Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.33 no.1 Texcoco 2015
Revision articles
Lasiodiplodia theobromae in Agricultural Crops in México: Taxonomy, Host, Diversity and Control
1Centro de Investigación en Alimentación y Desarrollo A. C. Coordinación Culiacán. Carr. A Eldorado Km 5.5. Campo El Diez, Culiacán, Sinaloa. CP 80110. México.
Lasiodiplodia theobromae is the causal agent of numerous plant diseases in a wide variety of hosts. Fruit and vegetable crops are particularly susceptible to infection by this fungus. Although in Mexico, L. theobromae is associated with dieback and fruit rot in fruits such as mango, grapes, papaya, rambutan, sapote mamey and citrus fruit, the number of reports of this fungus related to diseases in different plant species is still few. The taxonomy of L. theobromae has shown important progress, with the characterization of the intergenic spacer regions and the elongation factor 1 alpha, phylogenetic location and cryptic species differentiation have been clarified and an increase of reports in new plant hosts has been made. Thus, the objective of this review is to present the status of L. theobromae in Mexico.
Key words: Botryosphaeriaceae ; dieback; fruit rot; stem-end rot
El hongo Lasiodiplodia theobromae es el agente causal de numerosas enfermedades de plantas en una gran variedad de hospederos. Los cultivos hortofrutícolas son particularmente sensibles a la infección por este hongo. Aunque, en México L. theobromae está asociado con la muerte descendente y pudrición de frutos como mango, uva, papaya, rambután, zapote y cítricos aún es escaso el número de reportes relacionados con enfermedades causadas en diferentes especies vegetales. La taxonomía de L. theobromae ha mostrado importantes progresos, con la caracterización de las regiones espaciadoras intergénicas y factor de elongación 1 alfa se ha podido esclarecer su ubicación filogenética y su diferenciación con especies crípticas, lo que ha incrementado los reportes en nuevos huéspedes vegetales. El objetivo de esta revisión es el dar a conocer el estatus de L. theobromae en México.
Palabras clave: Botryosphaeriaceae ; muerte descendente; pudrición de frutos; pudrición del pedúnculo
Lasiodiplodia theobromae (Pat.) Griffon and Maubl. is the species type of the genus Lasiodiplodia, which is a fungus that was described for the first time around 1890 by Saccardo, affecting cocoa pods (Theobromae cacao) in Ecuador (Crous and Palm, 1999). This fungus is highly diverse and has a large range of hosts, including monocotyledons, dicotyledons and gymnosperms, especially from the tropics and subtropics. It is a pleomorphic and ubiquitous fungus, as such it has had more than one synonym (Abdollahzadeh et al., 2010; Wang et al., 2011).
The diseases caused by this pathogen include dieback, cancer, gummosis, leaf blight, and root rot on woody plants and crops (Pitt and Hocking, 2009; Shahbaz et al., 2009). L. theobromae is saprophytic but is considered a latent pathogen, found as an endophyte in healthy plant tissue and becoming a pathogen when the host is debilitated or stressed (Rubini et al., 2005; Mohali et al., 2005). It has also been reported as an opportunist pathogen in humans causing subcutaneous, ocular infections as well as infections of the internal organs (Rebell and Forster, 1976; Maslen et al., 1996; Summerbell et al., 2004; Woo et al., 2008). At a global level there are records of L. theobromae affecting fruit crops such as mango (Johnson, 1992), avocado (Pegg et al., 2003), papaya (Queiroz et al., 1997), banana (Alves et al., 2008), rambutan (Sivakumar et al., 1997), lychee (Liu et al., 2005), grapes (van Niekerk et al., 2004), soursop (Lutchmeah, 1988), cashew (Cardoso et al., 2002), citrus fruit, peaches (Damn et al., 2007) and longan (Serrato-Días et al., 2014) among others, causing economical loses in various stages of production.
In recent years, its phylogenetic relation with cryptic species has been established through the analysis of DNA in fragments of the intergenic spacer regions (ITS) and the elongation factor 1 alpha (EF-1α), which has allowed for clearer results of its location with regard to similar or closely related species (Pavlic et al., 2004); Alves et al., 2008; Abdollahzadeh et al., 2010).
The etiology of the disease is crucial for epidemiological studies and for a better understanding of the distribution and importance of this fungus, as well as to establish strategies and treatments for its effective control. The objective of this article is to describe the status of L. theobromae affecting various fruit and woody crops in Mexico as well as to compile the published results of the affected crops focusing on the aspects of biology, pathogenicity, epidemiology and strategies of control for this fungus.
Taxonomy
The fungus Lasiodiplodia theobromae is classified within the Ascomycota in the order Botryosphaeriales and the family Botryosphaeriaceae (Schoch et al., 2006; Slippers et al., 2013). It shows an uncommon sexual state (teleomorph) Botryosphaeria rhodina; however, there are no recent descriptions of its sexual state, which is why it has been reported that this information has been lost (Phillips et al., 2008). The sexual state of this fungus needs to be clarified, the results up until now have been inconclusive given that there have not been any subsequent reports that confirm this connection (Alves et al., 2008).
Diplodia theobromae was known as a synonym of L. theobromae (Alvarez, 1976; Denman et al., 2000). In recent years, phylogenic studies based on the ITS regions, carried out by Zhou and Stanosz (2001), Slippers et al. (2004) and Phillips et al. (2008), show that the clades of these two genera are in fact separate. Furthermore, morphologically the two genera are clearly distinct. For example, the marks of the conidia are only present in Lasiodiplodia in the same manner the sexual stage, has only been reported in L. theobromae. Therefore, there is no reason to consider these two genera as synonyms (Phillips et al., 2013).
For a long time Botryodiplodia theobromae Pat. (1892) was considered as the basionym of L. theobromae, which was described in the Theobroma cacao plant in Ecuador; however, Crous and Palm (1999) examined the original type conserved in Pennsylvania and found an Ascomycota valsoide by which the name Botryodiplodia (Sacc.) Sacc. is considered nomen dubium (uncertain name). On the other hand, the holotype of L. theobromae has not been found in any herbarium (Pavlic et al., 2004), as such it is presumed to have been lost with time. Phillips et al. (2013) designated a neotype isolated from an undefined fruit from the coral reef of the east coast in Papua New Guinea, far from the local of the holotype and the original substrate (cocoa tree), considering this neotype a strain of reference for L. theobromae.
Regarding its teleomorph, with the passing of time the superior taxonomy of the genus Botryosphaeria (sexual state of Lasiodiplodia theobromae) has had various modifications. Slippers et al. (2013) indicate that at the beginning the genus was assigned to the family Melogrammataceae, within the order Sphaeriales; subsequently, it was situated in the family Pseudosphaeriaceae that grouped taxa with a single loculus and multiscale ascostramata, within the order Dothideales; following that, the subfamily Botryosphaerieae was created and it was placed here; however, it was not placed within an order. A year later, it was assigned to the order Myriangiales, and subsequently the subclass Dothideineae was created within the new order Pseudosphaeriales and the new family Botryosphaeriaceae. One of the main reasons for this reorganization in the classification of Botryosphaeria was because of the confusion with regard to the ontogeny and morphology of true perithecia, ascostromata and interstitial tissue (Denman et al., 2000; Slippers et al., 2013).
In the last couple of years, mainly due to the availability of molecular tools based on the recombinant DNA, a more solid taxonomy has emerged for this group of fungi. Until a decade ago, the position of the genus in the highest classification of the ascomycetes had not been solved (Denman et al., 2000). Phylogenic studies show the position of the family Botryosphaeriaceae in the class Dothiodeomycetes, placing it within Botryosphaeriales, a new order and independent from the orders Pleosporales and Dothideales (Crous et al., 2006). Currently 6 families are recognized within this order: Botryosphaeriaceae (with 17 genera), Phyllostictaceae (Phyllosticta), Planistromellaceae (Kellermania), Aplospore-llaceae (Aplosporella, Bagnisiella), Melanopsaceae (Melanops) and Saccharataceae (Saccharata) (Slippers et al., 2013).
The analyses based on the DNA sequencing have originated significant changes in the nomenclature, identification and circumscription of species in Botryosphaeriaceae. These changes are the results of the implementation of a single nomenclature for all forms (sexual and asexual) of a species (Hawksworth et al., 2011), including the description of cryptic species based on the DNA sequences, where the morphological characters are not enough for this purpose (Pavlic et al., 2009; Sakalidis et al., 2011). Contemporary studies of the Botryosphaeriaceae family support this motion, based on the morphological characteristics typically used for the classification of species (form of the conidia or ascospores and its dimensions, septation and pigmentation) frequently not being too reliable. The ecological and geographical data are also difficult to interpret when some species have various hosts, and a host has various species (Slippers et al., 2009). Therefore, the majority-if not all-of the taxa include the DNA sequence and its phylogenetic inference in order to redefine these classifications (Slippers et al., 2013). For example, Liu et al. (2012) based themselves in the amplification and sequencing of the various regions of the genome using the oligonucleotides NS1 and NS4, LROR and LR5, ITS4 and ITS5, EF2-728 F/ EF2-968F and Bt2a and Bt2b (that amplify the region of the small subunit of the nuclear ribosomal gene, a segment of the large subunit of the ribosomal RNA gene, the intergenic spacer regions of the rDNA, a segment of the elongation factor 1-alpha and a segment of the β-tubulin gene, respectively) with the objective of rearranging the Botryosphaeriaceae family, accepting 29 genera and 1485 species, clarifying that species not yet described and some complex of species are still missing. The genus Macrovalsaria was also rearranged into this family, which is monotypic, that is to say with a single specie M. megalospora, which is only found in a sexual state and genetically close to Lasiodiplodia spp. Some consider this genus as the sexual state of Lasiodiplodia spp.; however, more in depth studies would be needed to confirm this proposal (Liu et al., 2012).
Recent changes in the taxonomy of fungi, according to the nomenclature of algae, fungi and plants (Melbourne Code), establish only one name for each species of fungi, given that during more than 100 years the code allowed the names of the asexual and sexual phase of a single species (Rico, 2011). The genus Lasiodiplodia is considered valid and is found on the list pending approval by the committee of fungi nomenclature, which could occur in the next international congress of botany in China in 2017 (Kirk et al., 2013; Wijayawardene et al., 2014).
Biology
Physiology and Morphology. The main characteristic that distinguishes the genus Lasiodiplodia from other closely related genera is the presence of pycnidia, paraphyses and longitudinal striations in mature conidia. Around 20 species have been described based on the morphology of conidia and paraphyses. The most recent descriptions of these species, other than the morphology, are based on the sequencing of the intergenic spacer regions of the rDNA (ITS) and elongation factor 1 alpha (EF-1) (Damm et al., 2007; Netto et al., 2014).
The morphology of its ascocarp is a dark brown to black, aggregate, with a thick dark brown wall and hyaline in internal layers, of 250-400 µm in diameter. The asci is bitunicate, stipitate, with 8 spores, of 90-120 µm longitude. The ascospores are biseriated, hyaline, aseptated of 30-35 x 11-14 µm. The conidiomata is stromal, simple or aggregate, immersed in the host and once mature, it emerges a dark brown color, unilocular, thick or thin brown wall, with setoso frequency, up to 5 mm wide, central ostiolo, unique, papillado. Paraphyses hyaline, cylindrical, partitioned, occasionally branched with the ends rounded up to 55µm long and 3-4 µm wide (Phillips et al., 2013).
The conidiophore are hyaline, simple, sometimes septated, rarely branched, cylindrical. The conidiogenous cells are hyaline, of a thick wall, smooth, cylindrical to sub-obpiriforms, holoblastic, with one or two rings. The conidia are subovoids to ellipsoidal, with apex amply rounded, that narrow to truncate at the base, wider mid-upper third, of thick walls, with a granular content, at first hyaline and aseptated, turning a dark brown once mature, with 1 septum, showing deposits of melanin on the interior surface of the wall longitudinally disposed given a striated appearance with measurements 21.5-31-5 x 13-17 µm and one portion of 1.9 Length/Wide (Figure 1) (Pitt and Hocking, 2009; Phillips et al., 2013).

Figure 1 Structures of Lasiodiplodia theobromae in an isolate taken from papaya. A) Mycelia and pycnidia in PDA at 14 days of growth. B) Mature and immature conidia at 14 days of growth.
The colonies in the culture medium are moderately dense, with aerial mycelium, beginning white, turning a gray-olive color at 7 days, and with time acquire a black color. The temperature of growth for L. theobromae is 15 ºC minimum, 28 ºC optimally and 40 ºC maximum (Slippers et al., 2004; Alves et al., 2008). The sporulation of the fungus is favored by photoperiods of more than 16 hours of light exposure, which allows for the formation of pycnidia; on the contrary, an exposure of less than 4 hours of daily light, in a period of 23 days, inhibits the sporulation of the fungus (Perera and Lago, 1986). The presence of nitrogen in the culture medium favors sporulation; Saha et al. (2008) evaluated the concentration of nitrogen in various culture mediums, finding that the potato dextrose agar (PDA) with added tea root extract induces rapid growth and higher mycelium, in addition to a higher concentration of spores than the rest of the evaluated mediums.
For several years, the physiology of the isolates in the separation of species of the genus Lasiodiplodia has been controversial. For example, Alves et al. (2008) distinguished a L. parva and L. pseudotheobromae of L. theobromae based on the ability of the first two to produce a pink pigment in the PDA medium at 35 ºC; they also reported that L. pseudotheobromae at 35 ºC produced an intense pink pigmentation in the PDA, in addition to the three species growing at 10 ºC. Therefore, the physiological characteristics have a limited value to determine the separation of species given that there exists great variability in the physiological characteristics between the isolates of a single species.
Phylogenetic Studies. Considering the similarity in diverse DNA sequences, diverse genera would be grouped with Lasiodiplodia to the point of considering them synonyms. Phillips et al. (2013) considers Macrovalsaria in the group of this genus. This was also pointed out by Liu et al. (2012), although no sufficient evidence was found in the LSU and SSU regions in order to make this change and establish it as a synonym.
On the other hand, joined to cosmopolitan presence, the ample number of hosts and the morphological variability of L. theobromae, various cryptic species exist. For example, Pavlic et al. (2004) described L. gonubiensis Pavlic, Slippers & Wingf based on the morphology and dimensions of its conidia and on the sequencing of the ITS regions. Subsequently, Burgess et al. (2006) described L. crassispora, L. venezuelensis and L. rubropurpurea based on the ITS regions and EF1-α and the morphological characteristics. Other species considered cryptic are L. parva and L. pseudotheobromae (Alves et al., 2008), which are separated by the size and form of the conidia. In L. pseudotheobromae the conidia is larger and more ellipsoidal than in L. theobromae. Also, L. parva is easily distinguishable from the other two species given that its conidia are smaller. L. mahajangana is another species that is considered cryptic, which is phylogenetically close to L. theobromae but morphologically distinct, given that the first has conidia relatively smaller-17.5-11.5 µm (Abdollahzadeh et al., 2010).
The SSR markers have been recently used to examine genes and genotypes, reproductive means and speciation of several fungi, including Botryosphaeria spp. and its anamorphs (Burgess et al., 2006; Mohali et al., 2005). An investigation suggests that geographic barriers exist for the exchange of genes between L. theobromae, based on the SSR markers, with isolates from Venezuela, Mexico and South Africa (Mohali et al., 2005). Shah et al. (2010) analyzed 30 isolates of L. theobromae of the pear culture in India, finding a high genetic diversity between the isolates stemming from various geographical zones and little genetic diversity between the isolates of the same geographical zone.
Other studies of genetic diversity propose that two cryptic species of Lasiodiplodia (L. theobromae and L. pseudotheobromae), have not been found and studied in the same host and therefore it has not been possible to establish if, at any moment, any hybridization between them has occurred (Begoude et al., 2010). Al-Sadi et al. (2013) found a moderate level of genetic diversity in populations of three species of Lasiodiplodia from various hosts and geographical origins, and a high number of genotypes (not specified) of L. theobromae, L. hormozganensis and L. iraniensis in Oman, UAE (United Arab Emirates). They also found that L. hormozganensis differed mostly from L. theobromae with regard to genetic diversity, suggesting that L. hormozganensis was erroneously identified for a long time as L. theobromae, due to the only form of identification available being by the morphological characters of the species and that the first is a cryptic species of the second.
Pathogenicity and virulence. The species L. theobromae is more virulent in comparison to other genera and species of the family Botryosphaericeae. For example, Úrbez-Torres et al. (2008) found that L. theobromae is more virulent than Diplodia seriata in the grapevines crop, given that it caused greater damage to the inoculated stems. In mango fruits, L. theobromae showed mid-high virulence in comparison to L. egyptiacae and L. pseudotheobromae (Ismail et al., 2012); in contrast, Marques et al. (2013) described L. theobromae with medium virulence compared to L. hormozganensis with this being the most virulent, causing damages of 33.6 mm in diameter on the fruit.
Umezurike (1979) mentions the cellulite activity of the fungus, which attacks the plant in a similar manner to a soft rot fungus, using the starch and other saccharides present in the initial substrate of the wood before the degradation of the cellulose and hemicellulose, although it does not degrade the lignin.
Symptomatology and epidemiology. In the fruit rot and stem-end rot, the disease is conditioned to high temperatures and relative humidity (Ploetz 2003). The damages caused by L. theobromae in mango fruit are initially diffused, aqueous-sunken dispersing from the stalk in the shape of fingerprint projections, which darken and rapidly coalesce around the base of the stalk forming undulating edges. The necrosis occurs below the cuticle, invading the pulp of the fruit and mummifying it. Pycnidia are first observed on the stalk and later on the fruit; furthermore, the injuries may gush a brown ooze (Ploetz, 2003; Ventura et al., 2004).
The main means of entry for L. theobromae into the hosts is through the wounds produced by work tools, insects or natural causes (Ploetz, 2003). It has been reported that during periods of rain there is a greater production of spores, which may be disseminated by raindrops and wind (Vázquez et al., 2009). The fungus colonizes the vascular system and advances ahead of the visible symptoms (Burgess et al., 2006; Shahbaz et al., 2009). The fungus survives on dead tissues on the tree or the ground (Pegg et al., 2003) and especially on mummified fruits (Ploetz, 2003). The incidence of L. theobromae is influenced by the temperature (above 30 ºC), to hydric stress and low nutrition levels of the plant (Khanzada et al., 2005). When the fruits are infected on the tree, the pathogen may remain latent until the fruit has matured. In post-harvest, the fruits may be infected by being placed on the ground after being harvested or through physical contact of a healthy fruit with a diseased fruit (Ventura et al., 2004).
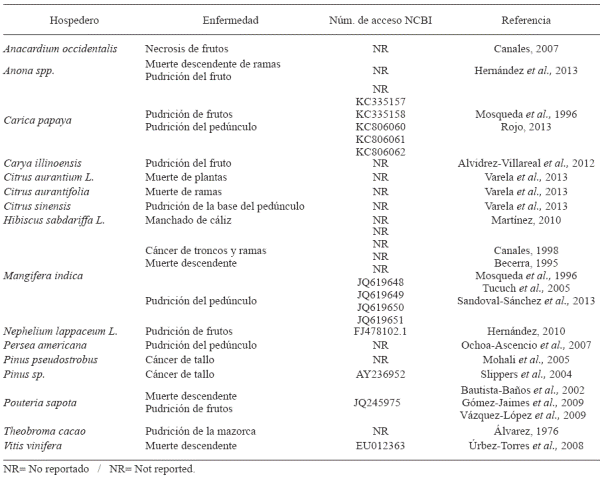
Reports in Mexico
Lasiodiplodia theobromae has been reported in Mexico causing various diseases in various crops, mainly fruit producers (Table 1). The oldest report found for this pathogen is that made by Álvarez (1976) affecting the cultivation of cocoa (Theobroma cacao). Other crops that he also reported it are: avocado (Persea americana), papaya (Carica papaya) and rubber tree (Hevea brasiliensis Muell.) under the name Diplodia theobromae, in sugar cane (Saccharum officinarum L.) such as Diplodia cacaoicola, custard apple (Annona cherimola Mill) and peach (Prunus persica) such as Diplodia natalensis causing fruit rot and gummosis, respectively.
The majority of the descriptions for this pathogen have been based on morphological characteristics and it was until this past decade that the identification was supplemented with molecular biology techniques. Between the reports based solely on morphology, there are those made on the cultivation mango (Mangifera indica) in the state of Veracruz, where the environmental conditions aggravate the situation given that the rain does not permit for the trees to recuperate their foliage (Mosqueda et al., 1996). Also, Romero (1993) made a description of plant phatogenic on agricultural crops in Mexico and reported B. theobromae affecting the fruits of mango, custard apple, cotton, yucca, sweet potato and barbasco. Other reports with the name of Botryodiplodia theobromae are those of Becerra (1995) affecting the cultivation of mango in Michoacán, Nayarit and Veracruz, where the humidity favors the appearance of the phytopathogen. Tucuch et al. (2005) reported B. theobromae as the causal agent for dieback in mango in Campeche, where the disease presents itself with greater intensity when the relative humidity is above 80 % and a temperature between 26 to 32 ºC, presenting a progressive and downward drying with black dots on the bark; grayish brown injuries start on the leaves causing intense defoliation leading to the death of the tree. In addition to the previous reports, there also exist others where Lasiodiplodia sp. was only assigned as a causal agent in cashew (Anacardium occidentalis) causing necrosis in fruits in Campeche, Quintana Roo and Yucatán (Canales, 2007). Canales (1998) also describes the cancer of the trunk and branches in mango under the name Botryodiplodia sp., as well as inducing flowering by causing hydrological stress on the branches and causing a girdling effect.
On other crops, based solely on morphological characteristics, are included those made by Bautista-Baños et al. (2002) where B. theobromae is reported as the causal agent for the rot in mature mammee; Martínez (2010) reports L. theobromae affecting roselle (Hibiscus sabdariffa); Hernández (2010) reports it as the causal agent for the black spot in rambutan (Nephelium lappaceum) in postharvest; Hernández et al. (2013) report it associated to dieback in soursop (Annona spp) branches, although proof of pathogenicity proving that it is the although it is not yet needed pathigenicty tests to prove it is the causal agent of the disease. Varela et al. (2013) describe it as the causal agent in the death of bitter orange plants grafted with various citrus species in nurseries, of the death of branches in Mexican lemon trees and of the stem-end rot in orange fruit.
Between the reports that, in addition to the morphological characteristics, consider some molecular characterization are those of Úrbez- Torres et al. (2008), who identified L. theobromae as causing cancer of the vine in the north of Mexico and the southern United States; for its identification and characterization they based on the ITS1-5.8s and ITS 2 regions, a partial region of the beta-tubulin gene (β-tubulin), and part of the elongation factor 1-alpha gene (EF1-α). Vázquez et al. (2009) reported L. theobromae affecting sapote twigs and identified the pathogen through morphological characteristics and the sequencing of the ITS regions. In the pecan tree (Carya illinoensis), L. theobromae was found in association with a coleopteran as the causal agent for the fruit rot; the fungus was identified based on the morphological characteristics and the sequencing of the ITS regions (Alvidrez-Villarreal et al., 2012). In the mango crop (Mangifera indica) in addition to L. theobromae, L. pseudotheobromae (cryptic species of the former) was also reported, causing stem rot and dieback, respectively. The identification of the species was based on morphological characteristics and in the amplification and sequencing of the ITS regions (Sandoval-Sánchez et al., 2013). In papaya (Carica papaya), Rojo (2013) identified, through morphological characteristics and the sequencing of ITS regions, L. theobromae as responsible for the postharvest stem rot.
Control. There have been various studies carried out in order to control L. theobromae once it has been detected in a crop. Li et al. (1995) evaluated fungicides against L. theobromae and Botryosphaeria dothidea which cause gummosis in peaches and apricot, finding that the fungicide metil-tiofanato inhibited the growth of mycelia, the germination of conidia and controlled the development of the disease in apricot trees; they also reported that the fungicides sprayed, metil-tiofanato 70WP and carbendazim 50WP can be used as an auxiliary treatment in order to prevent the infection of the pathogen.
On the other hand, Tamayo (2007) recommends the usage of calcium hypochlorite and carboxin/captan in order to prevent possible rot or the manifestation of the fungus in the nursery, and before storage the fruit must be submerged in a prochloraz-based solution. Tamayo (2007) also recommends a pre-harvest spray of copper-based fungicides, benomyl, metil-tiofanato, carbendazim or tiabendazole on a rotational basis in order to avoid the appearance of pathogen populations resistant to the fungicides.
In a sensibility evaluation study of L. theobromae towards two groups of fungicide, it was concluded that 91.6 % of 120 isolates stemming from papaya orchards were sensible to the active ingredients of the carbonates of the methyl benzimidazole type (benomyl and tiabendazole). Regarding the group of fungicides of the inhibition type by demethylation (Imazalil, prochloraz, tebuconazole) great variability was found with regard to the grade of sensibility of the isolates analyzed, concluding that L. theobromae is less sensible to this group of fungicides (da Silva et al., 2012).
In post-harvest, Barbosa-Martínez et al. (2002) evaluated the effect of ozone, iodine and chlorine in the germination of spores of L. theobromae isolating the mango fruit and found that in the application of iodine (500 mg.L-1) the germination of spores of L. theobromae was of 10 %; meanwhile, in the application of ozone (2.2 mg.L-1) and chlorine (360 mg.L-1) the germination of spores was of 30 and 40 %, respectively.
Tovar et al. (2013) reported that the combination of washing and the subsequent application of tiabendazole reduced the incidence of the diseases caused by L. theobromae up to 81 % in sapote graft. The fungicides cyprodinil+fludioxinil, pyraclostrobin+boscalid, prochloraz, tebuconazole, and iprodione were efficient in inhibiting the mycelian growth of L. theobromae in vitro.
In citrus, Varela et al. (2013) reported the application of benomyl and copper oxychloride-based compounds against L. theobromae in the various cultivation stages. Canales (1998) suggests in order to control the cancer of the trunk and branches of mango, to carry out a surgery in the cancer until eliminating the damaged tissue and then apply Benlate(r), Tecto 60(r) or Derosal 50(r) in the wounds. Also with regard to dieback in mango, it is recommended to prune the wounds and spray with copper-based fungicides every 15-20 days. The products Captan, Maneb, Zineb and Benomyl may also be applied from the beginning of the flowering period up to a month before the harvest (Tucuch et al., 2005). Sanitary pruning and preventive treatment with copper oxychloride or sulfur as an active ingredient is recommended on cashew before beginning the flowering period; during the flowering period and the formation of the fruit, a systemic fungicide with a Fosetyl-al, Metalaxil-m or Triforine base may be used to reduce the incidence of necrosis on the fruit (Canales, 2007).
Conclusions
Lasiodiplodia theobromae is a pathogen with limited pathogenicity studies and a range of hosts in Mexico. The majority of the reports of L. theobromae have been based on morphological characteristics. The complex of cryptic species associated with L. theobromae require studies that associate morphological and molecular or genetic characteristics. The priorities of the investigation programs must focus on quantifying the impact that this fungus has on various crops, as well as determining the means of infection and the evaluation of susceptibility to chemical products in order to establish guidelines for an effective control strategy.
Acknowledgements
To project 211-163213 "El manejo integral del cultivo de papaya en México, un acercamiento innovador" financed by SAGARPA-CONACYT and to CONACYT for financing the studies of P.A. Picos-Muñoz.
REFERENCES
Abdollahzadeh J, Javadi A, Mohammadi-Goltapeh E, Zare R and Phillips AJL. 2010. Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia 25:1-10. [ Links ]
Al-Sadi AM, Al-Wehaibi AN, Al-Shariqi RM, Al-Hammadi MS, Al-Hosni I, Al-Mahmooli IH and Al-Ghaithi AG. 2013. Population genetic analysis reveals diversity in Lasiodiplodia species infecting date palm, citrus, and mango in Oman and the UAE. Plant Disease 97(10):1363-1369. [ Links ]
Alvarez, MG. 1976. Primer catálogo de enfermedades de plantas Mexicanas. Fitofilo 71: 169 p. [ Links ]
Alves A, Crous PW, Correia A and. Phillips AJL 2008. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Diversity 28:1-13. [ Links ]
Alvidrez-Villarreal R, Hernández-Castillo FD, Garcia-Martínez O, Mendoza-Villarreal R, Rodríguez-Herrera R y Aguilar CN. 2012. Isolation and pathogenicity of fungi associated to ambrosia borer (Euplatypus segnis) found injuring pecan (Carya illinoensis) wood. Agricultural Sciences 3:405-416. [ Links ]
Barbosa-Martínez C, León-García LP, Sepúlveda-Sánchez J and Nieto-Angel D. 2002. Effects of ozone, iodine and chlorine on spore germination of fungi isolated from mango fruits. Revista Mexicana de Fitopatología 20(1): 60-65. [ Links ]
Bautista-Baños S, Díaz-Pérez JC and Barrera-Necha L. 2002. Postharvest fungal rots of sapote mamey Pouteria sapota (Jacq.) H.E. More & Stearn. Postharvest Biology and Technology 24:197-200. [ Links ]
Becerra L. 1995. Enfermedades del cultivo de mango. Pp:83-101. : Mata B, Mosqueda V (eds.) La producción de mango en México. Editorial Limusa. México. 159 p. [ Links ]
Begoude BAD, Slippers B, Wingfield MJ and Roux J. 2010. Characterization of Botryosphaeriaceae and Cryphonectriaceae associated with Terminalia spp. in Africa, PhD thesis, University of Pretoria. 268p [ Links ]
Burgess TI, Barber PA, Mohali S, Pegg G, de Beer W and. Wingfield MJ 2006. Three new Lasiodiplodia spp. from the tropics, recognized based on DNA sequence comparisons and morphology. Mycologia 98:423-435. [ Links ]
Canales CR. 1998. Tecnología para la producción temprana de mango. 1 ed. Comité Editorial del Campo Experimental Edzná. 12 p. [ Links ]
Canales CR. 2007. Control de la necrosis en frutos de marañón Anacardium occidentalis en la península de Yucatán. Reporte anual de investigación e innovación tecnológica INIFAP. http://utep.inifap.gob.mx/tecnologias_agricolas.php (consulta, mayo 2014). [ Links ]
Cardoso J, Vidal J, dos Santos A, Freire F and Viana F. 2002. First report of branch dieback of cashew caused by Lasiodiplodia theobromae in Brazil. Plant Disease86(5):558. [ Links ]
Crous PW and Palm ME. 1999. Reassessment of the anamorph genera Botryodiplodia, Dothiorella and Fusicoccum. Sydowia 52:167-175. [ Links ]
Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Phillips AJL, Alves A, Burgess TI, Barber PAand Groenewald JZ. 2006. Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55:235-253. [ Links ]
Damm U, Crous PWand Fourie PH. 2007. Botryosphaeriaceae as potential pathogens of Prunus in South Africa, with descriptions of Diplodia africana and Lasiodiplodia plurivora sp. nov. Mycologia99:664-680. [ Links ]
Da Silva A, Brainer R, Michereff S, da Silva M and Saraiva M. 2012. Sensitivity of Lasiodiplodia theobromae from Brazilian papaya orchards to MBC and DMI fungicides. European Journal of Plant Pathology 132:489-498. [ Links ]
De los Santos de la RF, Becerra LEN, Mosqueda VR, Vásquez A y Vargas A. 2000. Manual de producción de papaya en el estado de Veracruz. Folleto Técnico No. 17. INIFAP-CIR 67 p. [ Links ]
Denman S, Crous PW, Taylor J, Kang C, Pascoe I and. Wingfield MJ 2000. An overview of the taxonomic history of Botryosphaeria and a re-evaluation of its anamorphs based on morphology and ITS rDNA phylogeny. Studies in Mycology45:125-140. [ Links ]
Gómez-Jaimes R, Nieto-Ángel D, Téliz-Ortiz D, Mora-Aguilera JA, Martínez-Damián MT and Vargas-Hernández M. 2009. Evaluación de la calidad e incidencia de hongos en frutos refrigerados de zapote mamey (Pouteria sapota (Jacq.) H. E. Moore and Stearn. Agrociencia 43:37-48. [ Links ]
Hawksworth D, Crous PW, Redhead S, Reynolds D, Samson R, Seifert K, Taylor J, Wingfield MJ, Abaci O, Aime C, Asan A, et al. 2011. The Amsterdam declaration on fungal nomenclature. IMA Fungus 2:105-112. [ Links ]
Hernández AM. 2010. Caracterización cualitativa de frutos de rambután (Nephelium lappaceum L.) almacenamiento postcosecha y patógenos asociados. Tesis doctoral. Colegio de Postgraduados. Montecillo, Estado de México. 50 p. [ Links ]
Hernández FLM, Gómez JR y Andrés AJ. 2013. Importancia, plagas insectiles y enfermedades fungosas del cultivo del guanábano. Campo Experimental Santiago Ixcuintla, INIFAP. Libro Técnico No. 1. Santiago Ixcuintla, Nayarit, México. 87 p. [ Links ]
Ismail AM, Cirvilleri G, Polizzi G, Crous PW, Groenewald JZand Lombard L. 2012. Lasiodiplodia species associated with dieback disease of mango (Mangifera indica) in Egypt. Australasian Plant Pathology 41:649-660. [ Links ]
Johnson GI, Mead AJ, Cooke AW and Dean JR. 1992. Mango stem end rot pathogens. Fruit infection by endophytic colonisation of the inflorescence and pedicel. Annals of Applied Biology 120:225-234. [ Links ]
Khanzada MA, Lodhi AM and Shahzad S. 2005. Chemical control of Lasiodiplodia theobromae, the causal agent of mango decline in Sindh. Pakistan Journal of Botany 37:1023-1030. [ Links ]
Kirk P, Stalpers J, Braun U, Crous PW, Hansen K, Hawksworth D, Hyde K, Lucking R, Lumbsch T, Rosman A, Seifert Kand Stadler M. 2013. A without-prejudice list of generic names of fungi for protection under the International Code of Nomenclature for algae, fungi, and plants. IMA Fungus4(2):381-443. [ Links ]
Li HY, Cao RB and Mu YT. 1995. In vitro inhibition of Botryosphaeria dothidea and Lasiodiplodia theobromae, and chemical control of gummosis disease of Japanese apricot and peach trees in Zhejiang Province, China. Crop Protection 14:187-191. [ Links ]
Liu A, Chen W and Li X. 2005. Changes in the postharvest physiology and lychee fruits latently infected by anthracnose fungus and the biological characteristic of the pathogenic fungus of the disease. Acta Horticulturae 665:365-371. [ Links ]
Liu J, Phookamsak R, Doilom M, Wikee S, Li Y, Ariyawansha H, Boonme S, Chomnunti P, Dai D, Bhat J, Romero AI, Zhuang W, Monkai J, Jones EBG, Chukeatirote E, Ko KTW, Zhao Y, Wang Y and Hyde KH. 2012. Towards a natural classification of Botryosphaeriales. Fungal Diversity57:149-210. [ Links ]
Lutchmeah RS. 1988. Lasiodiplodia theobromae causing fruit rot of Annona muricata in Mauritus. Plant Pathology 37:152. [ Links ]
Marques MW, Lima NB, Morais Junior MA, Barbosa MAG, Souza BO, Michereff SJ, Phillips JL and Câmara MPS. 2013. Species of Lasiodiplodia associated with mango in Brazil. Fungal Diversity61:181-193. [ Links ]
Martínez SC. 2010. Etiología e incidencia de hongos asociados al manchado de cálices de jamaica (Hibiscus sabdariffa L.) en Guerrero, México. Tesis de maestría. Colegio de postgraduados. Montecillo, Estado de México. 88 p. [ Links ]
Maslen MM, Collis T and Stuart R. 1996. Lasiodiplodia theobromae isolated from subcutaneous abscess in a Cambodian immigrant to Australia. Journal of Medical & Veterinary Mycology 34:279-283. [ Links ]
Mohali S, Burgess TIand. Wingfield MJ 2005. Diversity and host association of the tropical tree endophyte Lasiodiplodia theobromae revealed using simple sequence repeat markers. Forest Pathology 35:385-396. [ Links ]
Mosqueda VR, De los Santos RF, Becerra LEN, Cabrera M, Ortega ZDA y del Angel PAL. 1996. Manual para cultivar mango en la planicie costera del Golfo de México. Campo Experimental Cotaxtla. INIFAP-CIRGOC. Folleto Técnico No. 15. Veracruz, México. 130 p. [ Links ]
Netto MSB, Assuncao IP, Lima GSA, Marques MW, Lima WG, Monteiro JHA, de Queiroz BV, Michereff SJ, Phillips AJLand Camara MPS. 2014. Species of Lasiodiplodia associated with papaya stem-end rot in Brazil. http://dx.doi.org/10.1007/s13225-014-0279-4 (Consulta, febrero 2014). [ Links ]
Ochoa-Ascencio S, Vázquez M y Farías R. 2007. Identificación genético molecular de hongos asociados a la pudrición peduncular del fruto de aguacate en Michoacán, México. Memorias X Congreso Internacional/ XXXV Congreso Nacional de la Sociedad Mexicana de Fitopatología. Abs. [ Links ]
Pavlic D, Slippers B, Coutinho TA, Gryzenhout M and. Wingfield MJ 2004. Lasiodiplodia gonubiensis sp. nov., a new Botryosphaeria anamorph from native Syzygium cordatum in South Africa. Studies in Mycology50:313-322. [ Links ]
Pavlic D, Slippers B, Coutinho TAand. Wingfield MJ 2009. Molecular and phenotypic characterization of three phylogenetic species discovered within the Neofusicoccum parvum/N. ribis complex. Mycologia101:636-647. [ Links ]
Pegg KG, Coates LM, Korsten L and Harding RM. 2003. Foliar, fruits and soilborne diseases. p. 299-337. A. W. Whiley AW, Schaffer B and Wolstenholme BN (eds.). The Avocado: Botany, Production, and Uses. CABI Publishing, UK. 560 p [ Links ]
Perera E, Lago E. 1986. Effect of the light period on mycelial growth and pycnidia formation of Diplodia natalensis (Abstr.). Ciencias de la Agricultura 26:14-18 [ Links ]
Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, Akulov A and Crous PW 2008. Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia21:29-55. [ Links ]
Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZand Crous PW 2013. The Botryosphaeriaceae: genera and species known from culture. Studies in Mycology 76: 51-167. [ Links ]
Pitt J and Hocking A. 2009. Fungi and Food Spoilage. 3 ed. Springer. pp 125-127. [ Links ]
Ploetz RC. 2003. Diseases of Tropical Fruit Crops. CABI Pu blishing. Wallingford, UK. pp 76-77. [ Links ]
Queiroz F, Muniz M and Menezes M. 1997. Podridão da haste do mamoeiro 'Sunrise Solo' causada por Botryodiplodia theobromae no Estado de Alagoas. Summa Phytopathologica 23(1):44-45. [ Links ]
Rebell G and Forster RK. 1976. Lasiodiplodia theobromae as a cause keratomycoses. Sabouraudia 14:155-170. [ Links ]
Rico AL. 2011. Próximos cambios en la nomenclatura de algas, hongos y plantas. Boletín de la Sociedad Argentina de Botánica 46(3-4):381-385. [ Links ]
Rojo BI. 2013. Lasiodiplodia theobromae, Colletotrichum gloeosporioides y Colletotrichum capsici asociadas a pudrición del pedúnculo y antracnosis en papaya (Carica papaya L.). Tesis de Maestría en Ciencias. Centro de Investigación en Alimentación y Desarrollo A.C. Unidad Culiacán, Culiacán, Sinaloa. 69 p. [ Links ]
Romero CS. 1993. Hongos Fitopatógenos. Universidad Autonoma Chapingo 333 p. [ Links ]
Rubini MR, Silva-Ribeiro RT, Pomella AW, Maki CS, Araujo WL, dos Santos DR and Azevedo JL. 2005. Diversity of endophytic fungal community of cacao (Theobroma cacao L.) and biological control of Crinipellis perniciosa, causal agent of witches' broom diseaseInternational Journal of Biological Sciences 1:24-33. [ Links ]
Saha A, Mandal P, Dasgupta S and Saha D. 2008. Influence of culture media and environmental factors of mycelial growth and sporulation of Lasiodiplodia theobromae (Pat.) Griffon and Maubl. Journal of Environmental Biology 29:407-410. [ Links ]
Sakalidis ML, Ray JD, Lanoiselet V, Hardy GES and. Burgess TI 2011. Pathogenic Botryosphaeriaceae asociated with Mangifera indica in the Kimberley region of Western Australia. European Journal of Plant Pathology 130:379-391. [ Links ]
Sandoval-Sánchez M, Nieto-Ángel D, Sandoval-Islas JS, Téliz-Ortiz D, Orozco-Santos M, y Silva-Rojas HV. 2013. Hongos asociados a pudrición del pedúnculo y muerte descendente del mango (Mangifera indica L.). Agrociencia47:61-73. [ Links ]
Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW and Crous PW 2006. A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98:1041-1052. [ Links ]
Serrato-Díaz LM, Rivera-Vargas LI, Goenaga R and French-Monar RD. 2014. First report of Lasiodiplodia theobromae causing inflorescence blight and fruit rot of longan (Dimocarpus longan L.) in Puerto Rico. Plant Disease 98:279. [ Links ]
Shah MD, Verma KS, Singh K and Kaur R. 2010. Morphological, pathological and molecular variability in Botryodiplodia theobromae (Botryosphaeriaceae) isolates associated with die-back and bark canker of pear trees in Punjab, India. Genetics and Molecular Research 9 (2):1217-1228. [ Links ]
Shahbaz M, Iqbal Z, Sallem A and Anjum MA. 2009. Association of Lasiodiplodia theobromae with different decline disorders in mango (Mangifera indica L.). Pakistan Journal of Botany 41:359-368. [ Links ]
Sivakumar D, Wijeratnam RSW, Wijesundera RLC and Abeysekera M. 1997. Post-harvest diseases of rambutan (Nephelium lappaceum) in the western province. Journal of the National Science Council of Sri Lanka 25:225-229. [ Links ]
Slippers B, Crous PW, Denman S, Coutinho TA, Wingfield BD and Wingfield MJ 2004. Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 96:83-101. [ Links ]
Slippers B, Burgess T, Pavlic D, Ahumada R, Maleme H, Mohali S, Rodas C and Wingfield M. 2009. A diverse assemblage of Botryosphaeriaceae infect Eucalyptus in native and non-native environments. Southern Forests 71:101-110. [ Links ]
Slippers B, Boissin E, Phillips AJL, Groenewald JZ, Lombard L, Wingfield MJ, Postma A, Burgess Tand Crous PW 2013. Phylogenetic lineages in the Botryosphaeriales: a systematic and evolutionary framework. Studies in Mycology76:31-49. [ Links ]
Summerbell RC, Krajden S, Levine R and Fuksa M. 2004. Subcutaneous phaeohyphomycosis caused by Lasiodiplodia theobromae and successfully treated surgically. Medical Mycology 42:543-547. [ Links ]
Tamayo PJ. 2007. Enfermedades del aguacate. Politécnica 4:51-70. [ Links ]
Tovar PJM, Mora AJA, Nava DC, Téliz OD, Villegas MÁ y Leyva MSG. 2013. Control of Lasiodiplodia theobromae, the causal agent of dieback of sapote mamey [Pouteria sapota (Jacq.) H. E. Moore and Stearn] grafts in Mexico. Revista Fitotecnia Mexicana 36: 233-238. [ Links ]
Tucuch CFM, Palacios PA, Ku NR y Guzmán EC. 2005. Manejo del cultivo de mango en el estado de Campeche. Campo Experimental Edzna, INIFAP. Folleto Técnico. Campeche, Camp., México. 33-34p. [ Links ]
Umezurike G. 1979. The cellulolytic enzimes of Botryodiplodia theobromae Pat. Biochemistry Journal 177:9-19. [ Links ]
Úrbez-Torres JR, Leavitt GM, Guerrero JC, Guevara J and Gubler WD. 2008. Identification and pathogenicity of Lasiodiplodia theobromae and Diplodia seriata, the causal agents of bot canker disease of grapevines in Mexico. Plant Disease 92:519-529. [ Links ]
van Niekerk JM, Crous PW, Groenewald JZ, Fourie PHand Halleen F. 2004. DNA phylogeny, morphology and pathogenicity of Botryosphaeria species on grapevines. Mycologia 96:781-798. [ Links ]
Varela FSE, Orozco SM, Torres ARI y Silva AGL. 2013. Guía técnica para la identificación y manejo de plagas y enfermedades en cítricos. Universidad Autónoma de Tamaulipas 428 p. [ Links ]
Vásquez-López A, Mora-Aguilera JA, Cárdenas-Soriano E y. Téliz-Ortiz D 2009. Etiología e histopatología de la muerte descendente de árboles de mamey [Pouteria sapota (Jacq.) H. E. Moore y Stearn] en el estado de Guerrero, México. Agrociencia 43:717-728. [ Links ]
Ventura JA, Costa H and Tatagiba J. 2004. Papaya diseases and integrated control. p. 201-268. : Naqvi SAMH (ed.). Diseases of Fruits and Vegetables: Diagnosis and Management. Volume II. Kluwer Academic Publishers Dordrecht, United States of America. 704 p [ Links ]
Wang F, Zhao L, Li G, Huang J and Hsiang T. 2011. Identification and characterization of Botryosphaeria spp. causing gummosis of peach trees in Hubei Province, Central China. Plant Disease 95:1378-1384. [ Links ]
Wijayawardene N, Crous PW, Kirk P, Hawksworth D, Dai D, Boehm E, Boonmee S, Braun U, Chommunti P, D´Souza M, et al. 2014. Naming and outline of Dothideomycetes-2014. http://www.fungaltaxonomy.org/files/6813/9241/1345/Naming_and_Outline_of_Dothideomycetes_2014.pdf (consulta, mayo 2014) [ Links ]
Woo PC, Lau SK, Ngan AH, Tse H, Tung ET and Yuen KY. 2008. Lasiodiplodia theobromae pneumonia in a liver transplant recipient. Journal of Clinical Microbiology 46 (1):380-384. [ Links ]
Zhou S and Stanosz GR. 2001. Relationships among Botryosphaeria species and associated anamorphic fungi inferred from the analysis of ITS and 5.8S rDNA sequences. Mycologia 93:516-527. [ Links ]
Received: July 22, 2014; Accepted: December 25, 2014











 texto en
texto en