Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista internacional de contaminación ambiental
versión impresa ISSN 0188-4999
Rev. Int. Contam. Ambient vol.26 no.1 Ciudad de México feb. 2010
Artículos
Toxic effects of linear alkylbenzene sulfonate, anthracene and their mixture on growth of a microbial consortium isolated from polluted sediment
Efectos tóxicos del alquilbencensulfonato lineal, antraceno y su mezcla sobre el crecimiento de un consorcio microbiano aislado de sedimento contaminado
Gabriel PINEDA FLORES, Carmen MONTERRUBIO BADILLO, Manuel HERNÁNDEZ CORTÁZAR, Cirilo NOLASCO HIPÓLITO, Rocío SÁNCHEZ PÉREZ and Ignacio GARCÍA SÁNCHEZ
Instituto Politécnico Nacional, Centro Mexicano para la Producción más Limpia. Av. Acueducto s/n, La laguna Ticomán, Gustavo A. Madero, México D.F. 07340, México. gpineda@ipn.mx
Recibido junio 2008
Aceptado junio 2009
ABSTRACT
The aim of this study was to determine the effect of linear alkylbenzene sulfonate (LAS), anthracene and a LAS–anthracene mixture on the growth of a microbial consortium isolated from polluted sediment. The microbial consortium was grown in a sterile glass bottle with mineral medium containing 1 g/L of glucose. Microbial growth inhibition produced by LAS, anthracene and combinations of LAS and anthracene was determined by viable count in nutritive agar; inhibitory concentration 50 (IC50) was calculated. The concentrations evaluated were 0.16, 0.8, 1.6, 16 and 160 mg/L of LAS or anthracene. The LAS–anthracene mixtures were prepared by fixing either LAS or anthracene at 0.16 mg/L while increasing the other compound at the above concentrations. Microbial growth was sensitive to LAS at an IC50 of 8.22 and to anthracene at an IC50 of 5.2 mg/L. In the LAS–anthracene combination, if LAS concentration was fixed and anthracene concentration varied, IC50 (5.92 mg/L) was similar to IC50 for anthracene alone. In contrast, the inhibition effect was diminished when anthracene remained constant and LAS concentration was increased (IC50: 70.11 mg/L). The sediment microbial populations were capable of degrading the LAS–anthracene mixture if the concentration of both compounds were at 0.16 mg/L.
Key words: toxicity, LAS, anthracene, mixture, microbial consortium, sediment, pollution.
RESUMEN
El objetivo de este trabajo fue determinar el efecto del sulfonato de alquilbenceno lineal (SAL), antraceno y su mezcla sobre el crecimiento de un consorcio microbiano aislado de sedimento contaminado. El crecimiento del consorcio se obtuvo en botellas de vidrio con medio mineral estéril más 1 g/L de glucosa. La inhibición del crecimiento microbiano, producida por SAL, antraceno o la combinación de ambos, fue determinada por cuenta viable en agar nutritivo y se determinó la concentración inhibitoria 50 (CI50). Las concentraciones evaluadas fueron 0.16, 0.8, 1.6, 16 y 160 mg/L de SAL o antraceno. Las mezclas SAL–antraceno fueron preparadas manteniendo constante la concentración de SAL o antraceno en 0.16 mg/L mientras se aumentó la concentración del otro compuesto a las mismas concentraciones mencionadas. El crecimiento microbiano fue sensible al SAL y antraceno a una CI50 de 8.22 mg/L y 5.2 mg/L respectivamente. Cuando la concentración de SAL y antraceno fue evaluada, si la concentración de SAL se mantuvo fija y la concentración de antraceno varió, la CI50 (5.92 mg/L) fue muy similar a la CI50 para antraceno solo. En contraste, el efecto de inhibición disminuyó cuando el antraceno permaneció constante con concentraciones en incremento de SAL (CI50: 70.11 mg/L). Por otra parte, se observó que las poblaciones bacterianas del sedimento son capaces de biodegradar la mezcla SAL/antraceno cuando la concentración de ambos compuestos fue de 0.16 mg/L.
Palabras clave: toxicidad, LAS, antraceno, mezcla, consorcio microbiano, sedimento, contaminación.
INTRODUCTION
Pollution of freshwater sediment ecosystems is generally due to the presence of a mixture of chemical compounds (Alexander 1997). Detergents and polycyclic aromatic hydrocarbons are organic pollutants that accumulate in freshwater sediment, constituting pollutant mixtures (Smulders and Krings 1990, Aboul–Kassim 1992). Anthracene and linear alkylbenzene sulfonate (LAS) are some of the many pollutants present in different aquatic ecosystems (Comber et al. 2006, Hamdi et al. 2006). The toxicity of chemical compounds on aquatic organisms depends on concentration in both the sediments and the water, as well as in processes related to their bioavailability. Bioconcentration, biodegradation, desorption and solubilization processes that occur in these substrata determine the quantity of free compounds that will reach toxic levels in the organs of aquatic organisms.
Anthracene is a tricyclic aromatic hydrocarbon; its molecules fate in nature is of great environmental concern due to their potential toxicity, mutagenicity and carcinogenicity (Li et al. 2008). Anthracene is a hydrophobic substance which has been shown to be toxic to fish and algae (Moody et al. 2001). An increasing number of studies have been conducted on anthracene biodegradability to examine its elimination from ecosystems. An interesting and important observation made by Cerniglia and Heitkamp (1989) was that eukaryotic microbes such as Cunninghamella elegans use a cytochrome P–450 monooxygenase system or lignin peroxidase to break down aromatic hydrocarbon rings into the detectable product cisdihydrodiol (Pickard et al. 1999).
LAS is a surfactant produced in large amounts used around the world in detergent and personal care products (Jiménez et al. 1991, Schleheck et al. 2004). LAS has been shown to affect the flora and fauna of aquatic ecosystems. It has been observed that this compound denatures proteins in the cell membrane, altering the permeability of the membrane to nutrients and other chemical substances (Kimerle 1989). Due to its surfactant properties, LAS is adsorbed preferentially onto sediments (Sanderson et al. 2006).
The initial enzymatic attack in LAS biodegradation occurs by omega oxidation of the terminal carbon of the alkyl side chain. The enzymes involved in this reaction, although not yet identified, are probably associated with cell membranes. This enzymatic attack results in a carboxylated alkyl chain or sulfophenyl–carboxylate, which is further biodegraded through beta oxidation. Once beta oxidation has taken place, the molecule loses its surfactant properties because it no longer has a hydrophobic side chain. Following complete mineralization of the alkyl chain, the benzene ring is desulfonated and cleaved (White and Russell 1994, Schleheck et al. 2004).
Laboratory studies have demonstrated that these toxic organic compounds do not routinely biodegrade, as many of them are resistant to microbial degradation. This is due to microbial biodegradation is usually accessible only when they are dissolved in aqueous solution or at least in direct contact with water (Sun–daram et al. 1994). Fu and Alexander (1995) found that the desorption or solubilization of petroleum hydrocarbons can be accelerated with the addition of surfactants. As a result, bioavailability and therefore biodegradation of anthracene may be increased in the presence of LAS in bodies of water polluted with these compounds.
According to Ventullo and Larson (1986), cationic surfactants can produce alterations in heterotrophic activity in limnetic microbial populations. Therefore it is possible that the chemical structure and toxicity of LAS and anthracene would have the same effect on sediment microbial populations.
The hypothesis is that the LAS–anthracene mixture can inhibit the growth of the microbial consortium in sediment. It is possible that anthracene is more toxic than LAS, and that when they are mixed, having made the anthracene more soluble by increasing the surfactant concentration, the mixture will be more toxic than the separate compounds. On the other hand, it is expected that at a low concentration, microbial degradation of the mixture will be possible, since there will not be an inhibition effect on microbial growth.
It was determined the toxicity and kinetics of biodegradation of the LAS–anthracene mixture by microbial populations of natural ecosystems, with particular emphasis on microorganisms found in sediment, where these compounds are concentrated. The objective was to evaluate the acute toxic effect of LAS, anthracene and the mixture of the two compounds on the growth of a microbial consortium isolated from sediment, and to determine the potential of the LAS–anthracene mixture to be degraded by isolated microbial populations.
MATERIALS AND METHODS
Sampling area
The sampling zone was located in the municipality of Tlahuelilpan de Ocampo in the state of Hidalgo, México. Samples were collected in Irrigation District 63, located in the southeast part of the state to the north of México City, between 15° 44' and 20° 29' N and 98° 57' and 99° 21' W, with an average elevation of 1895 meters above sea level.
The sediments are in continuous contact with domestic, agricultural and probably industrial wastewaters. These wastewaters did not receive any prior treatment; thus microorganisms present in the sediments are in direct contact with a great variety of chemical pollutants. The water is subsequently used for irrigating agricultural crops; however, this water contains toxic hydrocarbons and other by–products associated to their breakdown by the microorganisms contained in the sediments, which will pollute the crops.
Screening and maintenance of the microbial consortium
The microbial consortium was isolated from sediment obtained at the same time as the wastewaters were sampled. One kilogram of sediment was collected and placed in a sterile carrier bag. The sediment was stored at 4 °C until required.
To maintain the microbial population, the ISO–9439 system was employed (Pineda–Flores et al. 2004). One gram of sediment was placed in a 250 mL sterile glass bottle, which contained 100 mL of mineral medium (composition in mg/L: KH2PO4 0.085, K2HPO4 0.22, Na2HPO4•2H2O 0.33, NH4Cl 0.05, MgSO4•7H2O 0.023, CaCl2 0.028, FeCl3•6H2O 2.5×104) plus 1 g of glucose as a carbon and energy source.
The microorganisms that constitute the microbial population were identified as Pseudomonas men–docina, Flavobacterium breve and Corynebacterium favescens by microscopy, colony morphology and various biochemical tests following the schemes of Weaver and King (Weyant et al. 1996) and Bergey's manual (Sneath et al. 1986).
The culture was maintained at 25 °C and agitated by magnetic stirring (150 rpm). The microbial population was maintained by introducing weekly subcultures of 1 mL of the microbial population into 100 mL of fresh sterile mineral medium containing 1 g of glucose. Aseptic technique was used for all transfers.
Toxic effects of LAS and anthracene on microbial growth
Once the microbial population had reached the exponential phase (with an absorbance of 0.09 at 650 nm), 1 mL of culture was serially diluted from 10–3 to 10–6 in assay tubes with 9 mL of sterile distilled water (Espigares et al. 1990). Each tube was added with 0, 0.16, 0.8, 1.6, 16 or 160 mg/L of LAS or anthracene (Aldrich Chemical Co., 98 and 96 % purity respectively, both sterilized by 0.45 µm membrane filtration). The samples were stirred for 5 seconds, then a 0.2 mL aliquot was taken and streaked onto nutritive agar obtained from Becton Dickinson and Company, Cockeysville, MD. Each set of plates was incubated at 25 °C for 48 hours and the colonies enumerated. The experiment was replicated five times.
To establish the interval of concentrations for the tests, growth of the consortium was evaluated with 1, 4 and 16 mg/L of each compound as described above. With 16 mg/L of each compound, a reduction in the growth of the consortium was observed (data not shown). For this reason, the choice of the range of concentrations was based on decimal reductions and decimal increases of 16 mg/L (0.16, 1.6, 16 and 160 mg/L). The 0.8 g/L concentration was included to complete the five concentrations needed as mini–mums to determine IC50.
The effect of the LAS–anthracene mixture was quantified following the method described above, except for the following difference: each assay tube contained either a fixed concentration of LAS (0.16 mg/L) plus 0.16, 0.8, 1.6, 16 or 160 mg/L of anthracene or 0.16 mg/L anthracene plus 0.16, 0.8, 1.6, 16 or 160 mg/L of LAS. This enabled the determination of the toxicity of the LAS–anthracene mixtures. Five repetitions were performed for each concentration. IC50 for LAS, anthracene and the mixture was obtained using linear regression by plotting the number of colony–forming units against the logarithmic concentration of LAS or anthracene.
Microbial degradation of LAS–anthracene mixture
The production of carbon dioxide by microbial degradation of the LAS–anthracene mixture was evaluated using the device and methodology described by Pineda–Flores et al. (2004). A sterile 250 mL glass bottle containing 100 mL of mineral medium was inoculated with 1 mL of microbial consortium adjusted to Abs652 = 0.09. The concentrations of LAS and anthracene used in the mixture were as described above. Carbon dioxide was measured every 12 hours for 48 h. The concentrations used for the LAS and anthracene mixture were 10–0.1, 12.58–1, 15.84–10, 19.95–100 and 23.98–200 mg/L. Three repetitions were performed for each treatment. In order to characterize degradation of the LAS–anthracene mixture, the maximum reaction velocity (Vmax), Michaelis constant (Km) and affinity constant (AC) were calculated from the Michaelis–Menten equation (Conn et al. 1987).
RESULTS
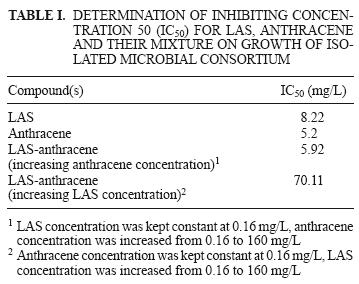
Figure 1 shows the toxic effect on the growth of the microbial population exposed to different concentrations of LAS and anthracene. The growth of the isolated microbial population was not inhibited with 0.16 mg/L of LAS or anthracene alone. When the LAS and anthracene concentrations were increased, toxic effects on the microbial population also did. CFU/mL declined as LAS and anthracene concentrations were increased from 0.8 to 160 mg/L. The LAS–anthracene mixtures produced a notable growth inhibition effect, as seen when figure 2 is compared to figure 1. The decline in CFU/mL was also correlated with an increase in LAS and anthracene concentration. The greatest inhibition, with 100 % of the microbial population killed, was observed with the mixture containing 0.16 mg/L of LAS and 160 mg/L of anthracene.
Table I shows that IC50 for anthracene was 5.2 mg/L, 3 mg/L lower than the IC50 for LAS. The IC50 of anthracene was similar to the IC50 of the LAS–anthracene mixture, where anthracene concentrations increased from 5.2 to 5.92 mg/L. However, the difference was much more pronounced than for the IC50 of LAS–anthracene mixture, whose 70.11 mg/L was almost 8.5 times higher than the IC50 of LAS without anthracene (8.22 mg/L).
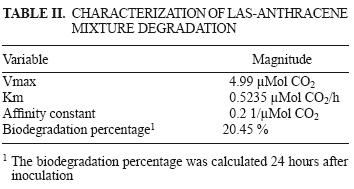
The kinetics of mineralization by the LAS–anthracene mixture is presented in figure 3. The maximum concentration of carbon dioxide produced in the system was observed at 24 hours. After this time, the mineralization fell abruptly and remained constant up to 48 hours.

Table II shows the results of the LAS–anthracene mixture degradation. It is to be noted that 20.45 % of the mixture was biodegraded by the microbial population within 24 hours.
DISCUSSION
There have been few studies of the effect of organic pollutants on sediment microbial populations. Toxicity data of chemical pollutants on microorganisms are scarce due to the considerable ability of microbes to resist or biodegrade organic chemicals. Some microbial populations are very sensitive to low concentration of LAS. Brandt et al. (2001) showed that ammonia–oxidizing bacteria isolated from soil are inhibited by 5–9 mg/L of LAS; inhibition was shown by its effect on microbial growth, specific growth rate and CO2 fixation. García et al. (2006) demonstrated that for LAS, an EC50 of 14 mg/L can be considered a toxic concentration for anaerobic microorganisms, and that the addition of LAS homologues to anaerobic digesters at surfactant concentrations higher than 5–10 g/kg of dry sludge gave rise to partial or total inhibition of methanogenic activity.
The microbial population isolated from sediment was previously exposed to high concentrations of LAS contained in the polluted water. Therefore it could be assumed that the polluted water would selectively isolate microbial populations which could grow in environments with a high LAS concentration. However, in contrast to the prediction, the microorganisms in this study were sensitive to low concentrations of LAS and the LAS–anthracene mixture, with IC50 values of 8.22 and 5.92 mg/L, respectively. Therefore, in this case prior exposure to polluted water did not contribute to an increase in microbial resistance. Jensen et al. (2007) demonstrated that concentrations of LAS in untreated sludge can range from 400 to 14,000 mg/kg dw.
The dose–response curves (Figs. 2 and 3) indicated that microbial growth was not inhibited at very low concentrations of LAS, anthracene and LAS–anthracene mixtures (0.16 mg/L). Increasing the concentration of all compounds and mixtures decreased microbial growth. This may be due to the LAS concentrations evaluated being less than the critical micelle concentration reported (410 mg/L, Brandt et al. 2001); therefore, it is not possible to suggest that there were surfactant–micelle interactions. The results suggest that the LAS toxicity may have been due to direct interactions of LAS monomers with the cell structure, causing an increase in membrane permeability, dissipation of ion gradients and membrane potential or leakage of essential cell constituents. Sartoros et al. (2005) reported that 20 mg/L of the surfactant Tergitol NP–10 may disrupt cell membranes by interacting with lipid structural components of bacterial cells isolated from soil polluted with polycyclic aromatic hydrocarbons.
Anthracene has a high octanol–water partition coefficient (log Kow = 4.1), and readily partitions into organic phases such as phospholipids, which are also found in bacterial membranes. This interaction provokes a hydrophobic region inside the bacterial membrane, which can act as a reservoir for anthracene accumulation (Bugg et al. 2000). It is suggested that the toxic effect of anthracene on isolated microbial populations in this study is due to the accumulation and mutagenic activity of anthracene, similar to the effect produced by polycyclic aromatic hydrocarbons on Salmonella strain YG1041 (Kummrow et al. 2006).
Evaluation of the effect of the mixtures showed clearly that there was no synergy between the compounds, and that the toxicity of the mixture decreases as the concentration of LAS is increased, contrary to the initial hypothesis. Martínez–Tabche et al. (1997) report a similar phenomenon with a mixture of crude petroleum and sodium dodecyl sulfonate: when the concentration of the latter was varied, toxicity of the petroleum on Moina macrocopa acetylcholines–terase activity was reduced approximately 100–fold (antagonistic effect).
Sundaram et al. (1994) note that adding a tensoactive agent to a polyaromatic hydrocarbon can delay biodegradation as the micelles of the former "protect" the latter by delaying or preventing its breakdown.
Laha and Luthy (1992) report that mineralization of phenanthrene is completely inhibited by the addition of 0.2 % of various surfactants. Similarly, Fu and Alexander (1995) state that mineralization of phenanthrene by soil microorganisms was inhibited after the addition of the anionic surfactant Neodol 25–35. Considering these reports, the increase in IC50 of the LAS–anthracene mixture when LAS concentration was increased was attributed to a reduction of anthracene bioavailability promoted by its adsorption of LAS. Because LAS concentration was below the critical micelle concentration, the LAS molecules did not form micelles. However, it is suggested that the LAS molecules interact with anthracene molecules, producing an interaction so strong that it reduces the bioavailability of anthracene, thus avoiding direct contact with the microorganisms and causing its toxicity to diminish. Johnsen and Karlson (2004) demonstrated that Novosphingobium subartica LH128 and Mycobaterium spp. VM572 only express their biological response to phenanthrene, fluorene, fluoranthene and pyrene when they are directly attached to crystals of these polycyclic aromatic hydrocarbons. A similar process has been described by Stelmack et al. (1999): when Mycobacterium and Pseudomonas strains are in direct contact with a nonionic surfactant–hydrocarbon mixture, they do not establish contact with the mixture and avoid its toxic effect. Jiang et al. (2005) demonstrated that 200 mg/L of LAS inhibited mineralization of phenanthrene (by 7 to 12 %) and its toxic effect on phenanthrene–degrading microorganisms in a water–lava–plant–air model ecosystem.
Since the degradation of the LAS–anthracene mixture is at a maximum at 24 hours (Fig. 3), it allowed the establishment of the optimum time at which samples should be taken during this study. Sampling was therefore performed 24 hours after inoculation. According to Ringelberg et al. (2001), the microbial populations present in sediment polluted with polycyclic aromatic hydrocarbons (PAH) are capable of degrading anthracene and other three–ring PAH in a bioslurry treatment system. The three–ring PAH were biodegraded from 115 ± 5.7 mg/kg to 56 ± 3.8 mg/kg in four months by the microbial populations present in the system. The microbial population isolated in this study had a greater capacity for breaking down the LAS–anthracene mixture, with degradation within 48 hours; however, the concentrations evaluated were low (0.16 mg/L of both compounds). In contrast, microorganisms isolated from soil or fresh water were able to degrade the single compounds up to 92 % for anthracene (Moody et al. 2001) and up to 90 % for LAS (Nishihara et al. 1997, Ying 2007); nevertheless, these results refer exclusively to the separate compounds.
Mineralization of the LAS–anthracene mixture was achieved by the microorganisms present in the polluted sediment. The capacity of LAS and anthracene to mineralize microbial populations is well known (Marchesi et al. 1994, Mutnuri et al. 2005, Perales et al. 2007). Still, it is important to consider that there can be non–cultivable microbial populations in the polluted sediment that make an important contribution to mineralization of the mixture.
CONCLUSIONS
The results showed that growth of the microbial population isolated from sediment was sensitive to LAS, anthracene and a mixture of the two. The toxicity of the LAS–anthracene mixture, expressed as IC50, diminished as the LAS concentration was increased, indicating that direct contact between microorganisms and the chemical is important to the toxic effect. The isolated microbial population also has the capacity to degrade the LAS–anthracene mixture if the concentrations of both compounds are low. When analyzing pollutant interactions in sediments, it is important to consider the chemical structure and concentration of the pollutants involved, as the mixture formed may be toxic or capable of being eliminated by the microbial populations present in the aquatic ecosystems.
ACKNOWLEDGEMENT
To Dr. Andrew Steele, Academic Haematology, Royal Free and UCMS, UK, for a valuable critical reading of this manuscript.
REFERENCES
Aboul–Kassim T. (1992). Impact of sewage disposal on the distribution and flux of detergents in Alexandria coastal waters, Egypt. Wat. Sci. Tech. 25, 93–100. [ Links ]
Alexander M. (1997). Microbial communities and interactions: a prelude. In: Manual of Environmental Microbiology (J. Ch. Hurst, Ed.). ASM Press, Washington D.C., pp. 5–13. [ Links ]
Brandt K.K., Hesselsoe M., Roslev P., Henriksen K. and Sorensen J. (2001). Toxic effects of linear alkylbenzene sulfonate on metabolic activity, growth rate and microcolony formation of Nitrospira strains. Appl. Environ. Microbiol. 67, 2489–2498. [ Links ]
Bugg T., Foght J.M., Pickard M.A. and Gray M.R. (2000). Uptake and active efflux of polycyclic aromatic hydrocarbons by Pseudomonas fluorescens LP6a. Appl. Environ. Microbiol. 66, 5387–5392. [ Links ]
Cerniglia E.C. and Heitkamp M.A. (1989). Microbial degradation of polycyclic compounds in the aquatic environment. In: Metabolism of PAHs in the aquatic environment ( V. Varanasi, Ed.). CRC Press, Florida, pp. 50–55. [ Links ]
Comber S.D., Conrad A.U., Höss S., Webb S. and Marshall S. (2006). Chronic toxicity of sediment–associated linear alkylbenzene sulphonates (LAS) to freshwater benthic organisms. Environ. Pollut. 144, 661–668. [ Links ]
Conn E.E., Stumpf K.P., Bruening G. and Doi H.R. (1987). Outlines of biochemistry. 5th ed. John Wiley & Sons, Singapore, 693 pp. [ Links ]
Espigares M., Román I., González–Alonso J.M., de Luis B., Yeste F. and Gálvez R. (1990). Proposal and application of ecotoxicity biotest based on Escherichia coli. J. Appl. Toxicol. 10, 443–446. [ Links ]
Fu M.H. and Alexander M. (1995). Use of surfactants and slurrying to enhance the biodegradation in soil compounds initially dissolved in non aqueous–phase liquids. Appl. Microbiol. Biotechnol. 43, 551–558. [ Links ]
García M.T, Campos E., Sánchez–Leal J. and Ribosa I. (2006). Effect of linear alkylbenzene sulfonates (LAS) on the anaerobic digestion of sewage sludge. Water Res. 40, 2958–2964. [ Links ]
Hamdi H., Manusadzianas L., Aoyama I. and Jedidi N. (2006). Effects of anthracene, pyrene and benzo(a) pyrene spiking and sewage sludge compost amendment on soil ecotoxicity during a bioremediation process. Chemosphere 65, 1153–1162. [ Links ]
Jensen J., Smith S.R., Krogh H.P., Versteeg J.D. and Temara A. (2007). European risk assessment of LAS in agricultural soil revisited: species sensitivity distribution and risk estimates. Chemosphere 69, 880–892. [ Links ]
Jiang X., Yediler A., Yufang S., Sun T. and Kettrup A. (2005). Effect of linear alkylbenzene sulphonate (LAS) on the mineralization, metabolism and uptake of 14C–phenanthrene in a model ecosystem (water–lava–plant–air). Chemosphere 61, 741–751. [ Links ]
Jiménez L., Breen A., Thomas N., Federle W.T. and Sayler S.G. (1991). Mineralization of linear alkylbenzene sulfonate by a four–member aerobic bacterial consortium. Appl. Environ. Microbiol. 57, 1566–1569. [ Links ]
Johnsen A.R. and Karlson U. (2004). Evaluation of bacterial strategies to promote the bioavailability of polycyclic aromatic hydrocarbons. Appl. Microbiol. Biotechnol. 63, 452–459. [ Links ]
Kimerle R.A. (1989). Aquatic and terrestrial ecotoxicoogy of linear alkylbenzene sulfonate. Tenside Surfact. Det. 26, 169–176. [ Links ]
Kummrow F., Rech C.M., Colimbrao C.A. and Umbuzeiro G.A. (2006). Blue rayon–anchored technique/Salmonella microsuspension assay as a tool to monitor for genotoxic polycyclic compounds in Santos estuary. Mutat. Res. 609, 60–67. [ Links ]
Laha S. and Luthy G.R. (1992). Effects of nonionic surfactants on the solubilization and mineralization of phenanthrene in soil water systems. Biotechnol. Bioeng. 40, 1367–1380. [ Links ]
Li X., Li P., Lin X., Zhang C., Li Q. and Gong Z. (2008). Biodegradation of aged polycyclic aromatic hydrocarbons (PAHs) by microbial consortia in soil and slurry phases. J. Hazard. Mater. 150, 21–26. [ Links ]
Marchesi R.J., Owen A.S., White F.G., House W.A. and Russell J.N. (1994). SDS–degrading bacteria attach to riverine sediment in response to the surfactant or its primary biodegradation product dodecan–1–ol. Microbiology 140, 2999–3006. [ Links ]
Martínez–Tabche L., Ramírez M.B., Germán F.C., Galar C.I., Madrigal O.M., Ulloa G.V. and Orozco F.M. (1997). Toxic effect of sodium dodecilbencensulpho–nate, lead, petroleum and their mixture on the activity on the acethylcholinesterase of Moina macrocopa in vitro. Environ. Toxicol. Water Qual. 12, 211–215. [ Links ]
Moody D.J., Freeman J.P., Doerge D.R. and Cerniglia C.E. (2001). Degradation of phenanthrene and anthracene by cell suspensions of Mycobacterium sp. strain PYR–1. Appl. Environ. Microbiol. 67, 1467–1483. [ Links ]
Mutnuri S., Vasudevan N. and Kaestner M. (2005). Degradation of anthracene and pyrene by microcrystals and non–aqueous–phase liquids. Appl. Microbiol. Biotechnol. 67, 569–576. [ Links ]
Nishihara T., Hasebe S., Nishikawa J. and Kondo M. (1997). Biodegradation of aniline, anthracene, chlornitrophen, fenitrothion and linear alkylbenzene sulphonate in pond water. J. Appl. Biotechnol. 82, 441–447. [ Links ]
Perales J.A., Manzano M.A., Garrido C.M., Sales D. and Quiroga J.M. (2007). Molecular structure and biodegradation kinetics of linear alkylbenzene sulphonate in sea water. Biodegradation 18, 567–578. [ Links ]
Pickard A.M., Roman R., Tinoco R. and Vazquez–Duhalt R. (1999). Polycyclic aromatic hydrocarbon metabolism by white rot fungi and oxidation by Coriolopsis gallica uamh 8260 laccase. Appl. Environ. Microbiol. 65, 3805–3809. [ Links ]
Pineda–Flores G., Boll–Arguello G., Lira–Galeana C. and Mesta–Howard, A.M. (2004). A microbial consortium isolated from a crude oil sample that uses asphaltenes as a carbon and energy source. Biodegradation 15, 145–151. [ Links ]
Ringelberg D.B., Talley J.W., Perkins E.J., Tucker S.G., Luthy R.G., Bouwer E.J. and Fredrickson H.L. (2001). Succession of phenotypic, genotypic and metabolic community characteristics during in vitro bioslurry treatment of polycyclic aromatic hydrocarbons–contaminated sediments. Appl. Environ. Microbiol. 67, 1542–1550. [ Links ]
Sanderson H., Dyer S.D., Price B.B., Nielsen A.M., van Compernolle R., Selby M., Stanton K., Evans A., Ciarlo M. and Sedlak R. (2006). Occurrence and weight–of–evidence risk assessment of alkyl sulfates, alkyl ethoxysulfates and linear alkybenzene sulfonates (LAS) in river water and sediments. Sci. Total Environ. 368, 695–712. [ Links ]
Sartoros C., Yerushalmi L., Béron P. and Guiot S.R. (2005). Effects of surfactant and temperature on biotransformation kinetics of anthracene and pyrene. Chemosphere 61, 1042–1050. [ Links ]
Schleheck D., Knepper T.P., Fischer K. and Cook A.M. (2004). Mineralization of individual congeners of linear alkylbenzene sulfonate by defined pairs of heterotrophic bacteria. Appl. Environ. Microbiol. 70, 4053–4063. [ Links ]
Smulders E. and Krings P. (1990). Detergents for the 1990's. Chem. Ind–London 19, 160–163. [ Links ]
Sneath P.H.A., Mair N.S., Sharpe M.E. and Holth J.G. (1986). Bergey's manual of systematic bacteriology. Williams & Wilkins, Baltimore, 898 pp. [ Links ]
Stelmack L.P., Gray R.M. and Pickard A.M. (1999). Bacterial adhesion to soil contaminants in the presence of surfactants. Appl. Environ. Microbiol. 65, 163–168. [ Links ]
Sundaram N.S., Sarwar M. and Islam M.R. (1994). Biodegradation of anionic surfactants in the presence of petroleum contaminants. Chemosphere 29, 1253–1261. [ Links ]
Ventullo M.R. and Larson J.R. (1986). Adaptation of aquatic microbial communities to quaternary ammonium compounds. Appl. Environ. Microbiol. 51, 356–361. [ Links ]
Weyant S.R., Moss C.W., Weaver E.R., Hollis G.D., Jordan G.J., Cook C.E. and Dareshvar I.M. (1996). Identification of unusual pathogenic gram–negative aerobic and facultatively anaerobic bacteria. 2nd ed. Williams & Wilkins, Baltimore, 796 pp. [ Links ]
White G.F. and Russell N.J. (1994). Biodegradation of anionic surfactants and related molecules. In: Biochemistry of microbial degradation (C. Ratledge, Ed.). Academic Publishers, The Netherlands, pp. 137–158. [ Links ]
Ying G.G. (2007). Fate, behavior and effects of surfactants and their degradation products in the environment. Environ. Int. 33, 272–273. [ Links ]














