g L-1. For practical purposes, it is desirable that the fermentation of waste organic be performed through dilutions to increase the homogeneity of alginate beads within the reactor and, consequently, allow the diffusion of CO2 and substrate through the beads.











 texto en
texto en 



