Introduction
Functional foods produce beneficial physiological effects when consumed on a regular basis because they are a source of biologically active components. In general, they are said to improve health, so they are used in the treatment and prevention of various diseases (Norberto et al., 2013). These properties are associated with the presence of phytochemicals such as glucosinolates (sulfur compounds in the Alliaceae but mainly in Brassicaceae), terpenoids (carotenoids, monoterpenoids and phytosterols) and several groups of polyphenols (mainly flavones, isoflavones, stilbenes, ellagic acid and anthocyanins) (Chen, Remondetto, & Subirade, 2006).
Anthocyanins give the red and blue color to plant organs such as fruits, flowers and leaves (Pojer, Mattivi, Johnson, & Stockley, 2013). This type of polyphenol has pharmacological properties such as reduction of coronary disease and anticancer, antitumor, anti-inflammatory and antidiabetic effects; in addition, they improve visual acuity and cognitive behavior. The pharmacological effects of anthocyanins are related to their antioxidant activity (Hopkins, Lamm, Funk, & Ritenbaugh, 2013; Kim, Joo, & Yoo, 2009).
An important source of anthocyanins is roselle (Hibiscus sabdariffa L.) calyces, which are consumed mainly as refreshing drinks (Prenesti, Berto, Daniele, & Taso, 2007). The color of Hibiscus calyces varies from yellowish green to deep red and is related to the content of these phytochemicals (Christian & Jackson, 2009). Thus, the varieties of dark calyces have an anthocyanin concentration five to seven times greater than that of clear calyces; on the other hand, the green and yellow varieties lack these compounds (Salinas-Moreno, Zúñiga-Hernández, Jiménez-de la Torre, Serrano-Altamirano, & Sánchez-Feria, 2012).
The environmental conditions during roselle production have a direct effect on the content of the main polyphenols present in the calyces. Depending on the production site, the amount of anthocyanins can even double in some varieties (Juliani et al., 2009). The increase in the anthocyanin content in the calyces could improve the nutraceutical quality and thereby increase the product’s value. Currently, in roselle marketing, the most important quality criteria are the acidity of the extracts and the anthocyanin content; the latter is related to the number of extractions that can be made from the calyces (Galicia-Flores, Salina-Moreno, Espinosa-García, & Sánchez-Feria, 2008).
Various types of stress in plants, such as pathogen attack, UV-B radiation, low temperatures, N limitations, and drought, as well as the application of pro-oxidant compounds such as H2O2, salicylic acid or heavy metals (copper, chromium, zinc, iron, nickel, cobalt), can cause changes in metabolism and thus modify the concentration of flavonoids (Xing, Huang, & Liu, 2010). Exposure to heavy metals can cause an increase in the production of reactive species or free radicals such as superoxide, hydrogen peroxide and hydroxyl radicals (Stohs & Bagchi, 1995). Under these conditions the genes of some plants are activated and modulate the biosynthesis of flavonoids such as anthocyanins; consequently, the levels of these phytochemicals increase during exposure to stress (Hernández, Alegre, van Breusegem, & Munné-Bosch, 2009). The inductive synthesis of anthocyanins is the result of the activation of genes and is carried out in order to increase the antioxidant response of the plant to maintain the physiological status of tissues, directly or indirectly affected by stress (Ling-Peng, Xin-Jiao, & Hai-Hu, 2012).
Anthocyanins have the ability to protect the plant against oxidative stress, due to their ability to stabilize the unpaired electrons of free radicals, this by donating hydrogen atoms. It has been reported that anthocyanins have greater antioxidant capacity than vitamins C and E (Hernández et al., 2009; Sytar et al., 2013). In addition, various types of anthocyanins such as 3-sambubioside of delphinidin and 3-sambubioside of cyanidin, present in roselle calyces, also have the ability to form complexes with metals and thus protect the plant against harmful effects (Castañeda-Ovando, Galán-Vidal, Pacheco-Hernández, Rodríguez, & Páez-Hernández, 2009; Galicia-Flores et al., 2008).
In some plant species such as Arabidopsis thaliana, in vitro exposure to essential metals (Cu, Zn and Mn) and non-essential ones (Pb and Hg) stimulated the synthesis of anthocyanins; this effect was proportional to the element dose (Baek et al., 2012). Cu at a dose of 250 μM was the most effective to increase polyphenol levels from 2 to 32.5 µg. g-1 of fresh matter (FM) and with Zn the maximum increase was with 1,000 µM (14 µg. g-1 FM). However, high doses of these elements reduced root and shoot length in A. thaliana (Baek et al., 2012). The application of other elements such as As also caused an increase in the anthocyanin concentration; thus, in Lemna gibba the highest content of these metabolites was found with 1.5 mg of As per L (45 mg. 100 g-1 FM) (Alves-Leão, Alves-de Oliveira, Arantes-Felipe, Santos-Fernese, & Soares-Gusman, 2014). In Atriplex hortensis var. Purpurea and A. rosea exposed to treatments with different concentrations of Cu, Ni, Pb and Zn, no changes were observed in the anthocyanin concentration (Sai et al., 2015). According to Alves-Leão et al. (2014), plants respond differently to heavy metal stress, depending on the type of metal, the concentration and the duration of the stress.
In roselle calyces it has been documented that the application of cobalt sulphate and nickel (20 to 25 mg. kg-1 of soil, respectively) increased the concentration of anthocyanins and other flavonoids (Aziz, Gad, & Badran, 2007); however, foliar application could make this response more efficient. On the other hand, Zn and Cu are nutrients sold as foliar fertilizers and, because they are essential elements, they help improve plant nutrition, which also results in a better agricultural yield. Studies on the induction of oxidative stress in roselle cultivation through the application of heavy metals to increase the anthocyanin concentration are limited, so the objective of the present research was to determine the effect of foliar application of Zn and Cu at high concentrations on calyx yield, anthocyanin concentration and physicochemical characteristics in three roselle genotypes.
Materials and methods
Study location
The experiment was established in the field under rainfed conditions in the town of Cajones, belonging to the municipality of Gabriel Zamora, Michoacán, Mexico (19º 10' 49" North latitude and 101º 58' 13" West longitude, at 503 masl). The area has an Aw0 climate, corresponding to warm subhumid with summer rains (García, 2004).
Establishment of the experiment and application of treatments
On July 8, 2015, the following roselle genotypes were sown: Criolla Guerrero (CG), Criolla Michoacán (CM) and Reina Roja (RR) (Figure 1), which are highly marketed in Guerrero and Michoacán. For the study, the seeds were provided by some producers of these states. The three genotypes were subjected to foliar sprays with Zn and Cu separately, and to the control (without foliar application, 0). The combination of genotypes with treatments generated nine combinations (CG-0, CG-Zn, CG-Cu, CM-0, CM-Zn, CM-Cu, RR-0, RR-Zn, RR-Cu), which were distributed in the field under a randomized complete block experimental design in a split-plot arrangement, with four replications. The large plot was the genotype and the small plot the micronutrients. In total there were 36 experimental units, each with three furrows 3 m long and 0.8 m apart. Two seeds per plant were sown every 0.5 m for a population density of five plants per m2. From 15 days after emergence, foliar applications were made up to dripping point with 300 mg. L-1 of zinc (Zn) and copper (Cu), plus a control without spraying. In total, four applications were made each with seven-day intervals.

Figure 1 Roselle genotypes used in the study: A) Criolla Guerrero, B) Reina Roja and C) Criolla Michoacán.
The sources of the micronutrients were: zinc sulfate heptahydrate (ZnSO4. 7H2O) and cupric sulfate pentahydrate (CuSO4. 5H2O). Additionally, 1 mL. L-1 of Inex® adherent was added to each mixture. For its application, a 15-L capacity pump equipped with an empty (hollow) cone nozzle was used.
Soil fertilization was carried out with 80 and 40 kg. ha-1 of N and P2O5, applied as urea (46-0-0) and triple calcium superphosphate (0-46-0). In addition, 100 % P2O5 and 50 % N were applied at 15 days after sowing (das), and the rest of the N at 45 das.
Determination of yield and its components
The harvest was carried out at physiological maturity, when the fruits started with brown coloration and the seeds presented black coloration. This stage varied among genotypes; in Criolla Guerrero and Reina Roja it occurred at approximately 145 das and in Criolla Michoacán at 156 das. Calyces without fruit were harvested from the central row of each experimental unit, and in these structures the following yield components were measured: length and diameter of calyces, number of calyces per plant and weight of 10 calyces. Subsequently, they were dried in a forced-air circulation oven at 50 °C until constant weight.
Determination of physicochemical characteristics in dry calyces
Aqueous extracts were prepared in duplicate from each replication of the different treatments, for which 15 g of calyces in dry matter basis were pulverized in a mill (IKA® brand, model MF 10) with 0.5-mm mesh. From the powder, 2.5 g were weighed and placed in 250-mL glass bottles (Schott Duran® brand), where 200 mL of distilled water were added. The mixture was left to stand for 24 h at room temperature in the dark, after which the extracts were placed in a water bath at 40 °C for 15 min. The obtained extracts were measured for: pH with a Beckman pH Meter® potentiometer (method 981.12), total soluble solids (method 932.12) with an ATAGO® digital refractometer (0 to 32 % scale), titratable acidity as a percentage of citric acid present (method 942.15) and ascorbic acid content (method 967.21) with 2,6-dichlorophenolindophenol. The determinations were made according to the methodology of the Association of Official Analytical Chemists (AOAC, 1980).
The anthocyanin concentration was determined with the Abdel-Aal and Hucl (1999) method, for which 100 mg of each pulverized sample were weighed and transferred to centrifuge tubes, after which 10 mL of acidified methanol extractor solution (methanol: 1.5 N HCl at a ratio of 85:15 v/v) were added. The mixture was left to stand for 24 h in dark conditions at 4 °C. Subsequently, to avoid variation in the volume of the extractor solution due to evaporation, it was filled to the initial volume. The samples were centrifuged at 5,000 rpm for 20 min in a Heraeus™ Biofuge Primo R centrifuge. A 1:10 dilution (100 μL of the concentrated extract + 900 μL of acidified methanol) was made. Finally, the absorbance of the diluted extract was read at a wavelength of 533 nm in a Genesys® 10 UV (Thermo Spectronic) spectrophotometer; the extractor solution was used as the target.
The anthocyanin content was estimated as equivalents of cyanidin-3-glucoside with the equation: A = (Ab/ɛ)(Ve/1,000)PM(1/Ph)(10 6 ), where A is the anthocyanin content, Ab is the absorbance of the extract, ɛ is the molar extinction coefficient of cyanidin-3-glucoside (25,965 cm-1.mol-1), Ve is the total volume of the extract, PM is the molecular weight of the cyanidin-3-glucoside (449) and Ph is the weight of the sample. The total anthocyanin content was expressed in mg of anthocyanins per g of dry matter of calyces.
Statistical analysis
The data of the variables were analyzed using an analysis of variance and Tukey’s range test (P ≤ 0.05), both with the Statistical Analysis System package (SAS, 2002).
Results and discussion
Calyx yield and its components
Applying Zn and Cu did not statistically modify (P ≤ 0.05) the length and diameter of calyces but reduced the number of calyces per plant in Criolla de Guerrero and Reina Roja. In these same genotypes, the Zn significantly increased the weight of the calyces, while the Cu favored this variable only in Reina Roja (Tables 1 and 2). This generated variation in yield in the genotype x micronutrient interaction; thus, in Criolla Guerrero the foliar supply of these micronutrients reduced yield; with Zn the decrease was 21 % and with Cu 12 %, although these reductions did not differ statistically (P ≤ 0.05). This result can be attributed to the fact that these elements caused, in the Criolla Guerrero plants, reductions in the size of the plant canopy (leaf area index) and in stem diameter; on average, these variables were 15 % lower than in plants without application.
Table 1 Significance level of the variables evaluated in roselle (Hibiscus sabdariffa) calyces.
| Factor | Yield | Length | Diameter | Number of calyces per plant | Weight of 10 calyces |
|---|---|---|---|---|---|
| Genotype (G) | ** | ** | ** | ** | ** |
| Micronutrient (M) | ns | ns | ns | ** | ** |
| G x M interaction | ** | ** | * | ** | ** |
*, ** P ≤ 0.01 and 0.05, respectively; ns = not significant (P ≥ 0.05)
Table 2 Comparison of means test of the variables evaluated in roselle (Hibiscus sabdariffa) calyces.
| Genotypes | Micronutrient | Length (cm) | Diameter (cm) | Number of calyces | Weight of 10 calyces (g) |
|---|---|---|---|---|---|
| Criolla Guerrero | 01 | 3.5 cz | 3.4 ab | 32.6 bc | 4.35 e |
| Zn | 3.6 c | 3.6 ab | 23.5 d | 4.78 d | |
| Cu | 3.8 bc | 3.7 a | 30.7 c | 4.05 e | |
| Criolla Michoacán | 0 | 3.1 c | 2.0 d | 34.8 ab | 3.42 f |
| Zn | 3.2 c | 2.0 d | 36.4 a | 3.27 f | |
| Cu | 3.3 c | 2.0 d | 34.3 ab | 3.08 f | |
| Reina Roja | 0 | 4.5 ab | 2.9 c | 17.3 e | 7.83 c |
| Zn | 4.8 a | 3.1 bc | 14.9 ef | 11.03 b | |
| Cu | 4.4 ab | 2.9 c | 12.9 f | 11.82 a | |
| Overall mean | 3.81 | 2.80 | 26.35 | 5.96 | |
| LSD | 0.80 | 0.48 | 3.03 | 0.37 | |
| CV (%) | 8.10 | 6.80 | 4.80 | 12.30 | |
10 = no application of micronutrients, Zn = application of Zn, Cu = application of Cu, LSD = least significant difference, CV = coefficient of variation.
zMeans with the same letters within each column do not differ statistically (Tukey, P ≤ 0.05).
Xing, Huang, and Liu (2010) mention that in some plants the use of heavy metals at high concentrations causes oxidative stress, due to the production of reactive species that damage cellular components such as DNA, proteins and lipids, which affects crop growth and yield. Some plants have a mechanism to tolerate heavy metal stress such as metal exclusion, metal accumulation and metal binding with proteins rich in cysteine and metalloproteins. In addition, some compounds in plants that act as markers can stimulate the antioxidant system to combat oxidative damage in tissues as part of their metabolism or defense mechanism (Thounaojam et al., 2012). Thus, in Criolla Michoacán, applying these elements did not cause significant changes (P ≤ 0.05), while in Reina Roja, Zn increased the yield by 15.9 %, so this combination presented the highest calyx yield (1,232 kg.ha-1) of all treatments evaluated (Figure 2).
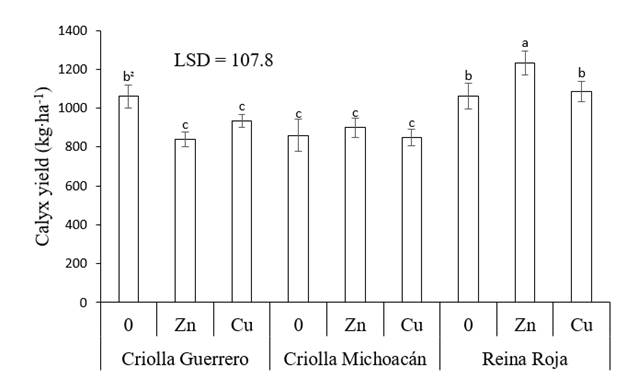
Figure 2 Dry calyx yield of roselle (Hibiscus sabdariffa) as a function of the genotype and application of micronutrients. 0 = no application of micronutrients, Zn = application of Zn, Cu = application of Cu and LSD = least significant difference. zMeans with the same letters between bars do not differ statistically (P ≤ 0.05).
The results indicate that the genotypes studied showed a different degree of sensitivity to high concentrations of foliar-supplied micronutrients (300 mg. L-1 per application), a response that can also be attributed to the differences among genotypes. In this sense, Reina Roja has thicker and larger leaves than the other two genotypes; likewise, Criolla Michoacán is the genotype with the longest growth cycle. Both characteristics may have reduced the stress caused by the application of heavy metals. Differences in the effect resulting from the application of these micronutrients have been observed in other agricultural crops such as wheat (Triticum aestivum L.), rice (Oryza sativa L.) and common bean (Phaseolus vulgaris L.), since only in some cases was the agronomic yield improved with the use of Zn (Ram et al., 2016).
Results similar to those of this work in Reina Roja with foliar application of Zn are reported by Aziz et al. (2007), who mention that in roselle with soil-supplied Co (20 and 40 mg. kg-1) and Ni (25 and 50 mg.kg-1), separately and combined, they obtained an increase in the yield of fresh and dry calyces of up to 137 %, where the yield component that was most favored was the number of fruits. Similarly, the cultivars ‘Jumbo,’ ‘Drakkar,’ ‘Cossair’ and ‘Pactol’ of Brassica napus L. subjected to concentrations of 2,000 µM ZnSO4 and 250 µM CdCl2 showed variation in dry matter production. ZnSO4 increased 'Jumbo' production by 71 %, while in the other cultivars it caused reductions of 34 % and Cd in all genotypes caused a 40 % reduction in their yields (Ghnaya, Charles, Hourmant, Hamida, & Branchard, 2009). This confirms the fact that each species responds differently to heavy metal stress (Alves-Leão et al., 2014).
Regardless of the application of heavy metals, it was found that Reina Roja presented the highest calyx yield, followed by Criolla Guerrero and Criolla Michoacán, this as a result of the Reina Roja calyces being the longest and heaviest. The genotype with the largest calyx diameter was Criolla Guerrero, while Criolla Michoacán had more calyces per plant, but both had the least weight (Table 2). This shows variation in yield and yield components among these genotypes. Similar results were found by Atta et al. (2011) in roselle ecotypes grown in the field under warm weather and seasonal rainfall conditions in Nigeria.
Physicochemical characteristics of calyx extract
Applying Cu and Zn at high doses caused highly significant differences (P ≤ 0.01) in titratable acidity and in anthocyanin and ascorbic acid contents, and in none of the genotypes was the pH or total soluble solids modified (Table 3). However, because the Criolla Guerrero calyces were less acidic, in any combination with micronutrients, they presented the highest pH values in comparison with the other treatments (Table 4).
Table 3 Significance level of the physicochemical characteristics of roselle (Hibiscus sabdariffa) calyces.
| Factor | pH | Total soluble solids | Titratable acidity | Anthocyanins | Ascorbic acid |
|---|---|---|---|---|---|
| Genotype (G) | ** | ns | ** | ** | ** |
| Micronutrients (M) | ns | ns | ** | ** | ** |
| G x M interaction | ns | ns | ** | ** | ** |
*, ** P ≤ 0.01 and 0.05, respectively; ns = not significant (P ≥ 0.05).
Table 4 Comparison of means test of the physicochemical characteristics evaluated in roselle (Hibiscus sabdariffa) calyces.
| Genotypes | Micronutrient | pH | Total soluble solids (%) | TA (%) | Ascorbic acid (mg∙100 g -1 ) |
|---|---|---|---|---|---|
| Criolla Guerrero | 01 | 3.93 az | 0.45 a | 8.3 d | 59.9 bc |
| Zn | 3.90 a | 0.45 a | 11.7 c | 39.2 ef | |
| Cu | 3.92 a | 0.42 a | 11.1 c | 33.0 f | |
| Criolla Michoacán | 0 | 3.38 c | 0.37 a | 21.2 a | 82.4 a |
| Zn | 3.36 c | 0.45 a | 23.1 a | 48.7 d | |
| Cu | 3.43 c | 0.42 a | 17.9 b | 44.2 de | |
| Reina Roja | 0 | 3.64 b | 0.37 a | 12.2 c | 66.7 b |
| Zn | 3.62 b | 0.40 a | 12.0 c | 62.2 b | |
| Cu | 3.58 b | 0.37 a | 12.2 c | 51.0 cd | |
| Overall mean | 3.64 | 0.41 | 14.4 | 54.15 | |
| LSD | 0.15 | 0.12 | 1.82 | 9.21 | |
| CV (%) | 1.3 | 12.90 | 5.23 | 5.74 | |
10 = no application of micronutrients, Zn = application of Zn, Cu = application of Cu, LSD = least significant difference, CV = coefficient of variation.
zMeans with the same letters within each column do not differ statistically (Tukey, P ≤ 0.05).
The titratable acidity response resulting from the supply of micronutrients varied depending on the genotype. In Criolla Guerrero an increase of 41 % was recorded with Zn and 33.7 % with Cu, while in Reina Roja no significant differences (P ≤ 0.05) were detected and in Criolla Michoacán the Cu reduced titratable acidity by 15.6 %. Of all the combinations of treatments, Criolla Michoacán without application of these metals and with Zn presented the highest percentage of titratable acidity, and the lowest was in Criolla Guerrero.
In most cases, the ascorbic acid concentration decreased when Cu and Zn were applied. Criolla Michoacán showed the greatest reduction, 40.9 % with Zn and 55.8 % with Cu. In Criolla Guerrero with Zn sprayings, the calyces presented 34.6 % less ascorbic acid and with Cu the decrease was 44.9 %, while in Reina Roja the Cu caused a 23.5 % decrease in ascorbic acid (Table 4).
With Zn and Cu, the anthocyanin concentration increased in all genotypes. The highest increase was recorded in Criolla Michoacán, where the calyces had 175 % more anthocyanins when Zn was applied and 187 % more with Cu. In Criolla Guerrero, Zn increased the anthocyanin content by 60 % and Cu by 39 %. The lowest increase occurred in Reina Roja calyces; with Cu, the anthocyanin concentration rose by 30 %, whereas with Zn it did not statistically differ (P ≤ 0.05) from the control treatment. However, due to the high anthocyanin content in the Reina Roja control, more than double that of Criolla Guerrero and Criolla Michoacán, this genotype recorded 22.7 mg∙g-1 anthocyanins in the dry calyces with the foliar application of Cu, higher than all treatment combinations (Figure 3).
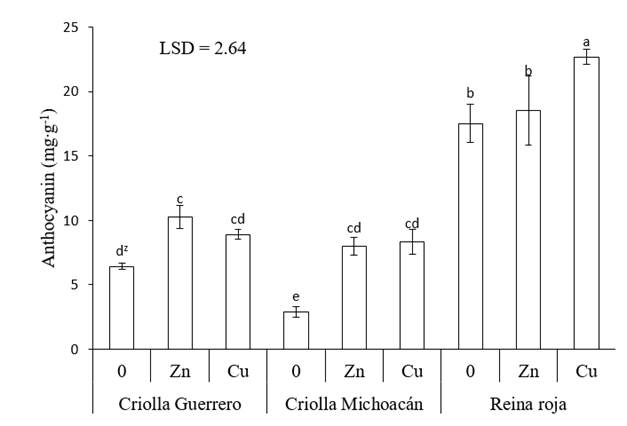
Figure 3 Anthocyanin concentration in roselle (Hibiscus sabdariffa) calyces as a function of the genotype and application of micronutrients. 0 = no application of micronutrients, Zn = application of Zn, Cu = application of Cu and LSD = least significant difference. zMeans with the same letters do not differ statistically (P ≤ 0.05).
Anthocyanins are pigments produced by secondary metabolism that can increase their concentration in plants in response to oxidative stress, caused by several factors, including exposure to high concentrations of metals (Azis et al., 2007). In the present study, the foliar administration of Cu and Zn solutions (300 mg.L-1) at concentrations higher than those required by the crops caused the three roselle genotypes to increase the anthocyanin content in their calyces with respect to the control. The antioxidative properties of anthocyanins arise from their high reactivity as electron donors, from the ability of radicals to stabilize and delocalize the unpaired electron and from their ability to chelate transition metal ions (Alves-Leão et al., 2014).
Results similar to those of the present study have been documented in several crops, such as duckweed (Lemna gibba) by exposure to arsenic (Alves-Leão et al., 2014), in Arabidopsis thaliana under in vitro conditions by addition of Cu, Zn, Mn, Pb and Hg to the culture medium (Baek et al., 2012) and in Capsicum annum with foliar application of Cu and Zn to the young leaves (Stavreva-Veselinovska, Ziranovik, & Djokic, 2010).
With the use of Zn and Cu the percentage of titratable acidity increased, which is important since the acidity has an antibacterial effect and contributes to the absorption of metal ions in the human body. In addition, the sensation of freshness perceived when drinking a cold roselle drink is related to this characteristic (Prenesti et al., 2007). Both Cu and Zn reduced the ascorbic acid content in all genotypes, an effect contrary to what happened with anthocyanins. In this regard, it has been found that the ascorbic acid content in roselle calyces is inversely proportional to the anthocyanin concentration (Salinas-Moreno et al., 2012). Although ascorbic acid also has antioxidant activity, it is lower than that of anthocyanins (Hernández et al., 2009; Sytar et al., 2013).
Conclusions
Applying Cu and Zn reduced calyx yield in the Criolla Guerrero genotype, in Criolla Michoacán they did not modify it and in Reina Roja only the Zn improved it; in turn, these elements increased the amount of anthocyanins and decreased the concentration of ascorbic acid. In Criolla Michoacán, the greatest increases in anthocyanins were recorded, so it was the genotype where the nutraceutical quality was improved the most. The titratable acidity in Criolla Guerrero and Criolla Michoacán was higher with Zn and Cu.
Reina Roja was the most outstanding genotype as it presented the highest calyx yield and anthocyanin concentration.











 texto en
texto en 


