Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista internacional de contaminación ambiental
versión impresa ISSN 0188-4999
Rev. Int. Contam. Ambient vol.30 no.4 Ciudad de México nov. 2014
Agronomic use of produced water in tomato plants (Lycopersicon esculentum L.) under greenhouse conditions
Uso agronómico del "agua producida" en plantas de tomate (Lycopersicon esculentum L.) bajo condiciones de invernadero
Fernando MARTEL-VALLES1, Adalberto BENAVIDES-MENDOZA1*, Rosalinda MENDOZA-VILLARREAL1, Alejandro ZERMEÑO-GONZÁLEZ2 and Antonio JUÁREZ-MALDONADO1
1 Departamento de Horticultura, Universidad Autónoma Agraria Antonio Narro, México *Autor para correspondencia: abenmen@gmail.com
2 Departamento de Riego y Drenaje, Universidad Autónoma Agraria Antonio Narro, México
Recibido mayo 2013;
aceptado agosto 2014
RESUMEN
Las estructuras geológicas productoras de hidrocarburos normalmente contienen aguas congénitas y al ser extraídas durante el proceso industrial de producción de gas o petróleo su composición es modificada y se le llama "agua producida". El objetivo del presente estudio fue caracterizar y verificar la factibilidad del uso de aguas producidas provenientes de la zona de exploración de gas de Sabinas-Piedras Negras de México, para cultivar plantas de tomate bajo condiciones de invernadero. Se establecieron tres tratamientos mezclando aguas producidas provenientes de tres estaciones productoras de gas (Buena Suerte, Monclova 1 y Forasteros), con agua de riego normal. Las proporciones de las mezclas fueron (mL de aguas producidas por L de agua de riego) 133, 3.4 y 125 respectivamente. Se incluyó un testigo en el que se usó solamente solución Steiner. Las aguas producidas se analizaron bajo la NOM-143-SEMARNAT-2003, al igual que los tratamientos. Los resultados mostraron que las mezclas con agua producida proveniente de las estaciones Monclova 1 y Forasteros eran factibles de ser utilizadas para la producción de tomate, ya que las variables morfológicas evaluadas no presentaron diferencias significativas comparadas con el testigo, aunque las plantas regadas con la mezcla con agua de la estación Forasteros mostraron disminución del peso seco de las hojas; pero la concentración promedio de minerales absorbidos por las plantas fue la que más se acercó al testigo. El tratamiento con la mezcla de aguas de la estación Buena Suerte no fue apta para uso agrícola porque afectó negativamente el diámetro de tallo, el peso seco de la hoja, la longitud de raíz, limitó la absorción mineral, además de causar la muerte del 58 % de las plantas.
Palabras clave: aguas congénitas, salinidad, agricultura, calidad del agua.
ABSTRACT
The geological structures used for hydrocarbon production typically contain congenital water whose composition is modified when it is extracted during the industrial production processing of oil or gas. This is known as "produced water." The aim of the present study was to characterize and verify the feasibility of using produced water from the gas exploration area of Sabinas-Piedras Negras, Mexico, to cultivate tomato plants under greenhouse conditions. The treatments were established by mixing produced water from three producing gas stations (Buena Suerte, Monclova 1 and Forasteros) with good quality irrigation water. The mixture proportions were (mL of produced water per L of fresh water) 133, 3.4 and 125, respectively. A control treatment consisted of Steiner nutrient solution. The produced waters and mixtures were analyzed under NOM-143-SEMARNAT-2003, a norm established for congenital waters. The results showed that the mixtures with produced water from the Monclova 1 and Forasteros stations were feasible for use in the production of tomatoes because the morphological growth parameters did not show significant differences compared with the control, although the plants irrigated with mixtures containing water from the Forasteros station showed decreased leaf dry weight. The average mineral concentrations absorbed by these plants were the most similar to those of the control plants. The treatment with the mixture of water from the Buena Suerte station was not suitable for agricultural use because this mixture negatively affected the stem diameter, leaf dry weight and root length and limited mineral absorption, causing the death of 58 % of the plants.
Key words: congenital water, salinity, agriculture, water quality.
INTRODUCTION
Congenital water is the water that is trapped in the pores of sediment at the moment of their formation. Geological structures producing hydrocarbons normally contain congenital waters (SEMARNAT 2003a). Congenital water is removed during the process of hydrocarbon production. This water can contain a large quantity of salts. Because this water does not evaporate or circulate between different strata, it has not been considered part of the hydrological cycle (Leet and Judson 1974, Llamas 1993). When this water is extracted during the process of gas and oil production, its composition is modified, and it is then called "produced water" (Manfra et al. 2010).
Produced waters show variation in their physiochemical composition and volume depending on the extraction site, the age and the geology of the formation from which the oil and gas is produced (Lee et al. 2002, Veil et al. 2004, Clark and Veil 2009). Various studies have indicated a great variability in the salinity characteristics and the content of elements of produced water, and such variability can be observed between hydrocarbon extraction sites in relatively close proximity (Benavides-Mendoza 2008). Similar variation occurs in the produced water derived from marine platforms (Veil et al. 2004, Manfra et al. 2010). Some sources of produced water contain as much as five or six times the salt content of seawater. They also may contain concentrations of Cl- of 150 000 to 180 000 mg/L (sea water contains an average of 35 000 mg/L) and show an average electrical conductivity (EC) of 3200 dS/m (Chave and Cox 1982). With these levels of salts, the water is toxic for many forms of life (Tinu and Amit 2011, ARPEL 2012), particularly for crop plants, where water with an EC greater than 3 dS/m or 2000 mg/L total dissolved solids (TDS) is considered saline (FAO 1994, GWPRF 2003, Clark and Veil 2009). In addition, produced water can contain compounds of low molecular weight, organic acids, condensers, oils and fats, aromatic hydrocarbons, such as benzene, toluene ethyl-benzene and xylene, polycyclic hydrocarbons (PAH) and phenols. When present in the water, these compounds contribute to the toxicity, individually or in combination (Veil et al. 2004, Clark and Veil 2009). Produced water can also contain chemical additives used during the drilling and production operations (Clark and Veil 2009). The concentration of metals in produced water varies according to the specific site, age, and geologic formation from which the petroleum or gas is produced, which affects the availability and accumulation of metals (Veil et al. 2004). Normally, the water derived from gas wells contains metal concentrations several times greater than that derived from oil wells (Jacobs et al. 1992). In 2002, 12.09 x 106 m3 ofproduced water was generated in Mexico (SEMARNAT 2003a), and in 2010, 12.04 x 106 m3 were produced, according to the information provided by Petróleos Mexicanos (Pemex 2010). As in Mexico, large volumes of produced water are also extracted in other oil producing countries; for example, in the USA, approximately 3.3 x 109 m3 of produced water were generated from nearly one million oil and gas wells in 2007 (Clark and Veil 2009). In Mexico, NOM-143-SEMARNAT-2003 (SEMARNAT 2003a) established the environmental specifications for the management of congenital water (produced water) associated with hydrocarbon exploitation. The norms establish the safe limits for compounds contained in produced water and the authorized forms and methods of disposal of these waters in Mexico. The most common technique used is to increase the output of hydrocarbons by injecting water into productive wells (SEMARNAT 2003a, CNH 2010). Other methods of disposal include injection into unproductive wells or discharge into bodies of fresh water, along the coast or into the ocean. In the U.S.A., a distinction is made between water from marine platforms and that derived from land-based wells (DOE 2012, USEPA 2012). The method used for sea-based wells is discharge into the sea after treatment, in accordance with the limits on chemical contaminants set by the EPA (1993). For land wells, produced waters are disposed of by injection underground or are channeled to evaporation or storage sites.
Alternatively, these waters may be useful for certain industrial and agricultural purposes (Clark and Veil 2009, DOE 2012). In the industry, these waters are sometimes used to control dust or fires. In agriculture, they may be used in irrigation or for applications in the livestock industry or for wild animals (Veil et al. 2004, NPC 2011). It is known that some types of produced water present a salt content that makes their use feasible for agricultural purposes. Such application has been tested experimentally (Veil et al. 2004, DOE 2012).
Mexico does not have sufficient information available about the composition of its produced waters, and no studies have been published to prove the possibilities of its use in crop cultivation. Therefore, the objective of the present study was to characterize and verify the feasibility of using produced water to irrigate agricultural crops. Specifically, we studied produced water derived from the oil- and gas-producing zone of Sabinas-Piedras Negras, in northern Mexico, using tomato plants cultivated under greenhouse conditions as an indicator of feasibility.
MATERIALS AND METHODS
The experimental work was conducted in a greenhouse located in Buenavista, Saltillo, Coahuila, Mexico, whose geographic coordinates are North latitude, 25 22', West longitude 101 00', at an altitude of 1760 meters.
Produced waters
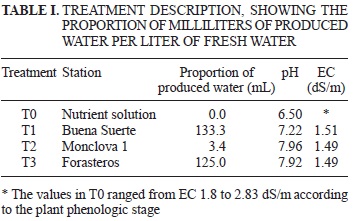
The produced water used for the present study was obtained from three Petróleos Mexicanos (PEMEX) gas-producing wells (Buena Suerte, Monclova 1 and Forasteros) located in the municipalities of San Buenaventura, Monclova and Abasolo, respectively, in the gas production area of Sabinas-Piedras Negras of Coahuila State, Mexico. Each of these stations gets portions of produced water from as many as 25 wells; therefore, the water from each station was a mixture from various nearby wells. These stations were selected because of the high electrical conductivity values of their produced waters.
To characterize the produced waters taken from the Buena Suerte, Monclova 1 and Forasteros stations, produced water samples taken from these stations were analyzed according to NOM-143-SEMARNAT-2003 (SEMARNAT 2003a). For comparative purposes, Steiner Solution (Steiner, 1961) at 75 % concentration was also analyzed under this norm. This analysis included the light, medium and heavy fractions of the hydrocarbons under the EPA methods 8015B-1996 (USEPA 1996) and EPA-8260C-2006 (USEPA 2006). The analysis also considered fats and oils and the different concentrations of Zn+2, Pb+2, Ni+2, Cd+2, Cu+2, Hg+2, As+3, Cr+3, total nitrogen, total phosphorus, nitrates, nitrites, and the sum of nitrogenous compounds, including the sum of ammoniacal nitrogen and organic nitrogen (Secretaría de Economía 2010). We also assessed the pH, biochemical demand of oxygen (BDO5), solid sediments, floating matter, total solids, total dissolved solids (TDS), total suspended solids (TSS) and total volatile solids (TVS). The techniques used to make the above determinations are listed in NOM-001-ECOL-1996 (SEMARNAT 1996) in the references section.
In addition, the above samples, plus a sample of the water used for irrigation, were analyzed to assess their quality as irrigation water (FAO 1994). The analysis included electrical conductivity, pH, total dissolved solids (TDS), and dissolved minerals (K+, Ca+2, Mg+2, Na+, CO3-2 and SO4-2), according to Normas Oficiales Mexicanas and Normas Mexicanas (CONAGUA 2014a, CONAGUA 2014b). The analysis also obtained the sodium adsorption rate (SAR) and the effective salinity (SE = Anions Sum - [Ca + Mg]).
Establishment of the experiment
To prepare the treatments to be applied, the EC from each produced water sample was determined; a dilution of the produced waters was made with the available fresh water in the greenhouse using a HANNA model HI 98129 conductivity meter to obtain a numerical EC value of approximately 1.5 dS/m (the average EC value of the applied fertilizing solution). After dilution, the pH of each sample ofthe mixtures was analyzed to verify the concentration of essential nutriment dissolved minerals. A Thermo Jarrel ASH inductive coupling plasma (ICP/AA) spectrometer was used for this purpose. The proportions in which the produced waters were used in the treatments for irrigation of the plants are shown in Table I; Steiner Solution (Steiner 1961) was used as the control (TO). The solution was applied in different concentrations according to the growth stage of the plant (ranging from EC 1.8 to 2.83 dS/cm).
Plant cultivation and treatment applications
The plants were cultivated in the greenhouse from June 23 to November 4, 2011. Tomato plants of the saladette type (Lycopersicon esculentum L.) cv. "Rio Grande," with a determined growth pattern, were used because this crop represents 56 % of the total tomato production in Mexico (SAGARPA 2010) and because it is a moderately salt-sensitive glycophyte species (Chinnusamy et al. 2005) and has a potential yield that is located in the middle of the other varieties (INIFAP 2014). The seedlings were produced in 200-cavity polystyrene trays, using a mixture of peat moss and perlite (3:1) as substrate. They were later transplanted into black polystyrene pots with a volume of 16 liters using the same substrate. To obtain plants with homogeneous vigor and growth, the plants were watered with the fertilizing solution only for 20 days before initiating the treatments. Water application was performed three times per day at 9:00, 13:00 and 18:00 h with the aim to keep the substrate wet and provide the plants with the nutrients needed for the treatments (Ikeda et al. 2002). At the start of plant growth, 400 mL was applied per plant per watering. This quantity was increased as the plants grew, until it reached 800 mL per plant per watering at the end of the cycle. The produced water treatment was applied in the first and third waterings, whereas in all cases, the fertilizing solution was applied in the second watering.
Morphologic variables assessed
The morphologic variables determined were the stem diameter (SD) (mm), measured at the first internode on the stem base utilizing a digital Vernier calibrator, the height ofthe plants (cm) (H), measured from the stem base to the terminal bud, and the root length (cm) (RL) from the base of the stem to the central root cap. The plant dry weight (g) ofthe aerial part (leaves plus stem) (PDW) was obtained at the flowering stage, and at the fructification stage, the dry weights of the leaves (LDW) and stems (SDW) were determined in separate measurements. The dry weight was measured after drying for 3 days at 60 °C by employing an analytical balance. To determine the number of fruits per plant (FN), five plants per treatment were chosen at random during the fructification stage. In these plants, the number of fruits was counted in each of six cuts. The production of fruit per plant (g) (FW) was the sum of six individual cuts during the harvest period between 93 and 128 days after transplantation.
Plant mineral content
To determine the mineral content (N, P, Ca, Mg, Na, Fe, Cu, Zn and Mn), five plants per treatment were chosen at random at both the flowering and fructification stages (93 and 128 days after transplantation, respectively). At flowering, root and aerial samples were collected, and at fructification, leaf, fruit and root samples were collected. The samples were dried at 60 °C in a dehydrating stove and later ground and subjected to acid digestion. The digestion extracts were analyzed using a Varian AA atomic absorption spectrophotometer, according to AOAC (1980). The phosphorus was determined via a colo-rimetric method using an aminonaphthol sulfonic acid reagent (ANSA) (Harris and Popat, 1954) and a Helios Epsilon spectrometer UV-Vis at a wavelength of 640 nm. The nitrogen was determined using the macro Kjeldhal method in compliance with standard techniques (AOAC 1980).
Statistical analysis
The experimental procedure was conducted under a completely randomized design, with 26 repetitions per treatment in the case of the morphology variables; however, in the case of the mineral analysis, only five repetitions were carried out. The experimental unit was a 16 L pot with a plant supplied with the respective treatment. For the statistical analysis, we utilized an analysis of variance (ANOVA) and Tukey's test (a < 0.05) to determine differences among the means using the SAS software (SAS Institute Inc. 2002).
RESULTS AND DISCUSSION
Analysis of produced water
The results show that the produced water coming from either the Buena Suerte or Forasteros Station had high hydrocarbon content according to NOM-143-SEMARNAT-2003 subsection 5.1.5.1 (SEMARNAT 2003a). According to these values (Table II), these waters could cause toxicity in the soil and crops and physiological problems such as germination inhibition, vegetal growth suppression or plant death (Powell 1997) if used as irrigation water, as reported by some authors (Adam and Duncan 2002, Quinones-Aguilar et al. 2003, SEMARNAT 2003b). None of the produced waters exceeded the permissible maximum limit of 25 mg/L daily average of fats and oils established for irrigation waters
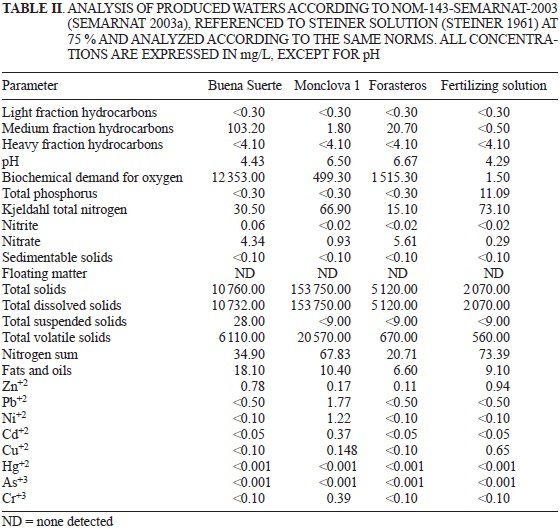
per NOM-001-SEMARNAT-1996 (SEMARNAT 1996). The produced water from the Buena Suerte station was outside the pH optimal range for use as irrigation water (FAO 1994, De Kreij 1999). All produced water has a high BOD, which indicates that it can inhibit microbial activity by decreasing the oxidation of the organic matter present in the water (Hudson et al. 2008). It was observed that the total volatile solids (TVS) and the total dissolved solids (TDS) and volatile solids (VS) ofthe produced waters in the Buena Suerte and Monclova 1 stations were above the limit ofNOM-001-SEMARNAT-1996 (SEMARNAT 1996). In addition, the total phosphorus in the produced waters from all of the stations was in no way optimal (SEMARNAT 1996), nor were the nitrates and nitrites, according to FAO (1994). On the contrary, the total nitrogen level in the water from the Monclova 1 station and in the fertilizing solution was above the values specified in NOM-001-SEMARNAT-1996 (SEMARNAT 1996). Regarding minerals, the water from Monclova station 1 was outside the permissible range for Pb according to NOM-001-SEMARNAT-1996 (SEMARNAT 1996) and over the toxic threshold according to the ARPEL (2012) guide. All other minerals were within the limits set by NOM-001-SEMARNAT-1996 (SEMARNAT 1996).
Table III shows the quality of the produced water from the three stations. The water treated with Steiner fertilizer solution at 50 % and the fresh water are also shown. The produced waters coming from Buena Suerte and Monclova l had EC values above the maximum limits for irrigation water (De Kreij and Van Den Berg 1990, FAO 1994, GWPRF 2003), indicating that when applied directly, these waters result in stress-induced salinity (Pessarakli 2011). Although the water pH from Buena Suerte and Forasteros was outside the optimum pH range, i.e., 5.5 to 6.5 (De Kreij 1999), indicating that some of the essential elements would not be available to the plants (De Kreij and Van Den Berg 1990), it was still within the recommended ranges for irrigation water according to FAO (1994). The produced water from Monclova l also presented high values of Ca+2 and Mg+2 (FAO 1994), which may cause precipitation of the phosphorus (Jones 2005). In addition, all of the waters had bicarbonate levels above the FAO limits (FAO 1994), which can promote the precipitation of Ca+2 and Mg+2 (Vivot et al. 2010). The produced water from the Forasteros Station also had a chloride concentration above the recommended limits (FAO 1994, SEMARNAT 2003a), which can induce cell necrosis (Razeto 1991). Additionally, the produced water from the Monclova 1 station exceeded the TDS and RAS (FAO 1994, SEMARNAT 2003a) so that when applied, this water may induce osmotic stress in the plants by the high concentration of TDS (Sa-ravanakumar and Ranjith, 2011). Likewise, the RAS with high concentrations of sodium ions displaces the calcium and magnesium (González 2000), leading to a decrease in leaf size (Jones 2005).
Table IV shows the results of the analysis of the fresh water used, the treatment waters (mixture of produced and fresh water), and the Steiner fertilizer solution at 100 % concentration used as the control. We observed that the ionic concentrations in the different mixtures of produced water solutions were lower than those recommended by Steiner (1961) for a fertilizer solution at 100 %. However, according to the ARPEL (2012) guide, they were within marginally adequate range for fertilizers. It was also observed that the concentrations of Mn, Ca, Mo, Fe, Cu and sulfates were lower in the three treatments than in the control, whereas the Mg concentration was lower in the Monclova 1 treatments (T2) and Foresteros treatments (T3). With respect to Zn, the Monclova 1 treatments and Buena Suerte treatments (T1) were equal. The concentrations of Na and chlorides were greater in the control than in the three treatments. Although the Na level surpassed the limit recommended by Steiner (1961), it was within the maximum permitted limits for general use in hydroponics (Jones 2005). The pH of the treatments was elevated in comparison to the control but within the limits of irrigation quality set by FAO (1994).
Morphology of the plants
Table V depicts the results of the morphological variables assessed in the tomato plants during the flowering and fructification stages. It was observed that in the flowering stage, there were no significant differences among the treatments in the response variables H, SD and PDW. At this stage, only the variable RL did show a significant difference, indicating that the variables measured in the plants of the Monclova l treatment were higher than the other treatments; most likely with the daily irrigation, the chloride concentration of the Buena Suerte and Forasteros stations could accumulate, causing reduced growth and cell necrosis (Razeto 1991), and the high concentration of carbonate in the plants irrigated with water from the outside station (FAO 1994) was able to precipitate the Ca and Mg, reflected in the lower biomass production (Barker and Pilbeam 2007).
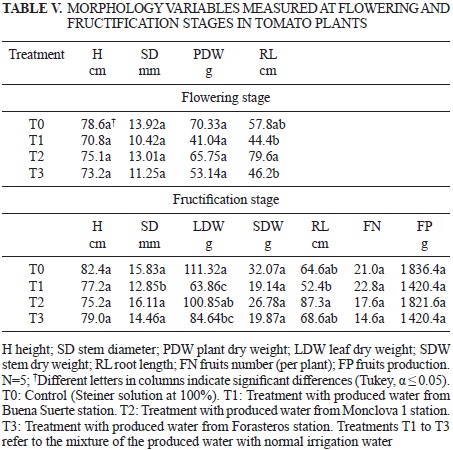
In the fructification stage, compared with the control SD, the LDW and RL in the Buena Suerte treatment were the lowest (with a difference of approximately 19 % and 43 %, respectively). Considering the RL, the treatment with the Buena Suerte water mixture was also lower but, in this case, only showed a significant difference with the Monclova 1 treatment; the RL in the Monclova 1 treatment was 67 % higher. In the rest of the assessed morphological variables (H, SDW, FN and FW), all ofthe treatments were statistically equal. This fact agrees with the results reported by Jackson and Meyers (2002). They reported that though it is feasible to use produced waters on plant growth, the yield of biomass and number of fruits is lower compared with that of plants treated with nutrient solution.
The results show that the plants treated with the Buena Suerte water mixture showed negative effects in some of the assessed variables (SD, LDW and RL) (Table V). We should note that 15 of the 26 plants in this treatment died. It is very likely that their death was caused by the harmful effect of the hydrocarbons and chloride (Razeto 1991, RamanaRao et al. 2012). The produced water utilized for this treatment contained a higher middle fraction of hydrocarbon contents (SEMARNAT 2003a) than did the other two stations (Table II) and was the least diluted of the three treatments (Table I). This finding is similar to the results of some studies that suggest that high hydrocarbon content in waters can cause toxicity in crops if used for irrigation (Adam and Duncan 2002, Quinones-Aguilar et al. 2003) and provoke physiological problems such as vegetal growth suppression and plant death (Powell 1997). In addition, the high pH of the water of the different treatments could inhibit the absorption of trace elements (De Kreij 1999), which, coupled with the highest concentration of Na, may cause nutritional imbalances in the plant (Yokoi et al. 2002).
Effect of treatments on the mineral content of the tomato plants
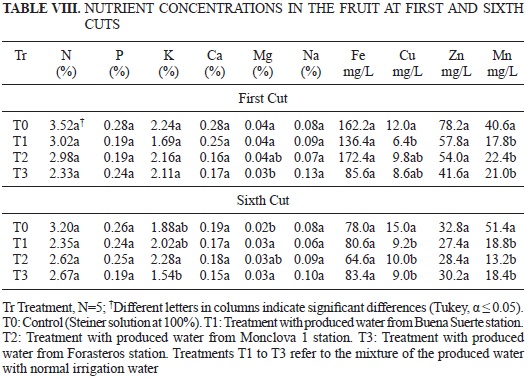
The results of mineral concentration analysis in the root of the tomato plants in either the flowering or fructification stages are shown in Table VI. The results of mineral concentration analysis in the aerial part at the flowering stage and in the stems and leaves in the fructification stage are depicted in Table VII. Finally, the mineral content in the tomato fruit, from the first to the sixth cuts, can be observed in Table VIII.
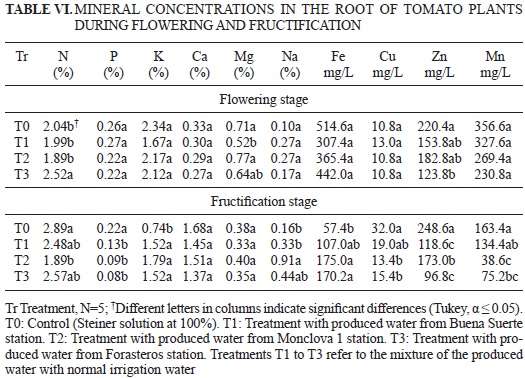
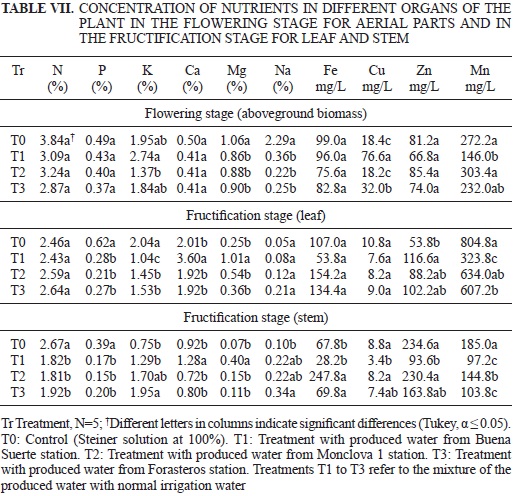
The concentrations of P, K, Ca, Na, Fe, Cu and Mn in the root were not affected by the treatments in the flowering stage. The nitrogen concentration in the plants irrigated with the Forasteros water mixture was greater than the other treatments, including the control. However, the plants grown in all treatments were within the normal range for root mineral content (Barker and Pilbeam 2007). A difference was observed, however, in the case of Zn, as the Forasteros treatment showed a lower concentration of that element. In the fructification stage, only Ca and Mg did not show a significant difference. It was also observed that the concentrations of P, Cu, Zn and Mn were lower in the three groups treated with the produced water compared with the control, whereas in the case of K and Fe, higher levels were exhibited in the treatments than in the control (Table VI). Concerning Mn, the observed results could have been due to the lower concentration of this element in the treatments with produced water than in the control, so the latter treatment may have limited Mn absorption (Table IV). Although differences were observed among the treatments regarding nitrogen, the pattern was not clear and remained within the normal range for the roots (Barker and Pilbeam 2007). Concerning the N, P, Ca, Fe, and Zn concentrations in the aerial part at the flowering stage, no significant differences were observed, but in the case of Na and Mg, the concentrations of these elements were significantly lower in the plants treated with produced water when compared with the control (Table VII). Because the mixtures with produced water showed higher bicarbonate concentration (Vivot et al. 2010), and K was above normal in the aboveground part (Salisbury and Ross 1992), we speculate that some type of competition in the absorption of different cations was present that favored K uptake. The Cu concentration in the Buena Suerte treatment was three times higher than in the control (Table VII), exceeding the toxic level for plants according to ARPEL (2012). Cui et al. (2010) noted that high Cu promote reactive oxygen species in concentrations that diminish growth by destroying membranes and add to the negative effects of the hydrocarbons mentioned previously (Razeto 1991, RamanaRao et al. 2012). We can attribute these negative effects of the hydrocarbons plus Cu on the variables of SD, LDW and RL (Table V), as we have discussed, to the death of 15 plants, which occurred during the growing period of the plants treated with the Buena Suerte water mixture.
No differences were observed in N, Na, Fe and Cu in the leaves at fructification; however, the stems showed significant differences in the concentrations of all these nutrients. Larger concentrations of P, K, and Mn in the plants of the control treatment were also observed compared with those treated with the produced water mixtures, and the same was observed in the stems. The foliage tissue also presented a lower Zn concentration compared to the control (Table VII).
Regarding the mineral content of N, P, K, Ca, Fe and Zn in the fruits of the first cut, no significant differences were found among the treatments. Only Mn presented significantly greater values in the plants of the treatment with produced water than in the control. No differences were observed in the N, P, Ca, Fe and Zn concentrations in the sixth cut. It was also observed that the concentrations of Cu and Mn were statistically greater in the control than in the rest of the treatments, but in the case of Mg, the control showed a lower concentration (Table VIII). In the case of Mn, the concentration of this element was greater in the treatment solutions utilized for watering the plants (Table IV), in the same manner observed in the fruits, stems and leaves in the fructification stage (Table VII).
CONCLUSIONS
Due to the high levels of electrical conductivity of the produced waters, these cannot be used directly for watering; however, the treatments assayed in this experiment (mixing produced water with fresh water to adjust the EC to 1.5 dS/m) proved that it is feasible to use these types of waters, when diluted with regular irrigation water, for tomato production under greenhouse conditions.
The water derived from the Buena Suerte station was unsuitable for use in watering due to the high middle-fraction hydrocarbon content and the high levels of Cu and chloride. In fact, the plants were damaged and some died due to the use of a normal irrigation mixture mixed with the water from Buena Suerte.
The produced waters from Monclova 1 and Forasteros are viable to be used for irrigation with previous dilution with another water source to reduce the electrical conductivity and mineral concentration.
It is necessary to conduct an analysis of the fruit mineral content according to NOM 143 to determine whether the concentration of the absorbed elements is feasible for consumption of the fresh fruit.
ACKNOWLEDGEMENTS
Activo Integral Burgos de PEMEX Exploración y Producción Región Norte allowed the use of the produced water for experimentation. Centro de Investigación en Química Aplicada (CIQA) provided support for the chemical analysis.
REFERENCES
Adam G. and Duncan H. (2002). Influence of diesel fuel on seed germination. Environ. Poll. 120, 363-370. [ Links ]
AOAC (1980). Official methods of analysis of the Association of Official Analytical Chemists. Association of Official Analytical Chemists. Washington DC, USA. 1018 pp. [ Links ]
ARPEL (2012). Disposición y tratamiento de agua producida. Asociación Regional de Empresas de Petróleo y Gas Natural en Latinoamérica y el Caribe. Guide. Alberta, Canada. 111 pp. [ Links ]
Barker A. V. and Pilbeam D. J. (2007). Handbook of plant nutrition. Taylor & Francis Group. Boca Raton, London, New York. 613 pp. [ Links ]
Benavides-Mendoza A. (2008). Proyecto de Manejo de Agua Congénita para el Desarrollo Sustentable del Activo Integral Burgos (Análisis de Variables Básicas del Agua). Universidad Autónoma Agraria Antonio Narro. Reporte Técnico entregado al Activo Integral Burgos de Pemex Exploración y Producción. Saltillo, México. 18 pp. [ Links ]
Chave A. D. and Cox C. S. (1982). Controlled electromagnetic sources for measuring electrical conductivity beneath the oceans. J. Geophys. Res. 87, 5327-5338. [ Links ]
Chinnusamy V., Jagendorf A. and J.-K. Zhu J.-K. (2005). Understanding and improving salt tolerance in plants. Crop Sci. 45, 437-448. [ Links ]
Clark C. E. and Veil J. A. (2009). Produced Water Volumes and Management Practices in the United States. [online]. http://www.ipd.anl.gov/anlpubs/2009/07/64622.pdf 01/03/2013. [ Links ]
CNH (2010). Documento Técnico 1 (DT-1). Factores de recuperación de aceite y gas en México, Comisión Nacional de Hidrocarburos [online]. http://www.cnh.gob.mx/_docs/DOCUMENTOTECNICO1FINAL.pdf 01/02/2012. [ Links ]
CONAGUA (2014a). Normas Oficiales Mexicanas. Comisión Nacional del Agua. [online]. http://www.cna.gob.mx/Contenido.aspx?n1=2&n2=16&n3=2&n4=11 04/25/2014. [ Links ]
CONAGUA (2014b). Normas Mexicanas. Comisión Nacional del Agua. [online]. http://www.cna.gob.mx/Contenido.aspx?n1=2&n2=16&n3=2&n4=141&n5=141 04/25/2014. [ Links ]
Cui X. M., Zhang Y. K., Wu X. B. and Liu C. S. (2010). The investigation of the alleviated effect of copper toxicity by exogenous nitric oxide in tomato plants. Plant Soil Environ. 56, 274-281. [ Links ]
De Kreij C. and Van Den Berg T. H. J. M. (1990). Nutrient uptake, production and quality of Rosa hybrida in rockwool as affected by electrical conductivity of the nutrient solution. In: Plant nutrition-physiology and applications. (M. L. van Beusichem, Ed.). Springer Netherlands, Wageningen, Netherlands, pp. 519-523. [ Links ]
De Kreij C. (1999). Bemestingsadviesbasis Substraten. Proefstation voor Bloemisterij in Glasgroente. Vestig-ing Naaldwijk, The Netherlands, 145 pp. [ Links ]
DOE (2012). Produced water management technology descriptions. Fact Sheet-Agricultural Use. Department of Energy of the United States. National Energy Technology Laboratory (NETL). [online]. http://www.netl.doe.gov/technologies/pwmis/techdesc/aguse/index.html 02/11/2012. [ Links ]
EPA (1993). Development document for effluent limitations guidelines and new source performance standards for the offshore subcategory of the oil and gas extraction point source category. [online]. http://yosemite.epa.gov/water/owrccatalog.nsf/1ffc8769fdecb48085256ad3006f39fa/969ed536d932393a85256d83004fd95c!OpenDocument 01/02/2013. [ Links ]
FAO (1994). Water quality for agriculture. Food and Agriculture Organization [online]. http://www.fao.org/DOCREP/003/T0234E/T0234E00.htm 07/05/11 [ Links ]
González R. (2000). Fertilidad de los suelos del Valle Cálido del Alto Magdalena. Sociedad Colombiana de la Ciencia del Suelo. pp. 80-99. [ Links ]
GWPRF (2003). Ground Water Protection Research Foundation, U.S. Department of Energy, and U.S. Bureau of Land Management. Handbook on Coal Bed Methane Produced Water: Management and Beneficial Use Alternatives. [online] http://www.ela-iet.com/EMD/CoalBedMethaneWater.pdf 10/27/2013. [ Links ]
Harris, W. D. and P. Popat P. (1954). Determination of the phosphorus content of lipids. J. Am. Oil Chem. Soc. 31, 124-127. [ Links ]
Hudson N., Baker A., Ward D., Reynolds D. M., Brunsdon C., Marquet C. C. and Browning S. (2008). Can fluorescence spectrometry be used as a surrogate for the Biochemical Oxygen Demand (BOD) test in water quality assessment? An example from South West England. Sci. Total Environ. 391, 149-158. [ Links ]
Ikeda H., Koohakan P. and Jaenaksorn T. (2002). Problems and countermeasures in the re-use of the nutrient solution in soilless production. Acta Hort. 578, 213-219. [ Links ]
Jackson L. and Myers J. (2002). Alternative use of produced water in aquaculture and hydroponic systems at naval petroleum reserve No. 3. Memoirs. The 2002 Ground Water Protection Council Produced Water Conference, Colorado Springs, CO, Oct. 16-17, 2002. [ Links ]
INIFAP (2014). Tomate. Variedades. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias [online]. http://sites.securemgr.com/folder11341/index.cfm?fuseaction=browse&id=920459&pageid=67 04/27/2014. [ Links ]
Jacobs R. P. W. M., Grant E., Kwant J., Marqueine J. M. and Mentzer E. (1992). The composition of produced water from shell operated oil and gas production in the North Sea. In: Produced water. Technological/Environmental Issues and Solutions. (J.P. Ray y F.R. Englehart, Ed.). Plenum Press, New York. 46, 13-21. [ Links ]
Jones Jr. J. B. (2005). Hydroponics: a practical guide for the soilless grower. 2nd ed. CRC Press, Boca Raton, London, New York Washington, D.C. 423 pp. [ Links ]
Lee R., Seright R., Hightower M., Sattler A., Cather M., McPherson B., Wrotenbery L., Martin D. and Whitworth M. (2002). Strategies for produced water handling in New México. [online]. http://wrri.nmsu.edu/publish/watcon/proc47/lee.pdf 04/24/2012. [ Links ]
Leet L. D. and Judson S. (1974). Fundamentos de geología física. Editorial Limusa-Wiley. México, D.F. 450 pp. [ Links ]
Llamas J. (1993). Hidrología general. Principios y Aplicaciones. Editorial Universitaria del País Vasco. Bilbao, España. 635 pp. [ Links ]
Manfra L., Maggi C., Bianchi J., Mannozzi M., Faraponova O., Mariani L., Onorati F., Tornambé A., Virno-Lamberti C. and Magaletti E. (2010). Toxicity evaluation of produced formation waters after filtration treatment. Natural Sci. 2, 33-40. [ Links ]
NPC (2011). Management of produced water from oil and gas wells. The National Petroleum Council, USA. [ Links ]
PEMEX (2010). Informe de Responsabilidad Social. Petróleos Mexicanos [online]. http://www.pemex.com/informes/pdfs/descargas/pemex_irs_completo_2011.pdf 03/10/2012. [ Links ]
Powell R. (1997) The use of plants as "field" biomonitors. In: Plants for Environmental Studies (W. Wang, J. Gorsuch, J. Hughes, Ed.). Lewis. Raton, FL, EEUU. pp. 47-61. [ Links ]
Pessarakli M. (2011). Handbook of plant and crop stress. 3a ed. CRC Press Taylor & Francis Group. Boca Raton London New York. 1195 pp. [ Links ]
Quiñones-Aguilar E. E., Ferrera-Cerrato R., Gavi-Reyes F., Fernández-Linares L., Rodríguez-Vázquez R. and Alarcón A. (2003). Emergence and growth of maize in a crude oil polluted soil. Agrociencia. 37, 585-594. [ Links ]
RamanaRao M. V., Weindorf D., G. Breitenbeck G. and Baisackh N. (2012). Differential expression of the transcripts of Spartina alterniflora Loisel (smooth cordgrass) induced in response to petroleum hydrocarbon. Mol. Biotech. 51, 18-26. [ Links ]
Razeto M. B. (1991). La nutrición mineral de los frutales: deficiencias y excesos. SOQUIMICH. Santiago, Chile. 105 pp. [ Links ]
SAGARPA (2010). Monografía de cultivos, Jitomate. Subsecretaria de fomento a los agro negocios. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación, México. [ Links ]
Salisbury F. B. and Ross W.C. (1992). Plant Physiology Wadsworth. 4a ed. Wadsworth Publishing Company. Belmont, California, USA. 682 pp. [ Links ]
Saravanakumar K. and Ranjith K.R. (2011). Analysis of water quality parameters of groundwater near Ambat-tur industrial area, Tamil Nadu, India. Indian J. Sci. Tech. 4, 660-662. [ Links ]
SAS (2002). JMP User's guide. Versión 5.0.1. SAS Institute Inc. [online]. http://support.sas.com/archive/installation/admindoc/installation/JMP501AdminGuide.pdf. 04/ 25/2012. [ Links ]
Secretaría de Economía (2010). Norma Mexicana NMX-AA-026-SCFI-2010. Análisis de agua - Medición de nitrógeno total Kjeldahl en aguas naturales, residuales y residuales tratadas. Método de prueba (Cancela a la NMX-AA-026-SCFI-2001). [ Links ]
SEMARNAT (1996). Norma Oficial Mexicana NOM-001-ECOL-1996. Que establece los límites máximos permisibles de contaminantes en las descargas de aguas residuales en aguas y bienes nacionales. Secretaría de Medio Ambiente, Recursos Naturales y Pesca. Diario oficial de la Federación. June 24 1997. [ Links ]
SEMARNAT (2003a). Norma Oficial Mexicana NOM-143-SEMARNAT-2003. Que establece las especificaciones ambientales para el manejo de agua congénita asociada a hidrocarburos. Secretaría de Medio Ambiente Recursos Naturales y Pesca. Diario Oficial de la Federación. March 3 2005. [ Links ]
SEMARNAT (2003b). Norma Oficial Mexicana NOM-138-Semarnat/SS-2003. Límites máximos permisibles de hidrocarburos en suelos y las especificaciones para su caracterización y remediación. Secretaría de Medio Ambiente Recursos Naturales y Pesca. Diario Oficial de la Federación. March 29 2005. [ Links ]
Steiner A. A. (1961). A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil. 15, 134-154. [ Links ]
Tinu A. and Amit L. (2011). Socio-economic & technical assessment of photovoltaic powered membrane desalination processes for India. Desalination. 268, 238-248. [ Links ]
USEPA (1996). US EPA 8015B. "Nonhalogenated Or-ganics Using GC/ FID." United States Environmental Protection Agency. EPA. Revision 2. 28 pp. [ Links ]
USEPA (2006). US EPA-8260C. "Volatile Organic Compounds by Gas Chromatography / Mass Spectrometry (GC/MS)." United States Environmental Protection Agency. EPA. Revision 3. 92 pp. [ Links ]
USEPA (2012). Oil and gas production wastes. United States Environmental Protection Agency. [online]. http://www.epa.gov/radiation/tenorm/oilandgas.html 04/13/2012. [ Links ]
Veil J. A., Puder M.G., Elcock D. and Redweik Jr. R.J. (2004). A White paper describing produced water from production of crude oil natural gas and coal bed methane. US Department of Energy. [ Links ]
Vivot E. P., Rugnaa C.M., Gieco A.M., Sánchez C.I., Ormaecheaa M.V. and Sequin C.J. (2010). Calidad del agua subterránea para usos agropecuarios en el departamento Villaguay, Entre Ríos. AUGMDOMUS. 2, 1-15. [ Links ]
Yokoi S., Bressan R.A. and Hasegawa P.M. (2002) "Salt Stress Tolerance of Plants." Center for Environmental Stress Physiology. Purdue University. JIRCAS Working Report. pp. 25-33. [ Links ]














