Introduction
Mexico detected the first SARS-CoV-2 case, causal agent of disease known as COVID-19, in Mexico City on February 28, 2020 (SSA, 2020; Méndez-Domínguez et al., 2020). Two months and eleven days after the outbreak in Wuhan, World Health Organization (WHO, 2021a) declared pandemic status, i.e., an epidemic with synchronous temporal and spatial occurrence respect to functional contagion relationships. Immediately, restrictions on economic and social activities were imposed in several Europe and Asia countries, due to 126559 COVID-19 cases with 4566 deaths in 113 countries (Dong et al., 2020). Five months after the outbreak, Public Health System in cities from Italy, Spain and other countries were on the brink of collapse. Limitations of infrastructure, specialized human resources, availability of commercial diagnostic kits, and validated clinical testing for SARS-CoV-2 were evident (WHO, 2021a). This was the result of adopting gradually a curative approach in detriment of the preventive principle (Velázquez, 2021; CEPAL y OPS, 2021; Frenk, 2003). In Mexico, preventive measures were implemented based on WHO recommendations for ambulatory populations. The ‘Healthy Distance Program’, ‘Sentinel Model’ for detection and estimation of suspected cases (Hernández-Ávila et al., 2020), and WHO’s Polymerase Chain Reaction (PCR) diagnostic protocol were immediately adopted for using in clinics and official laboratories. However, the feasibility of mass testing was questioned due to cost and infrastructure, the efficacy of which was evidenced during first quarter of 2020 in South Korea, Hong Kong and China for early SARS-CoV-2 detection and suppression of contagion chains (June-Ho et al., 2021). In November 2021, almost two years after the pandemic process, Mexico and rest of the world have gone through several stages of epidemic management and mitigation according to their governmental health structure, financial and operational capacity for to articulate WHO recommendations. The contrasting and inconsistent results require a cause-effect analysis from a comprehensive and systemic historical perspective.
In this context, the objective of this work was to analyze the SARS-CoV-2 pandemic process from a retrospective historical approach of epidemics in humans and plants, to compare the application of concepts, principles and mitigation strategies under the premise of that epidemiology is a transversal science unified by the population and the epidemiological system as a rational framework of a Health System. For this purpose, epidemics with extensive population and regional impact, but contrasting with respect to scientific, technological and social realities were selected.
1. COVID-19: Pandemic Management
Pandemic COVID-19. Impact and curative approach. Twenty one months after the pandemic outbreak (November 2021), more than 239 million and 5 million cumulative COVID-19 cases and deaths, respectively, have been reported in four ‘epidemic waves’ or cyclical events worldwide (Dong et al., 2020; WHO, 2021b). At least the last two waves occurred despite a mass immunization, but with an incipient doses number and poorly geographic distribution. However, the medical and clinical emphasis still prevails in the search for solutions to a complex problem that requires including economic, psychological, sociological, demographic, agro-productive, environmental, and technological-digitalization approaches (e.g., big data, web communication, etc.). The three epidemics of this century (Influenza A H1N1, SARS-2002, MERS and SARS-CoV-2) illustrate this reductionist approach focused on clinical solution, promoted by pharmaceutical companies and associated research centers. These zoonotic diseases suggest that anthropocentric activities were decisive for infectious agent transmission from animals to humans. Efficient prevention approaches, not just curative, would focus on epidemiological surveillance of primary ‘causality’ in animal microbiome for risk models.
Early epidemiological approaches and research. At international scale, SARS-CoV-2 detection was used for clinical and epidemic monitoring, without specific needs and strategies for medical treatment, in line with community preventive goals (Donget al., 2020; WHO, 2021a). On the other hand, scientific research was slowed or paralyzed due to voluntary or mandatory confinement. This impeded the infrastructure use and the application of interdisciplinary approaches to face the pandemic complexity from a holistic, systemic and multidimensional vision. Additionally, the strong scientific specialization has been detrimental to the interdisciplinary team formation. The appreciation that COVID-19 health crisis is responsibility of government and biomedical research systems has persisted, and still persists, among sociology, psychology, agriculture, demography, etc., researchers areas. Unfortunately, that vision also prevails in the health field.
Pharmaceuticals, immunization and antivirals. Almost two years after the onset epidemic, demands for a return to comprehensive preventive models through strengthening the Public Health Systems has disappeared. Rather, prevail a public agenda with predominance of pharmaceutical business vision. Thus, the focus is on mass vaccination and ‘herd immunity’, but SARS-CoV-2 reinfection in people with full doses suggests an immunity potential loss after 16 months, less than other coronaviruses such as SARS-CoV 2003, MERS or HCoV (Townsend et al., 2021). Furthermore, full immunity does not exist yet; cyclical epidemic processes have continued with a sequence of high-prevalence variants (Delta and Omicron are the current); and the occurrence of variable clinical conditions, including severe symptoms and deaths in immunized individuals. Therefore, it is now recognized that the potential for infection, re-infection or co-infection may be exhibit in immunized individuals, and that a vaccine eliminates the mortality risk over 90% in case of infection. Pharmaceuticals research was undoubtedly fast in the development of vaccines, but with mechanistic biological gaps derived of limited pathological virus understanding (Li et al., 2021). Despite these scientific gaps, another medical front is now being tested that will definitely eliminate the possibility of returning to sustainable health models, and maintain highest profitable curative approach to the prevention detriment.
At close of this paper, Merck and Pfizer had advanced clinical tests, not yet public, on antiviral pills (Molnupiravir and Paxlovid) against SARS-CoV-2 (Ap, 2021a; Afp et al., 2021a). On another front, the current pharmaceutical public agenda includes vaccination of children when their risk factors do not justify, and there are still high-risk populations without vaccines in countries lacking economic sufficiency and infrastructure for vaccines acquisition, which may represent sources of re-infection and new variants worldwide. As of October 28, WHO reported that only 0.4% tests and 0.5% vaccines administered worldwide were performed in low-income countries (Afp et al., 2021b). We are approaching to a public health cooptation for profitability, exploiting vulnerability and fear to COVID-19 as a dissuasive speech.
2. Prevention and public health
The WHO and COVID-19. In a historical perspective, it is necessary to state forcefully that neither WHO nor the human health institutions have been able to develop and implement effective public health models with emphasis on risk prevention (Figure 1). Successful preventive programs, concretized or initiated in the XX century (Cáceres, 2012), have been surreptitiously ignored despite the smallpox (1971), polio (1997) and measles (2016) eradication, the latter derived of a global program initiated in 1980 and which has prevented, according to the WHO, 17.1 million deaths in the world between 2000 and 2014 (OPS). Moreover, the emergence of anti-vaccine groups in Europe and US confirms the health organizations negligence to reverse a trend that undermines the prevention principle and favors economically profitable curative models (Velázquez, 2021; CEPAL and OPS, 2021; Frenk, 2003). Thus, past failures like H1N1 flu (2009) or Ebola (2014) evidenced fragile preventive models. Consequently, the management of COVID-19 pandemic has been questioned and controversial despite the significant financial amounts operated by WHO. In 2020-2021, this organization invested US$ 8482 million to address three fundamental health components: 1) Universal health coverage; 2) Health emergencies; and 3) Improved health and well-being (WHO, 2021a). By October 2021, 239 million infections since patient zero, on November 17, 2019 in Wuhan, China (Dong et al., 2020), suggests the global systematic negligence of prevention, and limited academical, research, and operational structures (Velázquez, 2021; CEPAL y OPS, 2021; Frenk, 2003). In this context, the WHO’s ‘new plan’ against the pandemic in less developed countries requires US$ 23.4 billion annually for operation, but lacks effective contingency plans, rigorous risk scenarios and massive data analysis for decision-making (Afp, 2021c).

Figure 1 Timeline of the most important epidemic/pandemic processes occurred in human and plant populations during 1300-2021. The accumulated curves of plant/human pandemics in the same period are shown. Source: Own elaboration with data from cited literature
Public health: Infrastructure and human resources. COVID-19 revealed the absence and weakness in infrastructure, technology and advanced research in public institutions to generate vaccines, even in developed countries. This compromised public health security in less than 10 major pharmaceutical companies. Between 2020 and 2021, these companies earned profits in excess of US$ 270 billion, with Moderna, mRNA-1273 vaccine creator, being the most benefitted with 50% (Carbajal, 2021). The epidemic evidenced, in addition to the lack of health workers and specialists in intensive care (e.g., pulmonologists, internists or emergency physicians), the scarcity of epidemiologists focused on research and mitigation of community risks, outside the hospital environmental, to develop preventive contagion models, complementary to diagnosis, confinement and monitoring of cases applied worldwide. Paradoxically, Mexico and many countries were at the forefront in educational and research models. As an example, the School of Public Health of Mexico (SPHM) founded more than 100 years ago, was created under the philosophy of principle prevention as a logical and rational alternative (ESPM, 2021).
A 1920 definition of public health clearly establishes the prevention principle adoption: ‘Public health is the science and art of preventing disease, prolonging life, and promoting health and physical efficiency through organized community efforts to clean the environment, control community infections, and educate the individual about the principles of personal hygiene; organize medical and nursing services for the early diagnosis and preventive treatment of diseases, as well as develop the social machinery that assures each individual in the community an adequate standard of living for the maintenance of health’ (Winslow, 1920). Although the evolution of public health concept is recognized, Julio Frenk emphasizes: ‘at the level of population analysis of public health, health conditions are addressed through epidemiological research’ (INSP, 2020). Consequently, a pandemic not studied by epidemiologists promotes ‘cure’ as only response for the disease, just as it currently occurs with COVID-19 pharmacological treatments.
Vaccines and global risk management. The mistakes in global risk management were also evidenced by COVID-19. The impartiality, or at least the apparent bureaucratism, of WHO and the European Medicines Agency, which have delayed the approval of Sinovac (CoronaVac), Cansino (Ad5-nCoV) and Sputnik V (Gam-COVID-Vac), vaccines created with participation of prestigious Chinese and Russian public institutions, is questionable. Disagreement that those countries involved have expressed as ‘unfair competition’ and ‘protectionism’ of the G-20 countries (Afp, 2021d). This delay is incomprehensible given that Sputnik V was the first vaccine produced worldwide and has been applied in over 70 countries, including Mexico. The China’s vaccines have been used in full doses for more than 1 billion people (75.7% of the population) and at least 20 countries (datosmacro, 2021a). A health crisis, by definition, implies rapid decision-making and comprehensive innovation in all processes associated with the solution. This geopolitical strategy is compounded by the pharmaceuticals company reluctance, supported by their countries, to release vaccine patents, evidencing economic interests over human health. This is an obvious consequence of dominant market economy. Consequently, humanity is trapped in its own structures and rules. So, it is not surprising the discouragement of the UN Secretary declaring: ‘not to have equitable distribution of vaccines is not only a question of being immoral - it is also a question of being stupid’ in response to ‘hoarding and nationalistic vaccination policies’ of the most developed countries such as USA, UK, France, and Germany (Navarrete, 2021; ONU, 2021).
Mexico and Cuba: Vaccine production. BIRMEX, a Mexican governmental institution, leader in the 50’s and 60’s in vaccine production (Mexico eradicated smallpox 20 years before the rest of the world in 1952), evidenced its unfeasibility to generate own immunologicals (BIRMEX, 2021). On the contrary, it is commendable that Cuba, the only Latin American country with developed vaccines, Abdala and Soberana 02, has applied 14 million 672 thousand 62 doses up to September 4 (52% of the population), and has been the first country in the world to initiate a National Children’s Campaign against COVID-19 once adult population was immunized (Noda y Chávez, 2021). This achievement is only understandable with a solid Health System, initiated in 1962, with an emphasis on prevention. This self-sufficiency example and public health security is an exceptional achievement considering the restrictions in infrastructure and laboratory material due to economic blockade imposed by the US since 60’s. This is inhumane and immoral considering current COVID-19 pandemic situation. It is paradoxical, but understandable from a health business perspective, that humanity suffers epidemics in XXI century in the midst of the amazing scientific revolutions flourishing. Also, it is ignominious that human epidemics occur without improvement of solid, sustainable and resilient public health models.
3. Human Epidemics and Pandemics
Pandemics and historical epidemic outbreaks. Throughout human history, infectious agents have coexisted and evolved with humans. Diseases have been documented since at least 1200 B.C. However, epidemic expression has resulted from the populations settlement in conditions that favor faster and effective contagion such as trade, migration, unhealthy conditions, poor nutrition, etc. Thus, a total of 19 pandemics (Piret and Boivin, 2019) and 200 local or regional epidemic outbreaks have occurred since the late Medieval Ages, highlighting the Black Death (1346-1353), Cholera (1846-1860), Spanish Influenza (1918-1920), H2N2 Influenza (1957-1958), SARS-CoV (2002), H1N1 Influenza (2009-2010), MERS-CoV (2012) and SARS-CoV-2 (2019-present) (Figure 1). However, these epidemic processes have been exempted of a rigorous comprehensive analysis from scientific, philosophical, social, economic and technological perspectives (Figure 2). On the contrary, there is a generalized disinterest for generate and systematize knowledge applying to public policies, educational models and the Public Health Systems strengthening. Historical successes and failures provide opportunities for analyzing and improving public health policies.
The emergence of pandemics is linked to the human rise over nature. The breakpoint was Industrial Revolution (1760-1840), a historical reference that represented the intensification of production through machines incorporated into manufacturing lines, this with the consequent radical changes in socio-cultural patterns, education, urban population growth, job insecurity, unhealthy conditions and expansion of technology and capital to poor countries. An inevitable framework for Karl Marx, in Das Kapital (1867), unraveled the merchandise or commodity paradigm of modern economy. Eventually, the international geopolitical interest realignment and the World War I were originated. It was the context for the Spanish Flu (1918-1919), first world pandemic occurred, caused by sick US soldiers displaced to Europe.

Figure 2 Timeline of technological, scientific, and social events linked to significant human and plant epidemics. Source: Own elaboration with data from cited literature.
The Spanish Flu (1918-1919) and human mobility. The Spanish flu was caused by the Influenza A (H1N1) virus, a variant identified in 2005 by genomics technologies (Figure 1). In only 18 months, a surprising synchronic contagion occurred in different regions of the world due to mobility of diseased individuals. Mortality was estimated at 50%. The secrecy of the disease for strategic reasons of countries participating in the First War World, contributed to the lack of risk communication. The Industrial Revolution represented the rise of modernity; accelerated-sustained growth of population since XVII century (Roser et al., 2013); and increase in anthropogenic mobility due to invented means of transportation (steamships, railroads, automobile, etc.) (Figure 2). Inevitably, these development dynamized the economy and enhanced socio-cultural interconnections between distant regions, e.g., tourism emerged, which would become an important factor in many economies and cause intense and human flows. This is the globalization beginnings and contemporary pandemic consequences. The SARS-CoV-2 dispersion during the epidemic onset proved the globalization weight on the contagion and the fast spread of an infectious agent, sufficing just over two months for a pandemic condition (WHO, 2021a). The fragility of global economy due to the disaggregation of supply chains was evidenced. A chip not produced in Asia stopped car production in America. The virus paralyzed the supply chains and the global economy.
Black Death (1346-1667), miasmatic theory and causality. Although the Black Death or Bubonic Plague historically had highest mortality rate (estimated 60%), the epidemic occurrence for many years was restricted mainly to Europe in overcrowded and unhealthy cities in coexistence with rats and fleas, the host-reservoirs from which the bacterium Yersinia pestis is transmitted to humans. Secondary individual-to-individual infection was effective due to direct bacterial skin exudates. This is a case of an infectious agent mobilized from Asia by trade (Figure 1). Although strictly the Black Death was not a pandemic, caused between 75-200 million deaths in the late Middle Ages (Figure 1). It occurred in a convulsive period characterized by wars (e.g., 100 Years’ War, Crusades, invasions, etc.). The theory of spontaneous or miasmatic generation prevailed (Caponi, 2002) and the Aristotelian vision permeated science, basically philosophical. Technological developments were essentially warlike, iconically illustrated with Leonardo Da Vinci (1452-1519) as inventor. The absence of solid scientific-technological bases delayed the possibilities of an efficient Black Death management. It was not until 1894, crucial stage in which miasmatic ideas were destroyed and microbiological etiology emerged, that Alexander Yersin, using the etiological approach of cause-effect association, discovered the causal agent Yersinia pestis and the zoonotic animal-human relationship. A. Yersin was a student of Robert Koch, discoverer of Mycobacterium tuberculosis which causes tuberculosis and to who are attributed the famous causation postulates widely used in phytopathology and medicine with some restrictions.
Black Death, vaccination, hygienism, and endemicity. The microbiological advances enabled the development of first vaccine against Y. pestis in 1897, i.e., 551 years after the first epidemic outbreak. However, the hygienist approaches of Adrien Proust was, among others, what helped to interrupt the primary chains of contagion involved with the Black Death, Cholera and Yellow Fever, clearly exposed in his book (1873): l’hygiène internationale: ses applications contre la peste, la fièvre jaune et le choléra asiatique. Proust succeeded in promoting hygienist public health policies, social distancing measures, quarantines, sanitary cordons in ports and confinement for infectious risks management (Figure 2). These strategies are applied for COVID-19 in a contrasting socio-economic context and that has been devastating for poor and emerging economies. Likewise, it has been desperate alternatives for a dismantled global Health System (Velázquez, 2021; CEPAL and OPS, 2021; Frenk, 2003), unable to prevent and mitigate risks despite today’s sophisticated scientific talent and great technological advances.
Consequently, despite the accumulated experiences, Black Death, Cholera, Yellow Fever, etc. are still present, in low-prevalence and spatially localized. For example, during 2010-2015, WHO reported 3248 associated cases to Black Death with 17.98% mortality. In 2017, outbreaks were reported in Madagascar, where it is considered endemic, Peru and Congo (WHO, 2017). The same institution estimates 3-5 million annual Cholera cases (Harris et al., 2012). This shows that an infectious agent evolves with the human being in a delicate biological-environmental balance and eventually transits from an epidemic condition of disequilibrium, to an endemic condition of low-prevalence with recurrent outbreaks. Therefore, epidemiological surveillance should be a fundamental strategy of a preventive Health System but with new paradigms linked to scientific-technological innovations (Mora-Aguilera et al., 2021a).
Cholera (1817-1961), Koch, pandemics and antibiotics. Cholera, another disease caused by a bacterium, Vibrio cholerae, also associated with overcrowding and unhealthy cities. Cholera had an important impact in Europe, particularly in London, England (1846-1860) at the time when microbiology was already contributing to etiology and mitigation measures. R. Koch, Nobel prize in medicine (1905) and considered the father of bacteriology, isolated the causative agent for first time (1883) from interdisciplinary studies in India. From 1817 to 1961 seven pandemic processes, associated with different variants of the bacterium, and endemicity in some regions of Africa and Asia, have been recognized (Harris et al., 2012). The 1961 pandemic, where Latin America was involved, progressed in three independent but overlapping waves associated to contrasting variants and SXT resistance factors. In this case, the mutagenesis induction by antibiotics was determinant in the occurrence of cyclic epidemic processes (Harris et al., 2021). Notoriously, the biological mutation, regardless of the microbiological organism, e.g., bacteria or virus, is inherent in diseases and their epidemics; and the cure of disease, that profitable resource promoted by pharmaceuticals, in this case sulphamethoxazole/trimethoprim are temporary solution and eventually causal of new variants resistant to available drugs.
Cholera, curative lesson and mutation. Medical cure is a trend analogous to the chemical control of plant pathogens (and pests in general) in agricultural crops. A toxic paradigm driven by companies, e.g., Bayer and Pfizer, which has own divisions for plants and humans. Pernicious circles at the expense of health. Consequently, the etiological level is no longer sufficient in epidemiological surveillance. The integration of molecular epidemiology is required for an efficient monitoring of races, variants, pathovars, etc. Not for variant detection purposes as prevails with current COVID-19 tests. The purpose should be inferential. Integrate the monitoring into risk models to forecast changes in variants prevalence and new pathogenic haplotypes emergence. COVID-19 has progressed in a succession of variants with differentiated parasitic fitness, at a faster rate than the generation of vaccines and drugs. The adaptive capacity of infection processes and the complex multiplication of SARS-CoV-2 (Li et al., 2021), makes it necessary transcend curative solutions of temporal effectiveness by implementing risk and forecasting models for COVID-19, integrating genomics to risk factors.
Cholera, contagione, epidemiorum, vaccines and inmunity. The main Cholera’s outbreaks occurred after the Enlightenment (1700-1800) and during the Industrial Revolution (1760-1840) (Figure 1). There was already an important advance in pathological and epidemiological theory and conceptualization in human diseases. The book ‘De contagione et contagiosis morbis et eorum curatione’ written by Girolamo Fracastoro (1546), described the ‘degeneration of the air’ associated with the spread of black death, leprosy, thysis, scabies, rabies, erysipelas, smallpox, anthrax, trachoma and other health problems of great impact at the time. Although the causality was not correct (Caponi, 2002), Francastoro is credited with embodying the transmissibility idea. Another reference of epidemiological interest was “Epidemiorum” by Guillaume de Baillou (1580), who described epidemics of measles, diphtheria and bubonic plague in Europe (Figure 2). There are also other transcendental scientific discoveries in biology and other disciplines, e.g., Leeuwenhoek invented the microscope without which etiological microbiology would have been impossible. His records of bacterial cells and organisms (1670-1683) were references for later more formal studies of R. Koch or L. Pasteur (Figure 1).
However, more than 200 years were required for the microbiology emergence based on the etiological causality principle (Caponi, 2002). In this context, it can be explained that the development of first Cholera vaccine (1885) was obtained 33 years after a major outbreak, contributing to limit mortality to 10 million people (Figure 2). In this period, the knowledge of immunity already existed and other vaccines had been generated against smallpox (1796, Jenner), anthrax and rabies (1881, 1885, Pasteur, considered the immunology’s father), and penicillin had been discovered (1897, Duchesne; 1928, Fleming) (Cáceres, 2012). These achievements marked a milestone in development of science showing the importance of scientific knowledge and humanistic essence. Diseases and epidemics seemed to have critical routes for their prevention. The millions of deaths would be unrepeatable experiences. However, 100 years later, with more than 250 million cases and 5 million deaths, COVID-19 has shown the opposite.
Cholera, J. Snow, contagion. The J. Snow period was decisive for modern medical epidemiology. It emerged as a science with conceptual and methodological population bases applied at the community level, understandable due to the absence of the hospitalarian systems. The organizational and functional hospital conception had its origin with the Crimean War (1853 and 1856), inseparable from the Florence Nightingale contributions (Turnes, 2009). The first London Epidemiological Society was founded in 1850, four years after the Cholera outbreak (Figure 2). John Snow played an active role in understanding the Cholera outbreak in London by studying for the first time the location of sick individuals and inferring the spatial dependence between sick individuals and the healthy population. That is, he demonstrated the contagion, a fundamental principle of epidemiology. He also showed the importance of population data, concretized in a community living together and sharing space and resources, to infer the solution without knowing the etiology of Cholera, which was elucidated 37 years later.
The ‘field’ and ‘community’ data is practically forgotten today. Clinical and hospital registration (or entry points at ports and airports, in Plant Health) is not sufficient. Identifying the infectious agent/pest in ‘pathway’ is a predominantly etiological approach disconnected from the principle contagion. The absence of spatio-temporal structured, convenient and integrated data in real-time to big databases for decision-making is the main limitation of current epidemiology in human, animal and plant health areas. Consequently, COVID-19 was no exception. The registration of positive cases has made possible to estimate SARS-CoV-2 lethality and mortality, and to describe the temporal epidemic dynamics in demographic populations, but it does not make possible the understanding and intervention of the contagion mechanisms that operate at community clusters, i.e., labor, family, social environments (Mora-Aguilera et al., 2021b).
Cholera, map, water. In 1854, on a paper map with traces of London streets, J. Snow located and counted the sick individuals number in the city. He included other spatial elements to explain the associativity of diseased cases. This allowed J. Snow to link the ‘water’ of supply sites with contagion: ‘As soon as I became acquainted with the situation and extent of this irruption of cholera, I suspected some contamination of the water of the much-frequented street-pump in Broad Street’. This rationalization, outside the reductionist paradigm of the sick-individual, made possible to analyze and conclude that the water distribution system was associated with Cholera cases dispersion in the city. Snow’s original Cholera map (Figure 3) shows conceptually and manually the abstraction to represent in a space the distribution and linkage of an epidemic event, becoming the precursor of dispersion pattern spatial analysis associated with contagion.

Source: Authors with historical data. ArcGis. https://www.arcgis.com/home/webmap/viewer.html?webmap=7523 7a7d4b7547c1b3addf9ffcd380fa.
Figure 3 A. Urban original map of cholera cases in London from J. Snow. B. Heat map developed using ARGIS enhancing that analytical interpretation. Red color indicates intense contagious activity. In yellow the main focus location
Nowadays, computational resources and specialized spatial software, e.g., Golden Surfer® (1985), ArcGis® (1999), QGIS® (2002) or Google Maps (2005), allow application in epidemiology to study contagion mechanisms. Various geostatistical methods and bi- or three-dimensional projections make possible to determine spatial dependencies or aggregation patterns using tools to add, join and superimpose layers of information with a single ‘click’. These developments suggest that spatial analysis is a simplistic process, but obviate the scientific conception of the problem in an epidemiological phenomenon context (Escobar-Gutiérrez et al., 2020; Ibarra-Zapata et al., 2019; López-Avalos et al., 2017). However, the precedent generated by J. Snow’s seminal idea, today so simple and logical, changed the paradigm for decision-making in the contagion spatial dimension, the first front of an epidemic process, and implied considering other environmental factors in addition to disease (Velázquez, 2021; CEPAL and OPS, 2021; Frenk, 2003).
Current cholera, zoonosis. Cholera and other historical epidemics continue to be studied, highlighting the deficient health models. In the last decade, research on Vibrio cholerae includes spatio-temporal models (Mari et al., 2012), climatic conditions (Escobar et al., 2015), and environmental reservoirs using remote sensing (Recault et al., 2019), among others. The scenario for Health Systems is a more adverse one given the recent emergence of zoonotic origin epidemics, a factor previously limited in the human health history where sanitation and hygienism had a fundamental epidemic mitigation role (Figure 1). Globalization, overexploitation of natural resources and anthropogenic environmental impact are some causes of these infectious biological bridges between animals and humans mediated mainly by highly effective viral agents with their structural simplicity and their sophisticated mimetic function to that of humans (García-Ruíz, 2021). Thus, at close this document, a coronavirus of the genus Deltacoronavirus (Porcine deltacoronavirus, PDCoV) was reported for the first time infecting humans in Haiti, a highly marginal region with contact between humans and backyard pigs, which represents another potential zoonotic risk. Previous human-adapted coronaviruses were restricted to genus alpha-coronavirus (HCoV-NL63) and beta-coronavirus (MERS-CoV; SARS-CoV 2003; SARS-CoV-2) (Lednicky et al., 2021).
4. Epidemics and pandemics in plants
Epidemics: Monoculture, dispersion, mobility. In the plant kingdom, regional and pandemic epidemic processes have also affected plant populations, both cultivated and native, during human history (Figure 1) (Mora-Aguilera et al., 2021a; Ristaino et al., 2021; Carvajal-Yepes et al., 2019; Parnell et al., 2017; Potter and Urquhar, 2017). Logically, the phenomena of mass parasitism have been accentuated with the agriculture evolution towards extensive monoculture systems and with the global dispersal of cultivated plant species from their origin centers. For example, in citrus crops, originating in Asia, the Citrus tristeza virus, endemic in the world, has caused more than 50 million plants deaths in America (more than 100 million worldwide) since first outbreak in Argentina and Brazil in 1930’s (CIPF, 2016), an epidemic caused by infected orange trees from South Africa (Figure 1) (Rivas-Valencia et al., 2008). Recent pandemics in humans have evidenced the role of human mobility and social proximity in fast continental and transcontinental pathogens dispersal (Davis et al., 2021), while in plant pathogens, with slow dispersal synchronous and discontinuous crops production cycles, the trade in plant products and sub-products has been more important, and since 90’s the long-distance dispersal due to extreme climatic variations (Chown et al., 2014; Mora-Aguilera et al., 2014a).
Analogous to human epidemics, the response of national (NPPOs) and regional plant protection organizations (PRMOs), and international organizations such as the International Plant Protection Convention (IPPC), associated with FAO, has not been effective either, they operate predominantly under classical commercial regulatory models (Mora-Aguilera et al., 2021a; Santivañez et al., 2013). Population restrictiveness and the etiological vision equally afflict both fields of epidemiology application, i.e., if in medical epidemiology the emphasis is on diseased-patient-hospital, in plant epidemiology it is restricted to disease-plant-field. Consequently, the history of crops epidemiology, and by extension the animal, has not been so different from the humans.
Various continental epidemics (i.e., restricted to one continent) and pandemics have impacted cultivated plants, increasingly in response to the need for big food volumes required to sustain urban growth since the industrial boom of XIX century. Similarly, at the early XX century, occurred the first epidemic outbreak in a non-cultivated host: native European Ulmus spp. caused by Ophiostoma ulmi, possibly by anthropogenic pressure on timber resources for construction purposes (Figure 1). This pandemic-endemic disease, with recurrent outbreaks, is estimated to have eliminated 30 million elms between 1970 and 1990 in UK, with severe economic and environmental consequences by destroying the tree population in some regions (Potter and Urquhar, 2017; Potter et al., 2011).
Historical pandemics in plants. In plant epidemics there are also present various infectious agents, including fungi, viruses and bacteria, which have high plant specificity. Although there are no reports of epidemic outbreaks in humans or animals with phytopathogenic origin, some diseases of medical interest have been reported, usually due to the effects of toxins from fungi, e.g., Aspergillus, Fusarium, Claviceps and Cladosporium, and in bacteria, the genus Pseudomonas stands out, which has pathogenic species in plants and humans (e.g., P. aeruginosa). In general, plant pathogens exhibits a biological barriers, which has not been the case between animals and humans. Among the diseases that have exhibited epidemic processes with millions of plants deaths, the following stand out: Phytophthora infestans - Solanum tuberosum (1845-1849), Hemileia vastatrix - Coffea spp. (1868-1882, 2009-present), Fusarium oxysporum f. sp. cubense - Musa spp. (1876, 1924, 1990-present), Ophiostoma ulmi - Ulmus americana (1919 - present), Moniliophthora perniciosa - Teobroma cacao (1987-endémico), Papaya ringspot virus - Carica papaya (1995), Citrus tristeza virus - Citrus spp. (1930-1937, 1990-present), Coconut lethal yellowing phytoplasma - Cocos nucifera (2000), Candidatus Liberibacter asiaticus - Citrus spp. (2009-present), among others (Mora-Aguilera et al., 2021a; Ristaino et al., 2021; Carvajal-Yepes et al., 2019; Potter y Urquhar, 2017; Mora-Aguilera et al., 2014b,c; Potter et al., 2011; Gonsalves et al., 2010; Rivas-Valencia et al., 2008) (Figure 1).
Drugs and agrochemicals: Cure and the same actors. Epidemics and pandemics in plants, in addition to the direct effect (e.g., the survival risk of native and cultivated stocks, and use of energy for genetic adaptations), have an important impact on quality and viability of human life, not only by limiting food production and safety, but also by the agroecological, environmental, economic, social and cultural impacts. On the other hand, a plant pathogen ensures survival, endemicity and epidemic potential through a wide range of hosts at the varietal, species, genus, family and order taxonomic levels. This infectious plasticity has generated an important agrochemicals market, emphasizing the cure to the detriment of preventive principle in a manner analogous to clinical management of diseases in humans.
Understandably, companies such as Bayer, a chemical-pharmaceutical company founded during the Industrial Revolution and with the largest global sales volume, have chemical divisions for plants (Cropscience, 45%), animals (Animal health, 3%) and humans (Pharmaceuticals, 41% and Consumer Health, 12%). In sales percentage, out of 41.4 billion euros in 2020, chemicals marketed for ‘pest control and crop protection’ exceed ‘prescription drugs’ for humans (45% vs. 41%) (Díaz, 2021). This is the challenge, changing the curative paradigm to disease prevention. That is, the epidemics prevention. It implies changing the current curative approach to risk management and mitigation. The curative approach is profitable but not sustainable.
Late Blight (1845-1850), varietal diversity, de Bary. In plant health history, the first epidemic challenge, addressed with a scientific approach was the Late Blight (‘potato murrain’ original name in Europe), a disease of potato (Solanum tuberosum), and a plant native to Andean regions of South America and carried to Europe in the XVI century by Spanish. Its epidemic status between 1845 and 1850 included GB, France, Holland and Belgium (Figure 4) (Dyer, 1874). But it had the highest impact in Ireland with a famine (known as ‘The Potato Famine’) resulting in 1.1 million people killed and 1.5 million migrants in Europe and US (van Esse et al., 2019) (Figure 1, 2, 4 and 5). This could be the first case of the plant diseases potential impact on human food security and the risk of accidentally spreading a pathogen associated with a specific plant genotype selected of native genetic variability. Peru and Bolivia, recognized as main origin centers of potato, have the greatest diversity of the 130 known wild species (CIP, 2021); and the region, with Mexico as a reference (Galindo and Gallegly, 1960) were for many years the only one with the sexual phase presence of the fungus, and consequently also of greatest variability. However, there are no epidemic records in this pathosystem in America in XVI and XVII centuries (Figure 1 and 5). Previously, Peronospora had been the fungal genus associated with the disease by English scientists such as Miles Joseph Berkeley (Berkeley, 1946), but Anton de Bary studied it in greater depth in the context of the public competition “Grand Prix des Sciences Physiques, proposé pour 1857” (Figure 5) organized by the Académie des Sciences (Matta, 2010). Anton de Bary, among other findings, observed that mycelial growth was associated with potato Late Blight, determined the life cycle and identified as Phytophthora infestans, currently considered a pseudofungi belonging to the class Oomicetes (Turner, 2005).
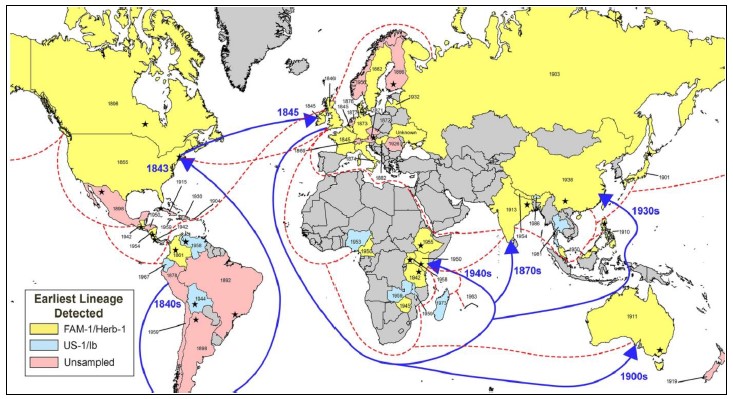
Source: Saville and Ristaino, 2021.
Figure 4 Worldwide dispersal of genotypic clade FAM-1 Phytophthora infestans (in yellow) causing the epidemic in potato (S. tuberosum) in 1840’s, responsible for the famine in Ireland with more than 1.1 million deaths; and the genotypic clade US-1 of later appearance (in blue). Dotted lines indicate estimated British sea routes for 1930. Blue lines suggest the possible routes and years of FAM-1 dispersion to Africa and Asia. The year in each country indicates the record of the collection in the herbarium and star indicates place with report of the disease.

Source: A. http://www.historylearningsite. co.uk/ireland_great_famine_of_1845.htm. B. Académie des Sciences. 1856. ‘Grand Prix des Sciences Physiques, proposé pour 1857.’ Comptes rendus 42: 161-163. C and D. Romero, 2004
Figure 5 A. Irish families affected by the famine in 1845 - 1849. B. Promotion of Grand Prix Des Science Physiques to study structure formation mode, physiological role, germination and spore penetration of parasitic fungi. C. Foliar symptoms of potato blight. D. Phytophthora infestans spore structure.
Blight, Millardet, variability and resistance. The solution proposed by de Bary was consistent with the hygienism applied in the containment of epidemics in humans in the mid-nineteenth century, e.g., by J. Snow in the Cholera management (1854); de Bary suggested the elimination of inoculum sources, a practice referred to in plant health as sanitization, consisting of destroying affected tubers. However, the infectious aggressiveness of the pathogen led to the development of cure/protection principle with the exhaustive use of Bordeaux mixture, developed in 1885 (copper sulfate, hydrated lime, water) (Millardet, 1885), considered the precursor of fungicides first generation with contact action. Currently, more than 150 years after the identification of this microorganism, the disease is endemic with regional epidemic outbreaks caused by gene migration from the organism, varieties resistance loss and development of fungicides resistance requiring the application of third and fourth generation products, mainly of systemic action (Mandipropamid and Azoxystrobin) (Lal et al., 2018; Romero et al., 2012); even so, global losses are estimated at US$ 6.7 billion annually (Lal et al., 2018; Haas et al., 2009).
As with SARS-CoV-2, the genomic technology applied in P. infestans has shown that the characterization of epidemics at the population level, under assumption of species homogeneity, is insufficient for the mechanistic understanding of parasitism and that it is necessary to determine subepidmic processes at the variants or genotypes levels (Davis et al., 2021; 2011). That is, to study the population genomic structure. For example, in P. infestans it has been possible to identify that a genotypic lineage, FAM-1, was responsible for pandemic process that began in 1840’s in Europe, becoming established in 144 years in six continents with 73% prevalence estimated with herbarium samples collected between 1845 and 1990 (Figure 4) (Saville and Ristaino, 2021). However, the microorganism is highly variable. Whole genome sequencing of P. infestans has revealed the complexity and high evolutionary and host adaptive capacity (Haas et al., 2009). This explains the intrinsic problem with irrational application of curative/protective principle resulting in pathogen resistance to fungicides due to mutagenic pressure. For example, metalaxyl loss of biological effectiveness has been reported over 3 and 12 years (Lal et al., 2018), despite the fungicides alternation in programs of 16-24 applications per potato production cycle (Romero et al., 2012).
Drug resistance, analogous to plant pathogens. The mutagenic capacity of P. infestans to fungicides is not exclusive to plant pathogens. According with WHO, developing antimicrobial drug resistance in humans (i.e., antibiotics, antivirals, antifungals and antiparasitics) is also a health threat to humanity (Solano, 2021). This organization warns that in 2050, if measures are not taken to curb antimicrobial resistance, deaths could reach 10 million at the intra-hospital level due to the ineffectiveness of curative drugs. However, this institution is omissive in holding the population responsible, implicitly with 2021 campaign ‘Spread the word, stop antimicrobial resistance’, for the improper use of drugs. The negligence of this agency and health institutions, for abandoning prevention as a public health strategy in favor of predominant curative approach, convenient for the pharmaceutical industry and private hospitals, is ignored. Clearly, microbial parasitic fitness adapted to plants, animals and humans allows organisms to evolve in the face of pressure that agrochemicals or drugs impose on them. Epistemologically, epidemiology emerges as a discipline that allows the introduction of systemic and holistic rationality in the sustainable solution of health problems in broad biological meaning. Epidemic of past and present indicate that we have not understood this.
Blight, Pasteur, science and causality. Unlike the Black Death, chronologically P. infestans had been preceded of great scientific discoveries by Hooke, Leeuwenhoek, Koch, Pasteur and other microbiologists. The theory of spontaneous generation was being severely questioned and finally discarded in 1862 with the famous experiment of boiling water in a swan-neck flask by Pasteur (Institut Pasteur, 2021). These biological advances, including Darwin’s evolutionary (1859, 1871) and Mendel’s genetic (1864) breakthroughs, among others, allowed the foundation of Nature (1869) and Science (1880) journals. This was the context of sciences ‘maturity’ that facilitated the elucidation of fungal parasitism mechanisms in potato plants, although de Bary performed some pathogenicity tests, his work was more descriptive, of this and other types of fungi such as those causing rusts and bunts. The pathogenicity test merit was for B. Prevost in 1807, even before R. Koch, publishing his results in ‘Mémoire sur la cause immédiate de la carie oú charbon desblés, et de plusieurs autres maladies des plantes, et sur les préservatifs de la carie’. Nevertheless, de Bary is accepted by the American school as the precursor of phytopathology. His achievements were recognized in Nature journal: ‘Prof. de Bary has worked out the scientific questions that occur as to the origin of the disease. It is owing to a fungus (Peronospora infestans)...’ (Dyer, 1874). In congruence with the foundational stage of microbiology where epidemics in humans drove development, the potato fungal epidemic and the severe famine in Ireland also promoted the phytopathology consolidation with the etiological principle of causality as central paradigm (Yuen, 2021).
COVID-19 Curve: The etiological paradigm vicious. As in human medicine, the etiological paradigm has strengthened the principle of protection (plants) and cure (humans) with the reductionist vision of causal identity as sufficient evidence to find the cure for diseases. The etiological paradigm has also distorted the epidemiological approach by emphasizing the epidemic curve study, constructed with progress of diseased subpopulation, when in fact prevention and health should be the principles of modern epidemiology (Figure 2). This restricted epidemiology view is clearly exhibited with COVID-19. There are countless web systems, the Johns Hopkins Coronavirus Resource Center being one of the most known (https://coronavirus.jhu.edu/map.html), that represent progressions of incidence and mortality over time with at least two conceptual problems (Mora-Aguilera and Acevedo-Sánchez, 2020): a. the curves refer to demographic populations of vast territories (e.g., country) without a functional relation of spatial contagious dependence, b. The diseased subpopulation (i.e., SARS-CoV-2 positive) is a clinical sample from individuals volunteers agree to testing, given the presumption disease, or are performed at hospitals for treatment reasons; thus, the infection rate is not dynamic, is underestimated, and does not apply to preventive mitigation model.
The epidemiological indicators have worked for descriptive purposes of epidemic status (e.g., hospital occupancy, number of beds used with ventilators, permissiveness of mobility and commercial activity, percentage value of increase or decrease in positive cases, etc.), but not for mechanistic purposes applicable to risk prevention. Moreover, the interest on COVID-19 epidemiological comprehension has decreased for the current mass vaccination expectations. Nevertheless, at the time of writing, Europe is exhibiting the fourth COVID-19 epidemic wave. As November 21, 2021, Germany reported that ‘entered a state of national emergency’ with an increase of more than 50,000 infections per day, according to Lothar Wieler, president of the Robert Koch Institute for Health Surveillance, and it was recognized that neither vaccination nor restrictions against unvaccinated persons ‘will be enough to stop the new wave of infections’ (Ap et al., 2021b).
Cure: Another vice of etiological paradigm. The etiological paradigm, and consequently the emphasis on disease, has become so deeply rooted that it has distorted the Plant and Health Systems, constituting, at best, reactive models for problems solution, not for prevention. Consequently, relegate in the agrochemical and pharmaceutical industry the contribution of curative medicine, and the abandonment or technical-scientific outdated of Surveillance Systems (Mora-Aguilera et al., 2021a; Mora-Aguilera et al., 2021b). Thus, it is not surprising that a recent study concludes that only between 1 to 3 SARS-CoV-2 infections per 100 cases were detected by Surveillance Systems in the US and several European countries during the first COVID-19 wave (Davis et al., 2021). Human’s pride, encouraged by great scientific and technological revolutions, does not allow us to understand our fragile ubiquity in the biological world. As Erich Fromm stated: ‘Man places himself above life’
Coffee rust (1869-1892), races and varieties. The epidemic history of Coffee rust (Figure 6A-C), originally known as ‘Coffee leaf disease’, a fungal disease of coffee plants (Coffea spp.) caused by Hemileia vastatrix, is analogous to Potato Late Blight in respect of the associative geographic spread of the pathogen-host, epidemic events alternation with endemicity, and constant emergence of new races. With Rust, it is mainly due to mutagenic pressure by resistant varieties, not by the fungicides effect (Alvarado-Alvarado et al., 2005). The genetic strategy has been privileged over the chemical due to the artificial depreciation of coffee in international markets with consequent reduction of the harvest value to the farmers by intermediary companies. The low profit margin of the crop for the majority of coffee farmers, even with the eventual increases in the international price of coffee, operates to the detriment of the agrochemicals products acquisition. This is the reason for the proliferation of organic coffee production. It is an obligated alternative to the sustained coffee depreciation. It also explains the absence of a profitable market for agrochemical companies. However, neither it is profitable for ‘seed’ companies (e.g. Syngenta).

Sources: A. https://fr-fr.facebook.com/pg/Lindoola-Coffee-Estate-225198544202156/posts/; B. MJ. Berkeley (1869); C. D. Morris (1879).
Figure 6 A. Deforestation and monoculture coffee plantations in Ceylon (Sri Lanka) in the coffee boom 1830-1870, scene of the first epidemic outbreak of coffee leaf rust caused by Hemileia vastatrix (1868-1892); B. Kidney-shaped and granulato-verrucose spores illustrated by MJ. Berkeley (1869); C. Integration of control strategies suggested in 1869 by ‘practical men of considerable knowledge and experience in coffee cultivation, aided, by careful scientific observation’.
In America continent, the coffee varieties production, similar to many perennial crops, has been predominantly an effort of national (e.g., EMBRAPA, Brazil; ICAFE, Costa Rica), state (UFLA/EPAMING, Brazil), or farmer associations (IHCAFE, Honduras; Anacafe, Guatemala; FNC, Colombia) (EMBRAPA, 2021). Questionably, Mexico lacks these institutionalized efforts since the 80’s, due to extinction of INMECAFE. The responsibility assumed by the Government and farmers has allowed free availability of genotypes, even between countries, without patents that put at risk the common germplasm property. This is contrary to many extensive grain and vegetable crops where seed companies impact on the production ‘agenda’. This is also contrary what currently prevails in pharmaceutical companies that hold patents for SARS-CoV-2 vaccines in the face of the State’s failure to generate them. This was illustrated with BIRMEX in Mexico in a previous section (BIRMEX, 2021). Government and farmers stewardship has allowed the mitigation of recurrent Coffee Rust and Citrus Tristeza virus epidemic outbreaks due to free availability of varieties in the America. For example, Costa Rica 95 (Costa Rica), Lempira (Honduras) and Castillo (Colombia) varieties, among others, were used in Mexico and other countries to reconvert Typica, Bourbon or Caturra coffee plantations, susceptible to H. vastatrix during the 2009-2015 epidemic outbreak in Central America (Mora-Aguilera et al., 2021a).
So, why do Rust epidemic cycles continue if there are no business visions that distort plant health models ? That is, the cure as a profitable principle in the plants and humans health. With this disease there are two experiences, agronomic and climate change, which are added to previously discussed idea of epidemic intensity in direct function with the pathogen-crop spread to new areas of productive adaptation. Regions of introduction where there is a smaller conglomerate of suppressive factors and biological competition, and a lower genetic diversity of the host. Coffee is presumably native to Kafa, Ethiopia with a history of cultivation since IX century, predominantly the species C. arabica (Ferreira et al., 2019). It is a high mountain region between 1,830 and 2,440 masl, 22 °C average temperature, and 510 to 1,525 mm annual precipitation. One-third of this territory and bordering areas are covered by tropical rainforest with three or more altitudinal levels (Ferreira et al., 2019; Zewdie, 2003). ‘The forest provides shade for the cultivation of coffee and commercially valuable spices that thrive when protected from frost and direct sunlight’ (Zewdie, 2003). Local cultures achieved to avoid regional dissemination of coffee seed/plants, until Dutch, French and British (XVI and XVIII century), mobilized it to their colonies (Ferreira et al., 2019; McCook, 2006).
Coffee rust: Biotrophic parasitism. The specificity and obligate or biotrophic parasitic specialization of H. vastatrix to species of the genus Coffea (Aime, 2006), which has a 124 species diversity suitable for a variable forest environment (Ferreira et al., 2019; Davies et al., 2011), suggest a close pathogen-host coevolution in the equatorial African (McCook, 2006). Phylogenetic studies with 18S rDNA and 28S rDNA gene has allowed postulating that rusts (Basidiomycetes: Uredinales) diverged 150 to 250 million years ago from ancestors adapted to the arboreal tropics, among which H. vastatrix was identified (Aime, 2006; Wingfield et al., 2004). The ancestry of this fungus and the life cycle in C. arabica, with rarely telial phase presence, sexual spore considered to be of winter survival and genetic recombination, suggests adaptation to benign tropical climates of Kenya and Ethiopia, rather than reflecting a primitive physiological condition. The sexual phase, however, possibly does exist in C. canephora and C. eugenioides, progenitors of C.arabica (Carvalho et al., 2011; Aime, 2006). The obligate parasitism coevolution, of supra-stomatic and non-systemic leaf infection, i.e., with interruption of infectious cycle by leaf senescence to the detriment of survival, coupled to wider genetic pool of host, possibly resulted in limited infective aggressiveness and low prevalence in Ethiopia, Kenya and other regions of equatorial Africa, a situation that prevails to present. Thus, absence of a massive epidemic process as it would operate against the fungus survival.
COVID-19: ‘Biotrophic’ parasitism, variants and lethality. The biotrophic type of H. vastatrix parasitism, with systemic nature and multiple tropism is also exhibited by SARS-CoV-2 (Saito et al., 2021) and the vast majority of plant and animal viruses. This type of highly specific parasitism involves a complex and dynamic pathogen-host coevolutionary process that must be understood to predict and prevent epidemic outbreaks (Robinson et al., 2013). The zoonotic origin of SARS-CoV-2 implies that the virus has only just begun adaptation and coevolution phase with humans. Nevertheless, mass infectious success has allowed high virus genomic variability (O’Toole et al., 2021; Shu and McCauley, 2017), and a complex population structure with clades differentiated by geographic regions, sex, age, symptoms present or absent, and lethality (Lee et al., 2021; Hamed et al., 2021).
It can be hypothesized that SARS-CoV-2 lethality and mortality are an adaptive transitional state and that evolution towards moderate variants would be expected, compensating the gradual loss of infectious severity with a high transmissibility rate and/or evasion mechanism to the immune system (Saito et al., 2021; Liu et al., 2021; Vashishtha, 2021). Thus, there is a succession of highly prevalent variants worldwide (many variants have no parasitic fitness and disappear): B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), and B.1.617.2 (Delta) (Saito et al., 2021; WHO, 2021c). The latter has been less aggressive-lethal (i.e., lower hospitalization rate) than the earlier virus variants, in absence of chronic virus-predisposing diseases (Álvarez-Maya et al., 2021). However, pathogenicity is apparently higher respect to speed and tropism.
The Delta increased transmissibility has been associated with effective viral recognition with host cells due to the P681R mutation of gene that synthesizes the S protein (Saito et al., 2021). The Delta variant also exhibits a shorter incubation and latency period, evading the immune system response (Vashishtha, 2021). Also, transmissibility is determinant because it increases viral multiplication events and therefore the likelihood of genomic variability; keeps the epidemic active; and may reduce the benefit of ‘herd immunity’ induced by vaccination (Liu et al., 2021). All genomic variation carries a risk of vaccine effectiveness loss. The risk is higher considering that these were generated with the original Chinese B1 lineage and predominant in the 2020 epidemic phase (Liu et al., 2021). The WHO recently announced 40% losses of vaccine effectiveness due to the Delta variant (Afp y Reuters, 2021).
COVID-19: Omicron, communication and business. In this context, the new variant B.1.1.529 (Callaway, 2021), ‘highly transmissible and of concern’ called ‘Omicron’ by WHO (WHO, 2021d), has triggered alarming global reactions even without sufficient scientific data, but not consistent with the evolutionary logic exposed. It is distressing that predictive models of emergence, establishment and prevalence of new variants in the SARS-CoV-2 population structure are still lacking. So far, models have not been developed despite the great progress in functional and quantitative genomics (https://www.gisaid.org/hcov19-variants/). The same researchers who reported the new variant expressed their doubts: ‘It is also unclear whether the variant is more transmissible than Delta, says Moore, because there are currently low numbers of COVID-19 cases in South Africa’, it is also necessary ‘to see what this virus does in terms of competitive success and whether it will increase in prevalence’ (Callaway, 2021).
The impacts of Omicron’s announcement have been immediate in the global economy with international stock market losses, cancellation of flights from South Africa, speculations about the antigenic effectiveness of current vaccines, etc. (EFE y AFP, 2021). These imply that little has been learned on current pandemic management (or conveniently not wanted to), and that there are no effective risk communication strategies at all decision-making levels. It also stimulates the commercial interests of large companies pressing for their ‘rescue’, offering in return ‘support, stability and evolution to value chains’ (Español, 2021; Fernández-Vega, 2021). But pharmaceutical companies are the most benefit.
AstraZeneca, Pfizer/BioNTech, Moderna and Novavaxya immediately expressed their ability to combat the Omicron variant. Creating a new vaccine would be ‘very fast’ (Afp y BBC, 2021). The media strategy worked. Immediately the share values of Pfizer and Moderna soared by 6.4% and 27.5%, respectively. On the German stock exchange, the shares of Pfizer’s partner BioNTech increased by 12% (Vivas, 2021). Irresponsibly, society cooptation is exercised with promotion of fear as a resource for obscure political and economic interests. The pandemic as a business opportunity, without a comprehensive structural solution for the Public Health System dismantled at global and regional level (CEPAL and OPS, 2021; Frenk, 2003). The WHO recognizes that COVID-19 evidenced needs for a new ‘international health architecture... to predict, prevent, detect, assess and respond effectively to pandemics in a highly coordinated manner’ (WHO, 2021e).
Epidemics and loss of pathogenic aggressiveness. The viruses’ aggressiveness loss by long host-adaptive processes is proved with human adenoviruses (HAdV-), causing common influenza, which could have their ancestor in non-human primate viruses (NHAdV-), with high specificity and not current evidence of interspecific transmissibility-infectivity (Bots and Hoeben, 2020). This is a scenario analogous to Malaria but with the protozoa Plasmodium falciparum and P. vivax, except that these exhibit highly restricted genetic variability (Sharp et al., 2020). The HAdV- adenoviruses are grouped into six species (A-G) with a total diversity of 103 types generated by mutagenic processes and homologous recombination (Robinson et al., 2013). Their specificity and relatively low aggressiveness, even asymptomatic, although with some lethal mutations (Robinson et al., 2013), is the reason for the adenoviruses use, with non-functional or functional replication, as vectors for the generation of vaccines against SARS-CoV-2 (e.g., Johnson & Johnson and AstraZeneca) and for oncolytic purposes (Bots and Hoeben, 2020).
In plants, the viral aggressiveness loss in adaptive response to the host is documented with Citrus tristeza virus, which modified the predominance of severe variants with lethal effects (e.g., T36), present in the South American epidemic outbreak in the 1930’s, with moderate asymptomatic variants (e.g., T30) during gradual and slow continental pandemic advance. Currently in Mexico, Citrus tristeza virus is asymptomatic with severe isolated outbreaks due to mobility of infected plants (Rivas-Valencia et al., 2010; Rivas-Valencia et al., 2008). These moderate viral variants circulate by acquisition-injection by at least four species of insect vectors (Hemiptera: Aphididae) (Loeza-Kuk et al., 2008), potentially conferring ‘cross-protection’ against eventual infection of severe variants, as has been implicated in Spain and Florida (Harper and Cowell, 2016). This natural ‘vaccine’ in plants was first found in Brazil (Müller and Costa, 1977), which however, in this region requires artificial plants ‘pre-immunization’ and use of resistant rootstocks to reduce the effect of Citrus tristeza virus severe races (Rui Leite, IAPAR. Br. Personal Communication), which remain ‘latent’ in the virus population structure (Loeza-Kuk et al., 2008).
COVID-19: Pathogenicity and biology. The previous sections proved that the pathogenicity and SARS-CoV-2 implications in epidemic processes must be understood in a community population environment for effective prevention and risk mitigation (Li et al., 2021). The clinical pathogenicity currently studied with mice, hamsters, and Vero cells (Lee et al., 2021; Liu et al., 2021; Saito et al., 2021) needs to be complemented with community epidemiological digital models to accelerate clinical understanding, natural and induced immune reaction (vaccines), and interrelated with chronic non-infectious diseases. Evolutionary studies of SARS-CoV-2 need to be strengthened for elucidating virulence, aggressiveness, transmission, tissue tropism, and disease expression. Additionally, coronavirus phylogeography from animal source(s), identification of variable genomic regions that determine their adaptability to humans and generation of predictive genomic models is urgent given the active migration in the last 20 years of several coronaviruses, notably the PDCoV, HCoV-NL63, MERS-CoV, SARS-Co 2003, and SARS-CoV-2 (Lednicky et al., 2021; Li et al., 2021; García-Ruiz et al., 2021).
Rust: Genetic diversity, movement and monoculture. In H. vastatrix, the hypothesis that coevolution over millions of years with 124 coffee species must had a strong multilineage effect (sensu Jensen, Borlaug and Gibler) similar to concept of mixing F4-F5 Castillo genotypes, a composite cultivar, employed by FNC Colombia (Alvarado-Alvarado et al., 2005), which resulted in a condition of low fungal-infectious prevalence (McCook, 2006). The selection and mobilization of C. arabica, and eventually of the fungus, by European colonizers between XVI and XVIII centuries (McCook, 2006), broken the original stabilizing effect. The cryptic sexuality of the fungus increased the risk, i.e., genetic exchange at the uredospore level in teliospores absence, which has been postulated as a specialization of H. vastatrix to C. arabica to ensure survival by producing more heterogeneous inoculum (Carvalho et al., 2011). However, it is possible that the fungus had a slow geographical dissemination because it does not infect the seed, presumably used for germplasm mobilization (contrary to the potato tuber by P. infestans). It was not until coffee plants were successfully grown in large plantations that it became necessary to transport plants. For example, although coffee had already been introduced at the backyard garden level and leaves were consumed, between 1640 and 1796 the Dutch reintroduced coffee seedlings from Java, one of their coffee colonies, to Ceylon (Boyle, 2014), and the British continued doing so between 1865 and 1880 (McCook, 2006).
Ceylon as a British colony, now Sri Lanka, was the first region to successfully grow coffee extensively as a monoculture on soils deforested for that purpose (Figure 6A) (Sabaratnam, 2010). This agroforestry shift to monoculture, combined with a gradual increase in cultivated area, favored the spread of fungus, causing the first epidemic outbreak in 1869-1892 (Figure 1). The diverse forest niche of coffee plantations (Zewdie, 2003) was drastically altered by artificial one, phenologically uniform and spatially continuous plant populations, physically exposed, with poor micro-environmental regulation (e.g., leaf radiation, high diurnal variation of relative humidity and temperature), water stress, erosion and soil fertility loss. All this caused by deforestation, estimated in 100 thousand ha (Boyle, 2014; Reddy, 2003). It is not only the movement of crops and pest-pathogens out of the origin center. Another conditioning factor for epidemics clearly emerged, agronomic and environmental management as predisposing factors. This is equivalent to the risk factors intrinsic to human populations like overcrowding, malnutrition, physical exhaustion, lack of hygiene, etc., which characterized the communities affected by the Black Death and Cholera during the European industrial boom. Notoriously, the disease became known as ‘Coffee Malaria’, endemic in the XVII century suburban London, because of the plant weakening without causing death (McCook, 2006).
Rust: Epidemic impact. The fever to cultivate coffee, called ‘coffee rush’ began in 1830 and peaked in 1870. Ceylon cultivated 111400 ha and exported 36 million kg in a year (746120 quintals) (Boyle, 2014). The disease reduced the area to 4600 ha (Reddy, 2003). Between 1869 and 1874 the disease had already been reported in southern India, and Sumatra, Java and Bencoolen in Indonesia, recognizing the epidemic capacity (Morris 1869). Ceylon, along with Brazil and Indonesia boasted the largest coffee production in 1860; but currently is ranked 35th in the world. The plantations loss in 15 years caused a radical change from coffee to tea consumption in England (Reddy, 2003; Schumann, 1991; Schieber, 1972). In 1824, the British introduced tea plants (Camellia sinensis) from China, a country that had already domesticated-cultivated and genetically improved the plant for almost 3000 and 1000 years, respectively (Pandey et al., 2021; Meegahakumbura et al., 2018), but it was not until 1867 that it was established as a crop in Ceylon. By 1893 it was exporting one million packets of tea and had displaced coffee completely. Unlike cultivated C. arabica (Scalabrin et al., 2020), the tea plant exhibits high genetic variability in domesticated lineages with stocks of up to 5100 (China and India) and 20000 (Global) accessions, including natural or artificial hybrids, mainly derived from origin centers (Meegahakumbura et al., 2018; Pandey et al., 2021). This may explain the absence of high impact epidemic reports and incipient technological applications in etiology and control (Pandey et al., 2021). Currently, Sri Lanka is the third-largest producer in the world.
Rust: Marshall, late epidemic intervention. During the most intense Rust epidemic phase, in 1880 the English cryptogamic botanist H. Marshall Ward, a contemporary of Anton de Bary, was sent to Ceylon by the British colonial government to support ongoing research work (Figure 6C) (Vines, 1906; Morris, 1879). Eleven years earlier (November 1869), the fungologist MJ. Berkeley had already reported, from Ceylon plant specimens: ‘it is not only quite new, but with difficulty referable to any recognized (sic) section of Fungi. Indeed it seems just intermediate between true moulds and Uredos ... We are obliged, therefore, to propose a new genus... Hemileia Berk. and Broome’ (Figure 6B) (Berkeley, 1869). Although this report was taxonomic and did not involve pathogenicity studies, there was the infectious causation notion implied by de Bary in P. infestans, so microbiological causation was accepted. Furthermore, Berkeley suggested ‘as the Fungus is confined under the surface of the leaves... it may be difficult to apply a remedy; but we should be inclined to try sulfhur...’. This recommendation was not assessed. Understandably, the pathogen did not spread beyond 1.5 ha in July 1869 (Morris 1879). In reality not much was known about parasitic mechanisms and there was not devastating epidemic history in coffee plantations. But more importantly, prevention did not exist as a comprehensible and applicable principle for a spatially delimited ‘pre-epidemic’ condition in focus condition. There was not notion that once an epidemic is temporal defined in a curve and reaches an exponential phase is impossible to stop the progress with preventive strategies (Figure 6C).
Rust: Population, infection and flattening curves. In Rust, as in Cholera and Black Death, high populations and overcrowding (i.e., monoculture) played a determining role as a contagion factor. Thus, as of few infected plants in May 1869, for ‘1873 all, or nearly all, the estates in the island were attacked’ and the yield reduction by disease went from a five-year average of 565 (1867-1871) to 364 kg ha-1 (1872-1876), 36% loss (Morris, 1879; Morris, 1880). Interestingly, similar maximum loss level (31%) was found in 2014 at the highest epidemic intensity phase in Chiapas, México (G. Mora, CP-LANREF. Unpublished). Regional large-scale experimental approaches to rust control were promoted by the Legislative Council of Ceylon, generating in 1879 a set of recommendations combining remedies, plot and regional prevention measures (Figure 6C) (Morris, 1879). But it was too late. Prevention precedes an epidemic. ‘Flattening’ the curve, a recurrent term during the first and second COVID-19 pandemic waves (the term is now omitted), is not found in any historical epidemic process. The exponential momentum of an epidemic obeys biological principles that make it impossible to ‘flatten’ a curve without total elimination of the diseased population. It would imply an absolute interruption of the contagion chain. Coffee growers with diseased or abandoned plants thought of this (Figure 6C). It was never applied. It was still believed that ‘where careful and intelligent cultivation is pursued they still offer a promising and attractive investment’ (Morris, 1879).
Rust: Pathogenic spread and Epidemiological System . Marshall encountered a late epidemic scenario in 1880. Logically, he was unsuccessful in his mission to control the epidemic. His contribution was the biological mechanicism of the disease in accordance with the plant-pathogenic approach of his contemporary de Bary. The development of plant and human epidemiology at this stage lacked of a formal scientific conceptual-methodological support. The Epidemiological System, i.e., host, pathogen, climate, and management (Mora-Aguilera et al., 2021a), a fundamental rational framework for understanding epidemics, emerged conceptually with E. A. Gäumann, in ‘Principles of plant infection’, a book published in 1946. Marshall, as a botanist, studied the fungus pathogenesis histologically, but proposed novel strategies to study the disease cycle through in vivo inoculations, and to infer infection from spore trapping in affected coffee plantations (Ayres, 2005). The non-experimental deduction of ‘degeneration of the air’ as an explanation for the ‘contagiosis’ of Black Death and other diseases proposed 254 years earlier by Girolamo Fracastoro (1546) was evidenced for first time with a fungus. Marshall inferred the epidemic as a product of inoculum released and dispersed by the wind: ‘the air must sometimes have turned red on windy days’. Understanding the contagion mechanisms is fundamental to developing mitigation strategies in any epidemic (Coria-Contreras et al., 2015).
COVID-19. Viral spread and ‘hygienism’. As in Rust and many phytopathogens, with SARS-CoV-2 volumetric air suction traps has been used to detect the virus with molecular techniques (RT-qPCR). In public transportation it was proved the dispersion capacity of viral particles in saliva droplets, justifying wearing mask as a preventive measure (Moreno et al., 2021). In the same study, and many others, the particles detection on surfaces justifies hand hygiene. The reluctance of these basic measures, paradoxically greater in developed countries, as well as the rejection of vaccination, is another evidence of deficient or limited risk communication approaches of the Public Health Systems, exacerbated in some countries, such as USA and Brazil, by negationist political leaders. The Austrian measures illustrate efforts to revert reluctance. With less than 66% of vaccination, the lowest in Western Europe, immersed in fourth epidemic wave and the new variant Omicron detection, will apply mandatory confinement and fines to people who reject vaccination against SARS-CoV-2 (Europa Press, 2021).
Rust: Prevention and sanitation in Marshall. With his mechanistic studies on Rust, H. Marshall concluded that prevention was the only viable mitigation strategy: ‘.... Not the killing of the parasite in the infected leaves...but preventing its development by covering preventively the surface of the leaves with various substances capable or making spores lose their viability or, at least, impeding their germination ’. For the same ‘preventive’ aim he suggested ‘sanitization’, by removing and incinerating damaged tissue, and diseased plants (not really necessary since the pathogen is not systemic), and invigorating the plant with organic nutrition, a practice already done in Ceylon because of soil fertility loss caused by deforestation (Figure 6A). However, effective prevention precedes an epidemic. His mitigation strategies, 11 years after the onset of epidemic, were not effective. Consequently, Ward had pressure from coffee growers and politicians. He defended himself by claiming that causality was his purpose (Ayres 2005). Wrong then and now. Etiological approaches do not mitigate an epidemic. But that was a time when scientists were visible to society and perceived as saviors. Snow, Proust, Pasteur, Koch, Jenner, de Bary, proved it. Nowadays, under the SARS-CoV-2 threat, for politicians and media perspective, ‘heroes’ are the pharmaceuticals.
Etiology without the mechanism is limited for disease control. Marshall understood this. He established that H. vastatrix germination was his vulnerable phase for controlling infection and suggested applying foliar chemicals prior to germ tube emission. Once the infection process was initiated, he determined that control was ineffective. Sulfur and lime were his only options. The Bordeaux mixture was not yet invented (Ayres, 2005; Schumann, 1991). Another important conceptual contribution of Marshall was that ‘the history of all great planting enterprises teaches us that he who undertakes to cultivate any plant continuously in open culture over large areas must run the risk of epidemics’ (Zadoks and Koster, 1976). Thus, extensive monoculture was recognized as an epidemic predisposing factor for first time. In 1970, the classic epidemic of ‘southern corn leaf blight’ (Bipoliaris maydis, syn. Helminthosporium maydis) in USA added another fundamental element: Genetic uniformity as the basis of crop vulnerability (Zadoks and Koster, 1976; Tatum, 1971). Extensive and genetically uniform population, which in essence represents the optimization of contagion, has become entrenched as an epidemiological dogma.
Rust: Genetic uniformity, Green Revolution, Surveillance. The uniform population is actually an anthropogenic attribute. The ‘southern corn blight’ epidemic, for example, dramatically highlighted the risk inherent in emphasis on monogenic or vertical genetic resistance, sensu Vanderplank, as the curative principle. It also represented the pre-eminently geneticist model failure of so-called Green Revolution as the unique and unequivocal model of modern agriculture (Ceccon, 2008). Additionally, it evidenced the omission of ‘seed’ companies by emphasizing the genetic approach, reducing costs and time, and underestimating the mechanistic understanding of infectious processes and the generation of new fungus physiological races (Tatum, 1971). This is a strong analogy on current technological trend of pharmaceutical companies, and their competition to control the immunological market against SARS-CoV-2. That is, a partial but profitable solution.
The ‘preventing’ purpose, but correctly applied in the outbreak condition, ‘pre-epidemic’ of Coffee Rust, based on the fungus infectious biology, was investigated for the coffee growing conditions in Mexico. The preventive principle underpinned the generation of automatic computerized algorithms to determine regional early warnings. The objective was to timely detect local foci and ‘interrupt chains of contagion’ to prevent occurrence of temporal regional epidemics with high disease intensity. This preventive model, operated by sanitary authorities of the federal government, was implemented to mitigate the epidemic outbreak in Mexican coffee production areas that originally started in Central America in 2009 (Avelino et al., 2015). A systemic-holistic Epidemiological System was the rational framework for establishing the prevention model applied to a National Epidemiological Surveillance Program (PVEF-Cafeto). This system was successfully used to control the epidemic outbreak of coffee Rust in 2013-2016 (Mora-Aguilera et al., 2021a).
Coffee rust: Four epidemic events. The global spread of H. vastatrix (McCook, 2006), has occurred in three stages: 1. 1870-1920 in the Indian Ocean; 2. 1950-1970 West Africa; and 3. 1970-1990 America. Recently, a fourth epidemic process occurred in America with significant impact in Central America, 2009-present (Figure 1). The aggressively colonialist activity had a fundamental implication in the first stage due to merchant transport, military displacements, and the collapse of Ceylon coffee plantation, by coffee investors migration (the British East India Company was consolidated in this period as a facilitator of investments and trade), and the return of Hindu Tamil harvesters, which could have mobilized plants. It is documented that British botanical expeditions actively contributed to the movement of coffee plants and other vegetables (McCook, 2006; Reddy 2003; Morris, 1879). In addition to the anthropogenic role, the involvement of monsoon winds from Ethiopia has been hypothesized on the pathogen displacement; however, the spore is not aerodynamic and Ethiopian forest barriers would limit the wind effect as proven for the Soconusco, Chiapas region (JJ. Coria. CP-LANREF, 2014. Unpublished data). The second and third dispersal stages may have been mainly due to the movement of varieties or germplasm for new commercial coffee varieties, establishment or varietal reconversion of plantations, activities that continue to the present (CIFC, 2021).
Rust: Climate in the fourth epidemic and zoonoses. The current Rust epidemic outbreak in America, although with possible involvement of active movement of harvesting crews between countries, is mainly attributable to the displacement of the thermal threshold required for H. vastatrix infection, and other pathogens, accumulating a greater favorable hours number (NHF) for spores germination and infection (Mora-Aguilera et al., 2021a; Mora-Aguilera et al., 2014a). This is due to sustained global temperature increase since the Industrial Revolution, which is estimated at 2.9 - 3.4 °C by 2050 in absence of corrective actions. Evidence on Hemileia - Coffea indicates that climate change may have fundamental implications on emergence and re-emergence of contemporary epidemics in humans, animals and plants.
Infective processes are more sensitive to climate variations at the micro-environmental level, which explain several regional epidemic events. This justifies the routinely inclusion of climate variables measurement and automatic NHF calculation for biological risk monitoring in coffee growing regions and other crops (Mora-Aguilera et al., 2021a). In addition, climatic disturbances will also have a strong impact on cultivated plants. Recent research has proved the risk of native coffee plant diversity reduction. It is projected that 50% of the 124 species could disappear by 2088 (Davis et al., 2019; Moat et al., 2019; Rodríguez, 2019). The highest damage would be for C. arabica, the most cultivated species for cup quality, due to restricted genetic variation in native and cultivated plants (Scalabrin et al., 2020). This would limit adaptive plasticity and implicitly resilience to increased pathogen (and pest) inoculum density, as apparently occurred with H. vastatrix. In a wider vision of anthropogenic effect on climate, deforestation (historically linked to coffee cultivation in several countries) (Figure 6A), urban pressure on rural areas, and unsustainable agricultural practices could be implicated in zoonotic diseases recurrent occurrence in humans by affecting the natural animal´s niches (Rulli et al., 2021; Lawler et al., 2021).
Rust: Epidemic impact and ‘Health Systems’. Local and transboundary migration, food security problems and increased diseases in coffee-growing communities as a consequence of coffee production losses, estimated at 10-55%, were Rust epidemic outbreak effects in Central America (Mora-Aguilera et al., 2021a; Lynch, 2019; Ruiz-de-Oña et al., 2019; Cerda et al., 2017). Colombia and Brazil, countries with better infrastructure and production technology, including programs to generate resistant varieties against H. vastatrix, nematodes, drought, frost, etc. as the preventive model basis were the least affected. This would be expected from a functional ‘Health System’, with cutting-edge research lines to support medium and long-term productive planning, including adaptive projections of imminent climate change (Alvarado-Alvarado et al., 2005; Embrapa, 2021). On the other hand, a dysfunctional ‘Health System’ had the predictable consequences for the rest of coffee-growing countries. The same global lesson for the current SARS-CoV-2 health crisis with a generalized and individualized curative model in detriment of community prevention and the absence of modern and effective Surveillance Systems.
Pathogen mutation and COVID-19 vaccination. The Coffee rust epidemic continues in Central America. Lempira (2017) and Costa Rica (2018) varieties, previously resistant to H. vastatrix, lost their resistance due to high population pressure of the fungus resulting in new races (World Coffee Research, 2021; 2017). This is equivalent to the gradual effectiveness loss of current SARS-CoV-2 vaccines upon successive epidemic waves that increase the likelihood of successful prevalent mutations (Afp y Reuters, 2021).
The genetic variability of an infectious agent is related to short infection cycles, high infection frequency, infection rate, and incidence in the host population structure. But it is also determined by the mutagenic pressure of control measures; in the case of Coffee rust, the genetic pressure of resistant varieties, and in potato Late Blight, fungicides. SARS-CoV-2 behaves like any other biological agent, enhanced by a human immune system that has not yet co-evolved with the pathogen for a long time. Surprisingly, SARS-CoV-2 has evidenced mutagenic capacity in a short time, possibly due to the global infectious synchrony in a population highly heterogeneous in risk factors and triggers, including ethnic status (Álvarez-Maya et al., 2021).
In the current scenario, the genomic revolution has been determinant in rapidly identifying the SARS-CoV-2 variants emergence (https://www.gisaid.org/). The ‘of concern’ variants of epidemic impact have emerged predominantly from countries with high epidemic intensity, spreading rapidly to more than 100 countries: GB (Alpha-2020), South Africa (Beta-2020), Brazil (Gamma-2020), and India (Delta-2020). Other variants of epidemic ‘interest’ have a local status in Peru (Lambda-2020) or Colombia (Mu-2021), or are of concern for their high genetic variation, such as Omicron reported in South Africa on 26 November (2021) and already present in 34 countries (https://www.gisaid.org/). These variants affect diagnostic protocols, clinical treatments, and the vaccines effectiveness (WHO, 2021c).
While genetic variability is intrinsic to the adaptive biological capacity of the virus, limiting mutagenic capacity is possible by restricting mass infectious processes through the widespread application of SARS-CoV-2 vaccines. This has not occurred. It has been repeatedly argued that inequitable distribution of vaccines may prolong the pandemic and increase the new variants risks. South Africa, the source of 2/5 of epidemic concern variants, has 25% of population vaccinated, compared to 5% in continental Africa (UN, 2021). A contrast with developed countries that on average have 65% of population with at least one dose against all the rest that exhibits 7% (Afp, 2021e). Anthropogenic actions can not only operate against nature, conditioning the humans, plants, and animals epidemics, but also against itself.
5. The epidemics and pandemics in history
Epidemios, population and surveillance in Hippocrates. The concept of epidemic ( epidemios = epi + demos), etymologically understood as that which is over a population, is first attributed to Homer in the Odyssey (600 B.C) in reference to a person who is in his country. Thus not in a disease context (Martin and Martin-Granel, 2006). It was Hippocrates in his book ‘ Of the epidemics ’ (Hippocrates, 400 B.C), who refers to clinical syndromes in different localities in Greece with respect to a population (people) . For example: ‘ In Thasus...the whole season being wet, cold, and northerly, people (ed. demos) were, for the most part, healthy during winter; but early in the spring very many, indeed, the greater part, were valetudinary. At first ophthalmies set in, with rheums, pains, unconcocted discharges, small concretions... ’. Note that Hippocrates establishes the associativity of the healthy and sick condition (rheums, pain, etc.) with space (Thasus), time (during winter, early spring) and population (people), fundamental rational frameworks of any epidemic process (Hippocrates, 400 B.C).
More interestingly, the prevention and cure (protection) principles underlie Hippocrates’s early descriptions of epidemiology. Surveillance of the healthy condition (healthy during winter) is the basis of prevention, as sick or clinical (early in spring… were valetudinary) is of cure. This is the structural flaw of current epidemiology focused on damage/disease curve (Figure 7), and intrinsically in the adoption of curative principle as a mitigation strategy. The analytical limitation of COVID-19 epidemic curves and the absence of preventive surveillance have been documented during the development of this paper. Epidemiology is not the record of epidemic data. Records only describe its temporal progress, which is valuable, but does not allow the development of mechanistic ideas (Zadoks, 1976).
Hippocrates employed epidemios as an adjective to refer to an established (‘preveiled’) or ‘circulating’ disease condition in the population in connection with the environment: ‘ In this state of things, during winter, paraplegia set in, and attacked many, and some died speedily; and otherwise the disease prevailed much in an epidemical (Greek ed. ‘ epidemios ’) form’ (Hippocrates, 400 B.C). The differentiation of a sporadic epidemic process, i.e., diseases that visit the population ( epidemeion = epidemic ), from established one, diseases that reside ( endemion = endemic ), implies that Hippocrates maintained constant health surveillance in the population. For this community health view, some consider Hippocrates as epidemiology’s father; others identify him as the first epidemiologist. Undoubtedly, he was the founder of medicine under the presumption of a physical and rational causality, an ethical practice ‘Hippocratic Oath’ and his humanistic vision, today so questioned by mercantile emphasis of health: ‘wherever the art of Medicine is loved, there is also a love of Humanity’ (Hippocrates 460-370 B.C.).
Source: Vanderplank, 1963
Figure 7 Progress of blight on potatoes caused by Phytophthora infestans. Top half shows the increase of x and bottom half the increase of log10[x/(1 - x)].
The original concept epidemios has evolved in semiotic terms for more than 2500 years, configuring complementary conceptual terms in accordance with the epidemiology scientific development. From adjective epidemios the noun ‘ epidemic ’ was generated, and in the second half of XIX century the terms epidemiology, epidemiological and epidemiologist emerged from French épidémiologie (1855), épidémiologique (1878), épidémiologiste (1896) (Martin and Martin-Granel, 2006).
Pandemios, Pandemick and dimensionality. The term pandemios also had origin in ancient Greece, outside the medical connotation, to refers ‘the public’ ‘that which concerns all people’ (https://bit.ly/3moj9Nj). However, it was not until the XVII century that term ‘pandemick’ was associated with a ‘vernacular’ disease in humans (Morens et al., 2021). The context suggests the regional occurrence of a disease. The scalability of an epidemic process to pandemic was actually possible until urban development, commercial trade and tourist mobility, reached high population interaction favorable to contagion. This scenario reached with the Industrial Revolution boom and Black Death, Spanish Flu and seven recurrent epidemic outbreaks of Cholera (1817 to 1961). Given the laxity in the concept application, historical recognized pandemic varies. For example, Cholera outbreak of 1831-1832 is recognized as the first pandemic but geographical spread only included Europe and Asia (Morens et al., 2021; Piret y Boivin, 2019). Other authors, with a more global criterion, consider the Spanish Flu. This disease impacted in the span of one year (1918-1919) in most continents in accordance with the increased human mobility and First World War troop movements. With the geographic, and massive effect on population criterion, 19 disease pandemics have been reported in history (Figure 1) (Piret and Boivin, 2019).
Pandemic characterization in plant diseases has also had an imprecise connotation, but coincides in global spread (Figure 1). However, there is not any case of global plant pandemic associated with a synchronous infective process (Figure 1). Comparatively, plant epidemics exhibit slow geographic dispersal from initial outbreak or focus and may even be restricted to one region due to host population topology factors: 1. Population discontinuity in time determined by annual crops, or by seasonality of phenology events, abruptly interrupting infection-contagion processes; 2. Spatial population discontinuity due to specificity of edaphoclimatic conditions required for a crop, diversity of cultivated species, and/or varietal genetic heterogeneity in a region; 3. The population is intrinsically fixed or immobile and depends on the environment or anthropogenic factors for the inoculum mobility. 4. There are regulatory and trade restrictions that prevent the transboundary movement of plants, products and/or subproducts by requiring a certificate or ‘phytosanitary passport’ recognized by World Trade Organization (WTO) and regional trade agreements. This is analogous to COVID-19 health or vaccination passport, already required by some countries, but not accepted, and considerate by the WHO as discriminatory. Indeed, this system, although effective in restricting the mobility of pathogens (and pests), in certain cases, has been used for protectionist purposes in agriculture (Sputnik et al., 2021).
Pandemic, conceptual precision. The concept of pandemic, previously presented, can be defined as an ‘epidemic process of synchronous occurrence, in the life time of the host and at an intercontinental or continental space, with respect to a functional relation of contagion from an identifiable primary source of the infectious agent, resulting in multiple sub-epidemic processes whose structures are determined by the Epidemiological System specific to a regional community cluster’. This definition has been restricted to pathogens but can be extended to other biotic agents (Mora-Aguilera et al., 2021a). In plants, a synchronous contagion process at the continental level is more expected. For example, CLas-Citrus spp., has progressed gradually in America from 2009 to the present since the detection in Brazil (Figure 1). Thus synchrony operates over a time period of n-productive cycles of the host. In humans, as has been evidenced with SARS-CoV-2, this process has been multi-continental, but can also involve one or more continents as occurred with several historical epidemics, e.g., Cholera or Black Death, and recently with Influenza A H1N1 (WHO, 2021f).
As the ‘directing and coordinating authority for global health action’ of the UN, the WHO is empowered to determine a pandemic status from a ‘disease outbreak’ or health emergency. However, its guidelines have been ambiguous or limited for specific cases (Morens et al., 2021). Thus, the WHO defines ‘Pandemic: increased and sustained transmission in general population’, in the context of a multistage strategy to direct actions of Public Health Systems with respect to influenza (WHO, 2005). In another definition, the same organization establishes that a pandemic is ‘The worldwide spread of a new disease’. In contrast, the International Association of Epidemiology defines it as ‘An epidemic occurring worldwide, or over a very wide area, crossing international boundaries and usually affecting a large number of people’ (IEA, 2021; Porta, 2014).
The pandemic definition must differentiate the strict epidemiological criterion from one inherent to justify the development and management of global public health policies as corresponds to the WHO. Thus, epidemiologically, the WHO definition that ‘a pandemic agent must be infectious, must be new, must spread easily, and must cause serious illness’ is not valid (Morens et al., 2021). In any country, for quantitative purposes required to an Epidemiological Surveillance System, the potential scalability of an epidemic constitutes one of n-risk factors, and the official declaration of an outbreak, emergency or pandemic should not impact the structure and conception of a risk model (Mora-Aguilera et al., 2021a; 2021b).
The chain of infection: Gäumann and Smith. The background of plant epidemiology, oriented to the classical infectious plant pathology approach, can be found with E. Gäumann (1946) in his book ‘Pflanzliche Infektionslehre: Lehrbuch der allgemeinen Pflanzenpathologie für Biologen, Landwirte, Forster und Pflanzenzuchter’ (Theory of plant infection: textbook on general plant pathology for biologists, farmers, foresters and plant breeders) (Figure 2). In Chapter 2, Gäumann integrated principles of plant infection related to environmental factors, incubation periods, infection threshold, colonization mechanisms, parasitic capacity, host susceptibility, basic principles of plant disease control (protection) and exclusion of potential pathogens. ‘Every epidemic develops according to its own rules, changes its character, expands and becomes malignant, decreases and becomes milder: it has an appearance of its own, its morphology, its own genius epidemicus’ (Gäumann, 1946). Its variable genius epidemicus, applies to the same pathosystem and is equivalent to Vanderplank’s (1963) ‘memory factor’, and the epidemic momentum used in this paper to refer an epidemic that enters a dynamic phase of progression, determined by an inoculum load (pest load) and infection rate, such that it exceeds a control or mitigation threshold. That is, an ‘escaping’ epidemic.
Gäumann in the same book (1946) stated that: ‘ Infection is considered as a fundamental biological problem of the threefold interaction of the constitution of host plants, the invasive potentiality of parasites, and the conditioning factors of the environment ’. This assertion gave rise to the classic concept of the epidemiological triangle also used in medical epidemiology. The main conceptual evolution includes the anthropogenic management in agriculture, and the conception as an open Epidemiological System with the healthy plant as the integrating focus (Mora-Aguilera et al., 2021a). The importance as a rational epidemiological framework is discussed in several contexts in this paper.
Gäumann is also credited with the introduction of a concept known as Gäumann’s chain of infection (1946). However, in medicine, T. Smith is credited with the same concept (Langmuir, 1961), previously presented in his book Parasitism and Disease (1934), in which he discussed it in the context of the parasite life cycle: ‘ We may divide the life of parasites into four critical stages or periods: 1. Their entrance into or invasion of the host; 2. Their multiplication within the body of the host; 3. Their discharge, emigration or excretion outwards; and 4. Their active or passive transfer or transmission to another host ’. Currently this concept is more widely used in medical epidemiology recognizing five or six events remarking the infectious agent and/or host in order to intervene or control an epidemic outbreak with preventive or curative strategies (CDC, 2006).
In plant epidemiology, the application of chain of infection has been replaced by three distinctive biological events involving both the population and environment: dispersal, pathogenesis and survival. These events complement epidemiological criteria for the correct sequential application in time, and in the selection of preventive and curative (protection) procedures for effective epidemic intervention (Figure 8).
The ability to conduct infection assays, without the constraints of human population intervention, has allowed for greater biological and mechanistic precision in understanding plant epidemics. However, at the global level, this does not justify the limited epidemic studies with metadata to understand the SARS-CoV-2 infectious dynamics at the community level, with a more solid rational framework than the chain of infection, in order to design mitigation strategies. In reality, the absence of Surveillance Systems, and the integration and automatic processing of big data in real-time, impede the systemic understanding of the current health emergency (Mora Aguilera et al., 2021a; 2021b). The hospital vision and the daily tests monitoring as indicators of epidemic status prevail, denoting the structural neglect of Health Systems and absence of effective WHO guidelines under a preventive approach.
Figure 8 Comparison of main population attributes (PN) in plants and humans that determine the contagion process (upper left box). Population characteristics intrinsic to an epidemic process independent of human, animal or plant nature (right box). Generic criteria adopted for COVID-19 pandemic that lack the systemic rational framework provided by Epidemiological System. The Figure below shows the sequential applicative conceptualization of prevention and cure principles for SARS-CoV-2 epidemic intervention. A putative threshold of viral endemicity is included as a function of a naturally activated and/or vaccine-induced human immune system.
Prevention and Cure: Whetzel and Proust. Herbert H. Whetzel introduced the terms inoculation, incubation and infection as events inherent to pathogenesis and their application in disease control in plant pathology. In that context, he proposed in 1926 eradication, exclusion, protection, and immunization as control categories, the latter in reference to varietal resistance (Newhall, 1980). These are now recognized as control principles. Exclusion had already been adopted as the basis of quarantine regulatory systems, e.g. in USA in 1912; and in Croatia, in 1377, ‘quarantine’ for 40 days in isolation centers for travelers and ship’s crew was introduced as a measure of Black Death exclusion (Tognotti, 2013). A. Proust, the hygienist, was an active promoter of an international health organization for Europe exclusion of diseases ‘imported’ by sea, such as Cholera, Black Death and Yellow fever (Howard-Jones, 1975). Eradication, in plant health, was first proposed in 1913 at the International Institute of Agriculture, predecessor of the FAO/UN, as a possible strategy but with doubts of its applicability (Nature, 1913). In human health, with a global and scientific approach, it has been promoted by the WHO, with Smallpox being the only human disease eradicated. It was achieved in 1977 through extensive vaccination campaigns (Roser et al., 2014).
With an applied vision in epidemiological surveillance (Mora-Aguilera et al., 2021a), these principles have been categorized into preventive (exclusion and eradication) and protective (cure in medicine), in which resistance is included, and their sequentiality has been established according to epidemic intensity and pathogen population thresholds. Figure 8 proposes the principles application for the SARS-CoV-2 epidemic and an endemicity threshold based on an immune system activated naturally and/or induced by vaccination.
Epidemic: Conceptual evolution. The epidemics definition, in plants and humans, and by extension to animals, has also evolved in history, analogous to population and contagion, its defining and determining principles. A selection of early references and definitions, based on the ‘A historical survey of botanical epidemiology’ review by Zadoks and Koster (1976), are included complemented with other references:
‘Epidemia fuiffe in pueris diximus: unum notavimus infolentia fymptomata…( hemos tenido una epidemia entre los niños: una centrada en síntomas del folículo )’ (Ballonii, 1571).
Has anyone until now observed contagious epidemics in plants? HL. Duhamel de Monceau (1728).
‘ Epidemics among man and animals appear suddenly and unexpectedly, spread destruction over whole regions for a while and then slowly disappear and so do plant epidemics ’ JG. Kühn (1858).
‘Epidemics (ed. in medicine) resemble each other in the extent of their range… Epidemics…derive their name from attacking large numbers at once’. S. Smith (1866).
‘Epidemics (ed. in medicine) are those peculiar affections which, springing up suddenly in some particular spot, spread over a certain portion of the habitable globe, and then disappear altogether’. J. Parkin (1873).
‘ The same behaviour in the world of disease among humans leads to epidemics, among animals to epizootics, and among plants to epiphytotics, generally conditions of exacerbation of some disease species… ’. F. Unger (1833).
‘By an epidemic (ed. in plants) we therefore understand the frequent incidence, the local concentration of an infectious disease within a limited period of time’. E.Gäumann (1946).
‘Epidemic is…any increase or decrease within the range 0 < y ≤ 1’. J.Kranz (1974). Note: 1=100% incidence / severity.
‘Epidemic refers to an increase, often sudden, in the number of cases of a disease above what is normally expected in that population in that area’. (CDC, 2006).
‘Epidemic is a process of contagion, colonization or occurrence of abiotic non-infectious events determined by a specific Epidemiological System that results in the loss of population health determined structural and functionally in time and space’. G. Mora and G. Acevedo (2021).
Population and infection in epidemiology. The population must be correctly defined in epidemiological studies. In an epistemological approach, biological structure and function should be established concerning interaction with the environment; and analytically, the variables and factors that will represent the population should be defined as ‘metadata’ in an epidemiological matrix, which includes other variables associated with the Epidemiological System. The relevance of this, to the specific health problem, will guarantee the population adequate parameterization in descriptive and quantitative analyses (Mora-Aguilera et al., 2021a). For example, age, sex, and ethnicity constituted structural factors and immune response constituted the population function in a recent comparative study of vaccine effectiveness in Omicron. It was concluded that this variant represents a 5.4-fold increased reinfection risk compared to Delta (Reuters et al., 2021).
Although infection is the plant epidemiology defining principle for ‘unknown reasons’ (Zadoks and Koster, 1976), the epidemics of non-infectious chronic processes e.g., diabetes, hypertension, etc., are recognized in medical epidemiology. Nowadays, the need for a holistic epidemiological approach has been promoted in plant health, with emphasis on health and plant as the integrating axis of phytosanitary processes. Thus, some Epidemiological Surveillance Systems operate multi-pest prevention, in the wider meaning, are sustainable and optimize operational, scientific and public policy planning strategies in an integral way (Mora-Aguilera et al., 2021a). The scientific formalization of this discipline began in 1874 with the German journal focused on medical epidemiology ‘Allgemeine Zeitschrift für Epidemiologie’ (General Journal for Epidemiology). While in plant epidemiology it began in 1913 with the French publication ‘Annales du service des épiphyties’ (Yearbook of the epiphytes service) (Zadoks and Koster, 1976).
6. The curve and the map: The same epidemic
The development of plant epidemiology as a scientific discipline can be attributed to Vanderplank with his seminal book “Plant Diseases: Epidemics and Control” (1963) (Figure 2). In contrast to the spatial approach to the Cholera analysis used by J. Snow (1854) in human epidemiology, Vanderplank approaches epidemiology from the temporal dimension, mainly to apply biological models (e.g., logistic) to disease progress curves to determine an ‘apparent’ infection rate. Vanderplank conceives the infection rate as the x-intensity of disease speed or increase epidemic per unit t-time (currently, y is the disease intensity). His chapter “How to trace the progress of an epidemic” was a fundamental idea (Figure 7), which today also seems as simple and logical as Snow’s Cholera map (Figure 3), but allows to understand the graphical abstraction of a curve to quantify changes in disease intensity over n-intervals of time and, from there, to establish strategies for disease control and forecasting. Also, for the first time the understanding of ‘disease in populations’ is subordinated to the development of control or mitigation strategies. Vanderplank developed quantitative epidemiology in order to assess the resistance of his potato germplasm (S. tuberosum) to Phytophothra infestans by comparing epidemic rates (Vanderplank. 1963). The approach applied to disease management was also shared by JC. Zadoks and J. Kranz, foundational epidemiologists of this discipline with their holistic and multivariate contributions, abandoning the epidemic rate curve as the only parameter for mechanistic interpretation of an epidemic. Today, the multivariate approach is little-understood and adopted in the systemic analysis of epidemics in all fields. SARS-CoV-2 is no exception. The intervention of the chain of infection or contagion is the closest similitude to such approach of understanding the structure and mechanics of a temporal epidemic for effective application of preventive and curative principle (Figure 8).
We cannot omit that the ‘epidemic curve’ has been the central focus SARS-CoV-2 epidemic, mainly during the first-two waves. Formal epidemiologists, physicists, mathematicians, political scientists, etc., argued (and still do) about modeling curves for ‘case’ forecasting and ‘curve flattening’. The problem was not (and still is not) the curve, but the absence of the curve analysis in the context of functional populations , i.e., epidemiology applied to the context of community clusters where functional contagion relationships are established. Consequently, space must be integrated into the modeling. Space and time define an epidemic integrally. Their individualization fragments the contagion mechanism comprehension. Moreover, the curve is a late process under the prevention principle. It is the focus(ci) and Yo parameter, sensu J. Kranz, that should be emphasized (Figure 8). The foci-space in a map that J. Snow understood perfectly in 1854 to mitigate the Cholera outbreak in London.
Descriptive curve and ‘case’ forecasting are not the mechanistic solution to the SARS-CoV-2 pandemic. The human community in its complex environmental, social, occupational, and mental and physical health interrelationships, associated with the SARS-CoV-2 population structure, in the correct spatio-temporal dimension, is the correct strategy based on historical epidemic experiences (Figure 8). That is a holistic and multivariate vision. The limitations and errors of SIR, SIER and other models that restrictively predicted dates of ‘increases’ and ‘curve flattening’ are then understood. This mathematical ‘fever’ of 2020 has now subsided. Even with mass vaccination and social restrictions, the pandemic and multiple waves have surprised with ‘momentum’, ‘genius epidemicus’, or ‘memory factor’ that eludes simplistic solutions and goes beyond a curve. Currently, there is not known model has been effective with an acceptable error margin. Not only in reference to statistical error, but also conceptual and intrinsic to the rational framework that emanates from the Epidemiological System. Vanderplank placed on the stage of plant epidemiology the temporal analysis possibility through the concept of ‘rate-r’ (Figura 7). He never succeeded or tried to ‘flatten curves’. They were always ‘curves’ with different ‘apparent infection rate’, depending on the genetics of a potato variety, because they are inherent to pathogenesis of n-processes, just like an infectious process in humans, which intertwine over time, and whose symptomatological visual expression (i.e., clinical symptoms) only estimates, hence the term ‘apparent’, the actual infection rate. Therefore, the asymptomatic subpopulation is not represented in the ‘curve’.
Epidemiology: Conceptual evolution. Just as the reductionist approach of the phytopathological (and medical) paradigm focused on the etiology and from there to look for the cure, in epidemiology it has been the curve, the rate of the curve, the model in pursuit the rate. That is, the dissociation of population structure and function, both in plant and human health. But also omitting that the objective of epidemiology is NOT the study of disease in populations (Vanderplank, 1963), but study of the healthy population as the main focus of Epidemiological System that allows the articulation of prevention and protection as risk management and mitigation principles of epidemics and pandemics (Figure 8) (Mora-Aguilera et al., 2021b). In this context, epidemiology is postulated as the multifactorial, multidimensional and dynamic study of relationships intrinsic to the Epidemiological System to elucidate rational strategies for epidemic prevention and health risk mitigation. The Epidemiological System emerges as the new object of study. This concept evolved from the classic epidemiological triangle or epidemiological triad associated with an infectious process in humans, animals and plants. The organization as an open system, with n-etiological factors and n-determinants of health, among other attributes, overcomes the restrictiveness of the triad (Mora-Aguilera et al., 2021a). This epidemiology vision considers biotic and abiotic factors as deterministic or probabilistic causality. Consequently, non-infectious processes are included in plants, such as insect-pests, or cardiovascular and metabolic imbalances in humans.
The holistic and integral approach to epidemiology ensures a rational framework for theoretical epidemiology and applied epidemiology in Health Systems (human, animal or plant). It allows the classical models integration based on infectious processes, e.g., Gompertz in plants, or SIR in humans, with multivariate probabilistic models suitable for estimating risks independently of the infectious or non-infectious cause. Likewise, the emerging disciplinary, epidemiology fragmented trend, i.e., molecular epidemiology, nutritional epidemiology, cardiovascular epidemiology, diabetes epidemiology, etc., is avoided (Frérot et al., 2018). Epistemologically, epidemiology is indivisible, holistic, systemic. The Epidemiological System, as a rational framework, adapts to the problem, forces to define the relevant factors and variables and establish the operative spatio-temporal dimensionality in the problem investigation, and the applicability in the solution. Thus, Epidemiological System determines epidemiology as a science.
In medical epidemiology, the ‘chain of infection’, not the epidemiological triad, i.e., pathogen, host and environment (CDC, 2006), is most often referred to as a rational framework for understanding an epidemic process and determining strategies ‘to break the chain of infection’. However, the global application of this strategy in COVID-19 has shown limitation in isolating the infection from social, environmental and demographic determinants. These aspects are remedied by Epidemiological System and are congruent with a study by M. Parascandola on the contemporary conception of medical epidemiology and the need for new analytical and methodological frameworks (Parascandola, 2011). More recently, M. Frérot et al. reviewed the conceptual epidemiology evolution from 1978 to 2017, identifying population, health, and disease as the most common terms, whereas infectious disease and massive phenomenon were absent as structural elements in the 102 definitions (Frérot et al., 2018). These comprehensive analyses show that epidemiology is moving toward recognizing the population and health preponderance in a comprehensive preventive and curative strategy. However, it will take time to recognize disease as an effect, not as an epidemiological purpose. For comparative purposes, selected definitions from the referred works and other complementary are included:
‘Epidemiology is the science of disease in populations’. Vanderplank (1963).
‘Epidemiology is a method of reasoning about disease that deals with biological inferences derived from observations of disease phenomena in population groups’. D. Lilienfeld (1978).
‘Epidemiology is the quantitative analysis of the circumstances under which disease processes, including trauma, occur in population groups, the factors affecting their incidence, distribution, and host responses, and the use of this knowledge in prevention and control’. A. S. Evans (1979).
‘Epidemiology is the study of the distribution and determinants of health related states or events (including disease), and the application of this study to the control of diseases and other health problems’. OMS
‘Epidemiology is the science of populations of pathogens in populations of hosts, and the disease resulting therefrom under the influence of environment and human interference’ J. Kranz (1973).
‘Epidemiology is the multifactorial, multidimensional and dynamic study of relationships intrinsic to the Epidemiological System to elucidate rational strategies for the prevention of epidemics and mitigation of sanitary risks’. G. Mora and G. Acevedo (2021).
7. COVID-19: A Vision of the Pandemic
The current SARS-CoV-2/COVID-19 pandemic, with active spread in more than 194 countries (https://coronavirus.jhu.edu/map.html; https://www.gisaid.org/), has shown strong limitations in the implementation of epidemiological principles applicable to health surveillance for risk prevention and management. The conceptual, methodological and scientific experiences derived from historical epidemics have not been systematically rationalized and a fundamental setback is exhibited with the transition from prevention, successful in the 50-70’s, to the current cure, abandoning the philosophical and scientific foundational principle of Public Health Systems with community emphasis. The cure emerges as the paradigm that sustains the current patient-disease model that systemically articulates, not only the vision of Public Health Systems, but also the entire hospital system and private medical practice, educational curricula and training, scientific development, technology and innovation, and government and organizational public policy in the health sector. It is the etiology and diagnosis preeminence, exacerbated by genomic medicine expectations in the patient-disease treatment. The health cooptation considered as business strategy and the omission of the State to preserve this universal right. Paradoxically, the State is being asked to face the health crisis, with an inoperative and disarticulated health model, without the private sector, and the WHO as a world leader in health management, assuming the cost of adopting the cure as new paradigm. In turn, the State, unloads on population failures in the containment of COVID-19, claiming low cooperation in the adoption of palliative, but not effective, measures for epidemic intervention. It is the human population enduring the disease threat, and being affected in the vast social, economic, spiritual, educational and cultural relationships, that bears the highest cost; however, that ‘cost’ is not entirely quantifiable in a global consumption economy that only assigns a value to commodities. The systematic deterioration of Health Systems and their discriminatory effects has been widely documented (CEPAL and OPS, 2021; Velázquez, 2021; Frenk, 2003). The consequence is illustrated by successive COVID-19 epidemic waves, regardless of the population immunized percentage, and changes in prevalence of new viral variants, the most recent being Omicron, reported for the first time on the African continent with 5% vaccination. While this is being written, the Netherlands and several European countries resumed for the umpteenth time new severe confinements, in the face of massive infection by Omicron, making up the fifth epidemic wave. In this context, health as a business is ignominious (Vivas, 2021). Speculating in the stock market with inexistence solutions for Omicron should be penalized as much as society is criminalized for apparent negligence to prevent COVID-19 (Europa Press, 2021).
The historical epidemics analysis, and COVID-19 pandemic/epidemic management, allows us to identify conceptual and methodological limitations that restrict the mechanistic understanding and scope of epidemic intervention strategies (Figure 8):
1. Population (P N ) defines an epidemic process in that it frames the contagion process. In COVID-19 it has been omitted that population has a multidimensional structure and function in time and space dimensions. The COVID-19 public web-based ‘surveillance’ or traceability systems articulate their indicators base on a territorial population that does not represent the actual contagion phenomenon. Thus, the curve or map of Mexico epidemic status, or any other country, is only a numerical representation of COVID-19 cases in time or space, but does not allow intervention since infection rates are not structurally and functionally real. The territorial population (i.e., sex, age, ethnicity, comorbidity, occupation labor, etc.) is valuable in spatio-temporal multivariate dynamic risk models. But not applicable for classical epidemiological models. The limitation is that countries, and WHO as the lead agency, lack digital Surveillance Systems designed to articulate real-time automated algorithms for preventive decision-making and regional community risk management (Figure 9) (Mora-Aguilera et al., 2021a; Mora-Aguilera et al., 2021b).
2. Contagion operates at the P N level by establishing relationships of continuous or discontinuous dependence measurable in space-time. Contagion, quantified in its biological, clinical and epidemiological determinants, provides the mechanistic attribute of an epidemic. COVID-19 has shown that primary infection (I p-t1,e1 ) is multiple, quasi-synchronous, and random in time (t) and transboundary space (e) The secondary infection (I S-t1+n, e1+n ) can be operationally quantifiable and detectable in a subpopulation-i (SP N-i ). Therefore, community contagion-j (Cluster j ) of the SP N-i , in turn structured in n-subclusters j of functional relationships (work, family, social, etc.), where early detection should operate for intervention of a community COVID-19 outbreak or focus, e.g. confinement (Mora-Aguilera et al., 2021b). It is then the epidemic spatial intervention the goal. Not the curve intervention. COVID-19 epidemics, represented as cumulative curves of clinical cases per country (Dong et al., 2020), violate population and contagion conceptions. Consequently, SIR (Susceptible, Infected or Recovered subpopulation), logistic, Gompertz and other models (Moein et al., 2021, Mwalili et al., 2020; Holmdahl and Buckee, 2020; Alanazi et al., 2020; Cooper et al., 2020) have failed in their predictive ability, and ‘curve flattening’ for mitigation purposes, lacks biological and operational support. Country/state scale curves are descriptive but not inferential. Population-contagion, the great infectious epidemics paradigm has been poorly understood and applied in COVID-19.
Source: Mora-Aguilera et al., 2021a.
Figure 9 Integrative model of digital technology applicable to the development and processing of data in a web-based Epidemiological Surveillance System.
3. The Epidemiological System (ES). Medical epidemiology restricts its rational framework to the ‘epidemic triad’ and more often to the chain of infection. Both are restrictive. They are defined by a deterministic infectious process and limited to 3 or 6 events. An Epidemiological System open to n-subsystems, with the healthy population (not the sick!) as the main axis and integrator of n-causal factors and n-determinants of health, in conjunction with the environment (Mora-Aguilera et al., 2021a); constitutes an alternative to establish the comprehensive rational framework for holistic-systemic intervention of epidemics occurrence risk (prevention) and for mitigation of the effects (prevention and cure).
4. The community is defined by n-individuals belonging to a territorial sub-population -i (SP N-i ) that exhibit functional relationships in time and space. They n-individuals are organized in n-clusters j and n-subclusters j defined by spatial dependencies determined by structural and functionally articulated roles and community values of co-responsibility and coexistence. The n-individuals of n-subclusters j operate as subjects of health and as subjects at risk of disease and should therefore be active participants in a preventive and risk mitigation model. Individuals of this subpopulation, integrated into a digital Surveillance System, can communicate their health status in real-time driven by their values and because it suits to their belonging community. Not by an external imposition (Mora-Aguilera et al., 2021b). Current epidemic management considers the population as a continuous unit and unconnected of multiple, concrete and quantifiable risk realities. A black-box of confused effects. It confers to the population a passive role on decisions and claim responsible for failures on the adoption of strategies decided without its involvement. Strategies that do not resolve the causation of Public Health Systems deterioration currently disarticulated obsolete and de facto privileging health as a business strategy and cure as a paradigm.
8. Epidemiological surveillance in human, animal and plant health. One health
In a previous work, the need for holistic-systemic Epidemiological Surveillance Systems (ESS), required for application of preventive models to regional epidemic risk processes in agricultural crops was presented (Mora-Aguilera et al., 2021a). The use of web technology and mobile applications allow the integration of data in structured matrices with rational criteria based on an Epidemiological System ad hoc to the plant health problem (Figure 9). The generation of automated relational and functional algorithms, i.e., early warnings, allows analysis and decision-making for prevention and management, i.e., mitigation of an epidemic. Data is generated in the field by multiple certified users to ensure traceability and quality of epidemiological information. These type 3 ESS differ of pathogen (pest) presence-absence systems (type 1) or quantitative spatial-temporal prevalence systems (type 2), which have strong regulatory support. Types 1 and 2 are those commonly used in Public Health Systems integrating information from primary and secondary health care centers. They include the sentinel model proposed by WHO and adopted by Mexico for Influenza A H1N1, and later adapted for SARS-CoV-2. Type 3 ESS have been developed in Mexico, with different application and consolidation levels, in coffee (Coffea spp.), blue agave (Agave tequilana) and citrus (Citrus spp.) by DGSV (SENASICA) responsible for national Plant Health (Moral-Aguilera et al., 2021a; Mendoza-Ramos et al., 2021; López-Bautista et al., 2020; Coria-Contreras et al., 2019; Flores-Sánchez et al., 2017).
The epidemiological principles of ESS type 3 were applied in development of a real-time digital prototype applied to the prevention of SARS-CoV-2 community transmission risks. The fundamental premise is that interruption of contagion chains is most effective at the community cluster-subcluster level due to the spatial dependence of risks identified, assessed, and automatically communicated to the immediate social environment. The horizontal integration of community actors in a digital system applied to the early detection of infection, confinement, clinical traceability, and safe reintegration of the individual into his/her family-social-labor cluster-subcluster would guarantee co-responsibility in essential public health decisions under the participatory prevention principle. The notion of population as a ‘herd’ or passive entity in the solution of a public health problem is abandoned (Mora-Aguilera et al., 2021b).
This prototype, called SMAT COLPOS, was applied in an academic-research work environment of Postgraduate Collage in Agriculture Science (CP) from September to December 2020, during a critical incidence phase of SARS-CoV-2 positive cases and maximum social-labor restriction in the State of Mexico and CDMX, typified by the Health Ministry with a ‘red traffic light’. The system successfully validated the fundamental premise and the risk management operative model. The second stage of the prototype development is upon the interest of Health Ministry. The management phase has not been easy, understandably in the absence of policies with cross-cutting health visions.
The ‘one health’, a goal for a new ‘health architecture’ that the WHO has recognized as an international need structurally articulated to countries to face COVID-19 pandemic and future epidemics, is easier in political conception than in operationalization (https://www.who.int/es/news-room/commentaries). The risk of turning politics into bureaucracy can be as dangerous for public health as the epidemic itself. The ‘one health’ for WHO applies to human and animal health, but the model is incomplete if plant health is not included because it is involved in food quality and safety, directly associated with human and animal health determinants, and also because it is essential condition in food security, linked to malnutrition and famine, which constitute risk factors for human diseases.
9. Perspectives
The SARS-CoV-2 coronavirus, causing COVID-19, zoonotic in nature, and with humans as the sole transmission vehicle, has surpassed all historical pandemic agents in host-adaptive parasitic speed; effective geographic dispersal of the primary inoculum; the complexity of the infective process; tissue, pathogenic, and clinical tropism; and population structure with highly dynamic lineages due to mutagenic capacity (Lee et al., 2021; Hamed et al., 2021; Saito et al., 2021; O’Toole et al., 2021). Worldwide reproduction rates of R t > 1 are congruent with more transmissible variants, such as Delta and Omicron, as an attribute of sublethal survival. For instance, Mexico has maintained maximum R t of 1.15, 1.26 and 1.01 in its three epidemic waves.
However, SARS-CoV-2 had not exhibit the mortality rate and case-fatality of other organisms despite the risk factors multiplicity inherent in current human populations. These include chronic non-infectious diseases and occupational, social and urban environments prone to contagion due to high mobility and social proximity (WHO, 2021a; Davis et al., 2021; Alvarez-Maya et al., 2021). Moreover, case-fatality has decreased from 11.1 - 15.2%, the maximum range at epicenters of first 2020 pandemic cycle, to 2.5% global average actual (Ritchie et al., 2020; https://ourworldindata.org/mortality-risk-covid#citation). In contrast, other recent epidemic/pandemic processes have exhibited higher levels, in absolute values, such as those associated to Ebola 40%, MERS 34%, Influenza A H1N1 10%, and SARS-2002 10%. There are not historical case-fatality estimates due to the incipient hospital system, with clinical traceability and epidemiological methodologies limited; however, it is possible to infer, based on estimated mortalities, that case-fatality was also higher in Black Death, Spanish Flu and Smallpox with 60%, 50% and 30% mortality of European population, respectively.
The seasonal influenza, predominantly caused by the Influenza A H1N1 virus (https://www.paho.org/es/temas/influenza-otros-virus-respiratorios), decreased from 2009 (10% maximum) to 0.1-0.2% annually in endemic condition (Ritchie et al., 2020; Roser et al., 2014; Wong et al., 2013). The endemic transition of SARS-CoV-2 is also highly probable due to its epidemic and pathogenic characteristics, with decreasing case-fatality as a natural adaptive process to the host. The later, not exclusively due to the vaccination process as has been implied. The restricted pathogenic aggressivity of Omicron, the most recent variant, but with the highest transmissibility in Africa, with less than 6% of the population vaccinated, shows the expected biological tendency of an obligated parasite as previously discussed in this work. (https://elpais.com/sociedad/2021-12-18/los-primeros-estudios-en-sudafrica-apuntan-que-omicron-se-contagia-mas-rapido-pero-con-sintomas-mas-leves.html).
However, the design of innovative preventive strategies at the community ambulatory level, linked to Epidemiological Surveillance Systems, is obligated for the following reasons:
The clinical course of the disease is unpredictable.
Clinical diagnosis is restrictive and may depend on the viral variant.
Similarity of some symptoms to other respiratory diseases
Requirement of specialized diagnostic tests
Absence of a vaccine with absolute and permanent effectiveness
Limited curative antivirals (Molnupiravir and Paxlovid)
Global and national politicization by health emergency management
Limited infrastructure and human resources in Public Health Systems
Cure is the paradigm of the patient-disease model. Costly and palliative.
Expectations of a ‘new normality’ by mid-2020, as a result of severe mobility restrictions and social distancing, were soon overtaken by successive epidemic waves. Some countries are currently exhibiting the fifth epidemic wave with return to confinement and additional measures previously employed. In Germany, Great Britain, USA, India and Brazil, the effects of subsequent waves were even greater, practically collapsing their Health Systems (Ap et al., 2021b; Dong et al., 2020). Surprisingly, the third pandemic wave, which started in the first quarter of 2021, proved that vaccination is not the absolute solution. Current public data have evidenced that SARS-CoV-2 infection may occur independently of immunization although with lower mortality risk. It is also clear that Delta and Omicron, the most recent variant, have reduced the vaccines immunological capacity (Townsend et al., 2021; WHO, 2021c). In countries, mainly in the G7 block, where 80% of the population, or more is vaccinated with full doses, it was found that ‘herd immunity’, originally established with a theoretical 60-70% threshold (Aschwanden, 2021), is not operating. This is explained by the temporary immunity conferred by vaccines, active virus variations, and asymmetric vaccination worldwide. In this context, Mexico ended the third epidemic wave with 46% of the population with a complete dose (SSA, 2021), and apparently faces the fourth epidemic cycle with 52% immunized, it is apparently facing the fourth epidemic cycle (Datosmacro, 2021b).
In this pandemic, cyclical context with strong social, educational, cultural, spiritual and economic implications, at least five explanations have been publicity given for the failure in resolving the health crisis caused by COVID-19 (ONU, 2021; Afp, 2021e; Navarrete, 2021; Afp, 2021d; Europa Press, 2021; Li et al., 2021; Afp et al., 2021a):
The amazing worldwide research, developed mainly at the clinical, immunological, histological and genomic levels, is coopted by the large pharmaceutical-consortiums in collusion with their governments.
The inequitable vaccines distribution with 80-90% of the world’s population, represented in 56 countries, without access to any biological. As a result, ‘herd immunity’ is not generated and the emergence of new viral variants may continue.
The refusal of pharmaceutical consortiums to release immunological patents. This makes impossible to include poor countries to the cure option.
Protectionist policies limit the authorization, by WHO and other agencies, of vaccines from China and Russia. Some countries / sectors of the population refuse to apply these vaccines, e.g., Sinovac and Sputnik V.
Society rejects, or partially adopts mitigation measures. Thus it justifies the implementation of coercive measures (fines, jail, access control to public places, etc.).
However, these arguments elude the real omission to resolve the health emergency. It is evident that at the global level, under WHO guidelines and the countries health policies, the SARS-CoV-2/COVID-19 pandemic/epidemic management lacks a comprehensive pansystemic model for a sustainable and resilient solution, based on systemic research, public policies, and Health Systems operative model innovations.
This comprehensive pansystemic model is absent and unfeasible based on current global COVID-19 management: the Public Health Systems have had a gradual over time deterioration in proportion to the abandonment of prevention as a foundational principle and the cure adoption as a profitable patient-disease strategy. Consequently, planning, operational and scientific support structures are not sufficiently solid for a harmonized international-regional decision-making, and for correct rationalization, implementation and intervention of the epidemic process. COVID-19 constitutes an opportunity to eliminate bureaucracy and innovate the Public Health System.
It is necessary to recognize that the vaccination/antivirals reductionist vision and the hygienist measures, which worked in other historical pandemic/epidemic contexts, do not constitute:
1. A systemic organic model.
2. A choice in the hypernode and multiple dependent global structure of the consumption economy.
3. The essence of a preventive health paradigm.
4. Sustainable solutions for an environment with strong anthropogenic intervention that triggers mutagenic and zoonotic microbiological effects and multiple health determinants.
5. A stimulus for the development of holistic-systemic Epidemiological Surveillance Systems.
The ‘one health’, a goal for a new ‘health architecture’ that the WHO has recognized as an international necessity in to face COVID-19 pandemic and future epidemics (https://www.who.int/es/news-room/commentaries), is possible if the systematic deterioration of the Public Health Systems is reversed, prevention is reinstated as the guiding principle, and the patient-disease model is reconfigured within an integral, inclusive and human model.
Conclusions
The SARS-CoV-2/COVID-19 pandemic had demonstrated that, despite the great scientific-technological advances, highly specialized human talent, and historical epidemics and pandemics experiences, which allowed the evolution of a conceptual theory and a methodological operational framework, private and Public Health Systems lack a pansystemic model to articulate rational and operational regional models . Thus a preventive epidemiological surveillance system, required for decision-making on risk management and epidemic intervention is missing or fragile. Public policies, in turn, showed the absence of State’s health plan vision due to the gradual adoption de facto of curative and individualized approaches promoted by the pharmaceutical industry. As a result, diagnostic, clinical, and hospital-based strategies have prevailed over effective community-based outpatient strategies. The fact that prevention anticipates and intervenes in community contagion chains is overlooked. It is the functional space relations for assessing and risk community intervention. The great paradigm of infectious epidemics, the population-contagion, has been poorly understood and applied in COVID-19.
Almost two years after the health emergency, the global strategy shows the following trends: 1. The human activities ‘normality’ has not been restored; 2. The pandemic maintains dynamic waves in time with a succession of SARS-CoV-2 variants with increasing transmissibility; 3. Countries with greater mobility, and density populated urban centers have had the highest contagious and mortality rates regardless of their economic development; 4. Social distancing and confinement, periodically recurrent strategies, and mass vaccination have not contained the pandemic process; 5. Public Health Systems have been recurrently overwhelmed in high COVID-19 incidence urban cities; 6. The research agenda is determined by pharmaceutical consortiums focused on generating of vaccines and antivirals under a curative clinical approach.
It is necessary to reinforce the biological, pathogenic and epidemiological understanding of SARS-CoV-2 through evolutionary studies to elucidate virulence, aggressiveness, transmission, tissue tropism, and disease expression. In addition, coronavirus phylogeography from an animal source(s); dynamic studies of variable genomic regions that determine human adaptability of new variants; and generation of predictive genomic models are urgent upon active zoonotic coronaviruses migration to humans on the last 20 years, including PDCoV, HCoV-NL63, MERS-CoV, SARS-CoV 2003, SARS-CoV-2.











 texto en
texto en 


