INTRODUCTION
Changes in the abundance and richness of species may affect the type and intensity of animal-plant interactions and may have cascading effects on the functioning of ecosystems (Ings et al., 2009; Barbour et al., 2016). Among the diversity of interactions, seed consumption by insects is the largest cause of plant mortality (Nurse et al., 2003; Nakagawa et al., 2005). Seed consumers reduce the quality and quantity of seeds produced by plants (Louda et al., 1990; Johnson et al., 2004). Nevertheless, in some cases when beetles do not affect the embryo, they may increase the viability of the seeds through mechanical scarification. These insects develop in the seeds leaving holes that facilitate the entry of water (Fox et al., 2010; Sanabria-Silva & Amarillo-Suárez, 2017). The seeds impose a strong evolutionary barrier for many insects because they are hard, have very little water content, have diverse chemical composition, as well as a great suite of secondary compounds (Amarillo-Suárez et al., 2017). In this way, seed-associated insect communities are expected to be very specific for each plant species.
Legumes are the dominant plant taxon in the tropical dry forest, offering a large amount of resources for insect specialization (Pizano & García, 2014). Hymenopteran parasitoids represent about 50% of the structure of the trophic networks in nature (Lasalle & Gauld, 1991). These interactions may be affected by several anthropogenic factors (Armbrecht, 1995; Didham et al., 1998; Hendrickx et al., 2007; González et al., 2015). Exotic species have a negative effect on the structure of interactions by, for example, increasing competition for resources with the native species (Didham et al., 2007), deviating natural generalist enemies of herbivores from native to exotic species that in turn increase herbivore abundance in the native species (Montero-Castaño & Vila, 2012). The introduction of exotic species can also affect the abundance of herbivores and therefore the abundance of their predators (Tallamy, 2004). Species that are generalists prefer to attack species (Parker & Hay, 2005), in consequence affecting the interaction networks (Sanabria-Silva & Amarillo-Suárez, 2017). In this study we compared the herbivores-parasitoid antagonist networks associated with two legume species the native Pseudosmanaea guachapele (Kunth) Harms, and the exotic and invasive Leucaena leucocephala (Lam.) de Wit, one of the 100 most invasive species in the world (Lowe et al., 2004) and in Colombia (Baptiste et al., 2010). The study took place in an area of tropical dry forest in Tolima, Colombia. We also compared the chemical composition of seeds in these two leguminous plants in order to establish possible relationships in the chemistry of the seeds and the community of insects associated with each species of legume.
MATERIALS AND METHODS
Study organisms. Pseudosamanea guachapele, Fabaceae is a Colombian native species that reaches 25 meters in height and one meter in trunk diameter. Its canopy is broad and has the shape of an umbrela. The tree produces white, hard seeds about ten mm long and five mm wide (Geilfus, 1989), and this species of tree is found in warm, temperate regions. In Colombia, the species is distributed in the Magdalena and Cauca River Valleys, the Atlantic coast, and in the watersheds of the Orinoco and Catatumbo rivers between sea level and 1,500 masl (Mahecha-Vega et al., 2012). This tree species has been found in ecosystems of tropical dry forest, tropical wet forest, and pre-montane wet forest. Usually, the tree´s wood is used as firewood. This species is also used to provide shade for crops such as cocoa, coffee and for livestock; and it is ideal for restoration of mature forests (CorAntioquia, 2008).
Leucaena leucocephala, Fabaceae is native to Central América and is considered an exotic invasive species in Colombia. The species is considered one of the 100 most harmful exotic invasive species worldwide (Lowe et al., 2004). It reaches 15 m in height and about 80 cm in diameter and develops brown seeds of 8 mm long by 5 mm wide (Mahecha-Vega et al., 2012). In Colombia the tree is located in warm and temperate areas from sea level to 1,300 meters in ecosystems such as tropical dry forest and tropical humid forest. It is broadly used in soil recovery and control of erosion and is also used as a living fence (Mahecha-Vega et al., 2012).
Data collection, experimental design and analyses. Sampling was carried out in the department of Tolima, Colombia during April 2015 the beginning of the dry season when we found the largest number of seeds available for the native P. guachapele. Leucaena leucocephala produce seeds all year around (senior author, personal observation for more than ten years). We collected seeds from L. leucocephala at 4° 15' N, 74° 44' W and from P. guachapele at 4° 15' N, 74° 37' W. From each tree species we collected mature seed pods (ten trees of P. guachapele and 20 trees of L. leucocephala) according to seed availability and from branches located at the four cardinal directions. The number of bags with seeds collected from each tree varied between one and five. Mature seed pods from each tree were preserved in hermetic plastic bags with a capacity of 3.7 L. All seeds were transported and processed at the Evolutionary Ecology and Conservation Laboratory at Pontificia Universidad Javeriana, Bogotá, Colombia.
Once in the laboratory, all ripe seed pods were opened. Seeds were set up in labeled hermetic plastic containers in a growth chamber at 28°C and 70% RH. Containers were inspected every 24 h for organisms emerging from the seeds; and once emerged, they were preserved in 97% alcohol. Insects were identified to the lowest possible taxonomic level using the keys and books of Borror & White (1970), Udayagiri and Wadhi (1982), Jhonson (1990), Fernández and Sharkey (2006), Manfio and Ribeiro-Costa (2016), and with the help of parasitoid wasp taxonomist Dr. Carlos Sarmiento from Universidad Nacional de Colombia and Curculionidae taxonomist Dr. Robert Anderson from Cannadian Museum of Nature.
To study the antagonistic interaction between herbivores insects and parasitoids in the exotic and native legume plants, we performed separate analyses for each host plant. We built two unweighted networks and calculated the following parameters for each one: (1) nestedness, a parameter that indicated whether a community was composed by subsets of generalist species interacting with each other, and specialist species interacting only with generalist species (Corzo et al., 2011). Values of nestedness ranged from a 100% to 0%; 100% was a perfectly nested network in which subsets of specialists interacted with generalists (Fortuna et al., 2010; Bascompte & Jordano, 2014); (2) connectance, defined as the proportion of interactions found in the network in relation to maximum potential interactions (Dunne et al., 2002; Delmas et al., 2019); (3) generality, which determined the average number of links of herbivorous per parasitoid (Blüthgen et al., 2008); and (4) vulnerability, which estimated the average number of links of parasitoids per herbivore (Blüghten et al., 2008). Networks were analyzed and plotted with the R program (R Development Core Team, 2013), bipartite package (Dormann et al., 2014).
We built one herbivore-parasitoid network for each legume plant. Regarding the type of herbivores that feed on the seeds (seed feeder), no direct interaction could be established between parasitoid species and a particular species of seed feeder. This was because more than one species of seed feeder emerged from the seeds. Thus, we generated record-based relationships by consulting literature on the associations between these trophic guilds (Hetz & Jhonson, 1988; Hagstrum & Subramanyam, 2009; Wood et al., 2016; Delgado-Machuca et al., 2019; Morales-Silva et al., 2019; Pérez-Benavides et al., 2019).
Last, we analyzed the chemical composition of the seeds in order to determine the differences between species The analysis of seeds was done according to the procedures described in detail by Amarillo et al. (2017). We used dry, clean seeds of P. guachapele and L. leucocephala with no evidence of insects developing in them. Differences in the composition of seeds were evaluated following the analyses proposed by Borges et al. (2008). We compared the abundance and type of chemical compounds between the two legume species with a Mann Whitney test. This analysis was performed with R software (R Development Core Team, 2013).
RESULTS
We collected a total of seven species of herbivores, all of them Coleoptera, and eight species of parasitoids, all of them Hymenoptera. Of this total, 85% of the herbivores and 100% of the parasitoid species were found in L. leucocephala, while 57% of the herbivorous and 87% of the parasitoid species were found in P. guachapele (Table 1). The seed feeding beetle Merobruchus paquetae (Kingsolver, 1980) was recently recorded emerging for the first time from seeds of the exotic legume L. leucocephala (Amarillo-Suárez & Camacho-Erazo, 2020). Two species of seed feeding beetles were exclusive to P. guachapele, and two were exclusive to L. leucocephala. Only one species of hymenopteran parasitoid was found in L. leucocephala. The remaining species of hymenopteran parasitoids were shared between the two networks (Table 1).
Table 1 Arthropods associated with the seeds of two legume species: the native species Pseudosamanea guachapele, and the invasive species Leucaena leucocephala in an area of tropical dry forest in Colombia. The "x" represents the presence of the taxon in the seeds of the host plant.
| Taxon | Trophic guild | Host | |
|---|---|---|---|
| L. leucocephala | P. guachapele | ||
| Coleoptera | |||
| Chrysomelidae | |||
| Merobruchus columbinus (Sharp, 1885) | Seed feeder | x | x |
| Acanthoscelides macrophthalmus (Schaeffer, 1907) | Seed feeder | x | |
| Merobruchus paquetae (Kingsolver, 1980) | Seed feeder | x | |
| Stator limbatus (Horn, 1873) | Seed feeder | x | |
| Curculionidae | |||
| Rhyssomatus sp. | Seed feeder | x | x |
| Cucujidae | |||
| Cucujidae sp. | Xylophagous | x | x |
| Cerambycidae | |||
| Cerambycidae sp. | Xylophagous | x | |
| Hymenoptera | |||
| Braconidae | |||
| Heterospilus sp. | Parasitoid | x | x |
| Stenocorse sudamericanus | Parasitoid | x | x |
| Eulophidae | |||
| Horismenus sp. | Parasitoid | x | x |
| Eurytomidae | |||
| Chryseida sp. | Parasitoid | x | x |
| Eupelmidae | |||
| Eupelmus sp. | Parasitoid | x | x |
| Ichneumonidae | |||
| Ichneumonidae sp. | Parasitoid | x | |
| Bethylidae | |||
| Bethylidae sp. | Parasitoid | x | x |
| Pteromalidae | |||
| Pteromalidae sp. | Parasitoid | x | x |
The interaction networks (Fig. 1) from both species had low connectance, nestedness, generality, and vulnerability. However, it is worth noting that the nestedness of the network in L. leucocephala was almost double than that in P. guachapele (Table 2).
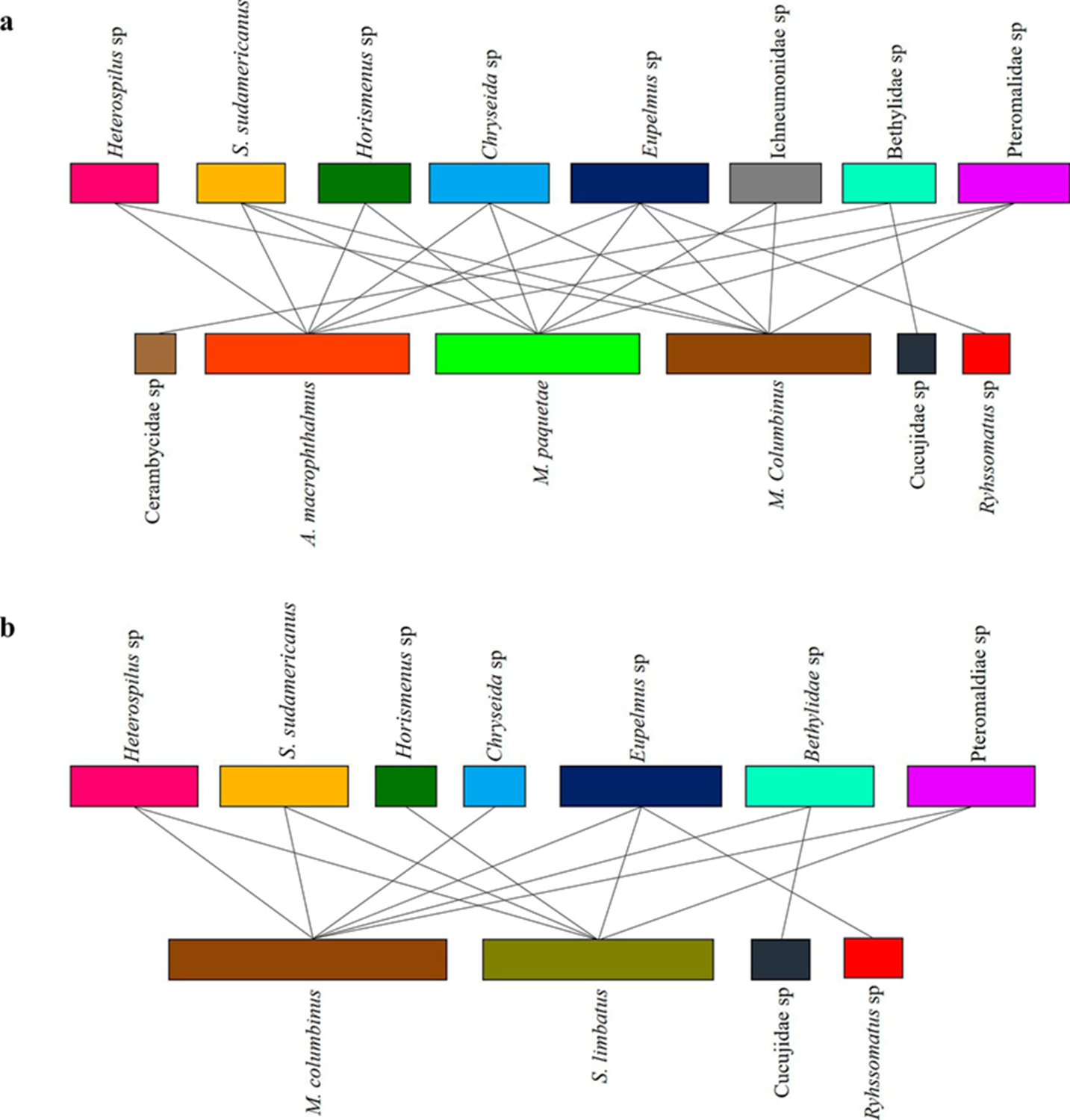
Figure 1 Antagonistic networks between herbivores and parasitoids associated with a) L. leucocephala and b) P. guachapele; each color represents a different species and the lines indicate the interaction between beetles and parasitoids.
Table 2 Metrics of the ecological networks of arthropods associated with two legume hosts in an area of tropical dry forest in Colombia. Parasit. Parasitoid richness Herb. Herbivore richness, N. Nestedness, C. Connectance, V. Vulnerability, G. Generality.
| Parasit. | Herb. | N | C | V | G | |
|---|---|---|---|---|---|---|
| L. leucocephala | 8 | 6 | 34% | 0,437 | 5,28 | 2,8 |
| P. guachapele | 7 | 4 | 18% | 0,464 | 4,84 | 2,07 |
We identified a total of 26 compounds in the seeds of the two hosts. The seeds of P. guachapele have twice as many compounds as those of L. leucocephala and the two species showed significant differences in chemical composition (Mann-Whitney U = 214.5, p = 0.02). Four steroid-type compounds, among which are campesterol, stigmasterol and gamma sitosterol (high concentration), were exclusive to L. leucocephala. Today those compounds are considered sources of cholesterol, which is essential to the growth and reproduction of insects (Behmer & Nes, 2003) and may additionally have an attractive effect on the bruchids found in the exotic species (Amarillo-Suarez & Camacho-Erazo, 2020). There were 16 compounds found in P. guachapele. The squalene, hexadecanoic acid, octadecanoic acid and some derivatives of the ester type compounds found in the native species P. guachapele are mainly precursors of fatty acids and they are necessary in metabolism (Lozano-Grande et al., 2018) (Table 3).
Table 3 Chemical compounds (%) found in the seeds of the two species Pseudosamanea guachapele and Leucaena leucocephala. RT, Retention Time.
| Name of the Compound | RT | P. guachapele | L. leucocephala |
|---|---|---|---|
| Decane | 3.46 | 0.21 | |
| Tetradecane | 8.32 | 0.25 | 0.83 |
| Hexadecane | 10.73 | 0.31 | 1.02 |
| Hexadecanoic Acid, Methyl Ester | 14.21 | 0.23 | 0.15 |
| Hexadecanoic Acid, Ethyl Ester | 14.87 | 1.77 | |
| 9,12 Octadecadienoic Acid (Z,Z) -,Methyl Ester | 15.83 | 0.78 | 0.16 |
| 9,12 Octadecadienoic Acid (Z,Z) | 16.3 | 4.88 | |
| 9,12 Octadecadienoic Acid (Z,Z) -,Ethyl Ester | 16.45 | 33.17 | |
| Octadecadienoic Acid | 16.59 | 1.25 | |
| Hexadienoic Acid Buthyl Ester | 16.64 | 4.83 | 0.37 |
| Isopropyl Linoleate | 18.1 | 3.42 | |
| Octadienoic Acid 2-Methylpropyl Ester | 18.35 | 0.56 | |
| Bacchotricuneatin C | 18.44 | 0.29 | |
| Tricosane | 19.24 | 0.34 | |
| 2 -Methyl -Z,Z -3,13-Octadecadienol | 19.6 | 0.15 | |
| Hexacosane | 20 | 0.24 | |
| Nonadecane | 20.47 | 0.13 | |
| Heptacosane | 20.75 | 1.34 | |
| Squalene | 21.71 | 0.36 | |
| Nonacosane | 22 | 1.18 | |
| Nonadecane | 22.38 | 1.27 | |
| Beta Tocopherol | 23.25 | 0.44 | |
| Vitamin E | 23.85 | 5.26 | 9.23 |
| Campesterol | 24.66 | 10.46 | |
| Stigmasterol | 24.86 | 15.25 | |
| Gama Sitosterol | 25.2 | 12.93 | 35.63 |
DISCUSSION
This study compared the antagonist networks between seed-consumer beetles and parasitoids of a native and an exotic host species. Exotic species can cause negative effects on native species, such as increased competition and deviation of herbivorous and parasitoids from native species (Didham et al., 2007). Parasitoid wasps exert top-down control over seed consumer communities (Cuevas-Reyes et al., 2007). If these parasitoids move towards exotic plant species, the rate of seed consumer invasion in native plant species would increase; there will be no control over these communities and consequently there will be greater seed predation, thus generating a decrease in the reproductive capacity of native plant species (Maron & Crone, 2006; West, 2012).
Although, we found no significant differences in the diversity of herbivores (X2 = 0.5; g. l, = 1 ; p > 0.05) and parasitoids (X2 = 0.13; g. l. = 1; p > 0.05) between the two species of legumes, this could be the case of the exotic species L. leucocephala that showed a greater diversity of herbivorous, and parasitoids compared to P. guachapele. Although we did not find a significant difference in the diversity of herbivores and parasitoids between the two species of legumes, the exotic species L. leucocephala showed higher diversity of herbivores and parasitoids compared to P. guachapele, perhaps because L. leucocephala produces seeds throughout the year (Grether et al., 2006; Sharratt & Olckers, 2012) while P. guachapele is markedly seasonal and produces fruits only for one or two periods during the year (Senior author, personal observations for more than ten years). Seasonality affects the structure of the community of insects associated with legume seeds, since in one species it facilitates permanent access to the resources available, in this case, the seeds of the exotic species L. leucocephala. In the absence of L. leucocephala, seasonal fluctuations in richness and composition of arthropods associated with native legume seeds are expected to occur.
One assumes that more insects consuming L. leucocephala seeds would help to control the species expansion. However, previous studies on seed germination in this species show that in addition to the known harmful effects of seed-eating beetles, bruchids leave a hole at the time of adult emergence. The hole increases water retention by seeds; and in cases where the embryo is alive, the germination time decreases (Fox et al., 2010; Sanabria-Silva & Amarillo-Suárez, 2017).
Acanthoscelides macrophthalmus (Schaeffer, 1907), a specialist bruchid of L. leucocephala with a distribution as extensive as its host plant, has been introduced in some places as a biological control agent of this leguminous plant (Sharratt & Olckers, 2012). However, evidence shows the efforts to control the exotic tree species are not sufficient (Tuda et al., 2009). Thus, L. leucocephala remains among the 100 most invasive species worldwide (Lowe et al., 2004) as well as in Colombia (Cardenas et al., 2017). Breeding experiments conducted by Castro (2014) show that A. macrophthalmus cannot use the native legumes P. guachapele, Senegalia riparia (Kunth), Parkinsonia aculeata (L.) and Acacia farnesiana (L.) that are sympatric with L. leucocephala for its development. However, L. leucocephala has acquired new parasitoids of A. macrophthalmus such as Stenocorse sudamericanus. Recent phylogenetic studies show that there is more than one species of Stenocorse. Stenocorse sudamericanus, is only found in Colombia (Delgado-Machuca et al., 2019; Zaldivar-Riverón et al., 2019), and is a parasitoid of seed beetles that parasitizes native legume seeds such as P. guachapele, S. riparia, Chloroleucon bogotense Britton & Killip, and A. farnesiana (Amarillo-Suárez, 2010; Pizano & García, 2014; Sanabria-Silva & Amarillo-Suárez, 2017). Thus, the native parasitoid Stenocorse sudamericanus successfully managed to colonize an exotic species such as L. leucocephala.
With regard to interactional networks, the more diversity there is, there will be greater connection in the structure of the communities that promotes the persistence and resilience of networks (Sauve et al., 2014). The intermediate values of connectance of our networks show that there is high stability in the communities of herbivores and parasitoids, since the dispersion of disturbances is limited (Kolchinsky et al., 2015). However, it also means that a small proportion of possible links occurs (Dáttilo & Rico-Gray, 2018) generating simplified networks, given the low richness of interacting species (Table 2). In our case the dispersion of disturbances is limited due to the low species richness, thus generating simplified networks. We found that most parasitoid species have more than one host insect, which explains the low levels of nestedness and suggests competition among the parasitoids that behave in both host plants as generalists. This could make the entire community more vulnerable, as supported by the values obtained in the vulnerability parameter of both networks (Dáttilo & Rico-Gray, 2018) (Table 3).
In addition, our networks are small and have low insect diversity because they are immersed in a remnant of tropical dry forest that has been used for livestock. Fragmentation generates cascading effects on the structure and stability of networks (Grass et al., 2018). Thus, some network parameters are more sensitive to network size (Vanbergen et al., 2017) since the vulnerability of having a small network with fewer herbivores per parasitoid. In our case we found the opposite, probably because the parasitoids we collected were generalist’s species (Fernández & Sharkey, 2006). For metrics such as vulnerability and generality it is important to consider the size of the network since they are very sensitive to the absolute sampling effort; that is, the larger the network, the more that observations per species will decrease as the number of possible links or connectivity increases (Kenny & Loehle, 1991; Blüthgen et al., 2008). In our case, we obtained low generality values, explained because we found fewer herbivores than parasitoids.
For metrics such as vulnerability and generality it is important to consider the size of the network since they are very sensitive to the absolute sampling effort, that is, the larger the network, the more the number of observations per species will decrease as the number of possible links or connectivity increases (Kenny & Loehle, 1991; Blüthgen et al., 2008). In our case, we obtained low generality values that can be explained because we found fewer herbivores than parasitoids.
Regarding the chemical composition of seeds, we found that the steroidal compounds campesterol, and stigmasterol are exclusive to L. leucocephala, and gamma sitosrerol almost triplicates its concentration in L. leucocephala. These compounds are a source of cholesterol, an essential molecule for the development, growth, and reproduction of insects (Behmer & Nes, 2003). Cholesterol must be dealkilated in order to be used by insects in their metabolism (Behmer & Elias, 2000). Pseudosamanea guachapele, the native species, has other sources such as squalene and hexadecanoic acid, which makes the production of fatty acids for metabolism easier (Lozano-Grande et al., 2018). Besides these large differences in chemical composition of seeds, it appears that it has effects on the specialist bruchids A. macrophthalmus and M. paquetae that only are found in seeds of L. leucocephala (Amarillo-Suarez & Camacho-Erazo, 2020), while the seed beetle Stator limbatus (Horn, 1873) only uses seeds of P. guachapele and other native legume plants in the same family (Amarillo-Suarez & Fox, 2006; Sanabria & Amarillo-Suarez, 2017).
Because ecological networks change across space (Bascompte & Jordano, 2014), across time (Olesen et al., 2008; Carnicer et al., 2009), and across host plants, as in this case, we expected that the networks we described here also change across space and time. Thus, it is necessary to continue the analysis of this variation at different spatial and temporal scales in order to measure the magnitude of these changes and to determine community effects at a broader scale. This will allow us to infer broader patterns of interactions and to develop management practices for exotic and native species in different landscapes and human altered ecosystems.
CONCLUSIONS
Invasive species such as L. leucocephala have managed to enter the pre-established ecological networks in the ecosystem, diverting parasitoids such as Stenocorse sudamericanus, native Colombian species that managed to successfully colonize an exotic species. In addition to this the ecological networks represented were immersed in a fragment of tropical dry forest which could cause the low richness and diversity that we found. Since the fragile tropical dry forest is home to the greatest diversity of legumes in the world (Gentry, 1995) and Colombia (Pizano & Garcia, 2014), it seemed necessary to continue studies that analyzed the several ways in which the exotic species L. leucocephala had become incorporated into ecological networks, like its interactions with pollinators, micorryzes, competition with other exotics, and native legumes, etc. This would allow the establishment of more responsible plans for the use and management of this legume, considered worldwide and nationally one of the 100 most harmful invasive species (Lowe et al., 2004). Only in this way can responsibly plans for the use and management of this harmful legume be defined (Lowe et al., 2004).











 nueva página del texto (beta)
nueva página del texto (beta)



