Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta zoológica mexicana
versión On-line ISSN 2448-8445versión impresa ISSN 0065-1737
Acta Zool. Mex no.81 Xalapa dic. 2000
Article
Effect of nectar-foraging ants and wasps on the reproductive fitness of Turnera ulmifolia (Turneraceae) in a coastal sand dune in Mexico
Leonel Torres-Hernández1, Victor Rico-Gray2, Citlalli Castillo-Guevara2 and Judith A. Vergara2
1Instituto de Investigaciones Biológicas, Universidad Veracruzana, Apartado Postal 294, Xalapa, Veracruz 91000, MEXICO.
2Departamento de Ecología Vegetal, Instituto de Ecología, A.C. Apartado Postal 63, Xalapa, Veracruz 91000, MEXICO.
Recibido: 5 de marzo 1999
Aceptado: 28 de junio 2000
Resumen
Evaluamos durante dos años el efecto de diferentes especies de hormigas sobre el éxito reproductivo (estimado como número total de frutos por temporada) de Turnera ulmifolia. El estudio se llevó a cabo en el matorral de duna costera en la costa central del estado de Veracruz, México. Los resultados muestran que (i) plantas asociadas con la especie de hormiga más grande (Camponotus abdominalis) produjeron más frutos que plantas asociadas con las especies de hormiga más pequeñas, y (ii) plantas asociadas con las hormigas sufrieron niveles de herbivoría menores, que plantas sin hormigas. Consequentemente, la presencia de hormigas no es sinónimo de protección para una planta, y el nivel de protección por hormigas dependerá del tamaño de las hormigas obreras en un gremio de hormigas visitantes. Más aún, cuando se excluyeron a las hormigas, aumentaron las visitas de avispas y abejas, las que efectuaron un nivel de protección mayor que aquel ofrecido por las especies pequeñas de hormigas. Sin embargo, el efecto diferencial de las avispas requiere atención futura.
Palabras clave: Interacciones hormiga-planta. Mutualismo. Turnera.
Abstract
We evaluated over two years the effect of different ant species on the reproductive fitness (estimated as end-of-season fruit set per treatment) of Turnera ulmifolia. Research was done on a sand dune matorral located on the coast of Veracruz, México. The results show that (i) plants associated with the larger ant species (Camponotus abdominalis) produced more fruits than plants associated with the smaller ant species, and (ii) plants associated with ants were subject to lower levels of herbivory, than plants without ants. Consequently, ant presence is not synonymous of plant protection, and the level of protection by ants will depend on the size of the worker ants in a guild of ant visitors. Moreover, when ants were excluded, wasps and bees increased their visits, exerting a higher level of protection than that offered by the smaller ant species. However, the differential effect of wasps needs further attention.
Key words: Ant-plant interactions. Mutualism. Turnera.
Introduction
A number of plants bearing extrafloral nectaries have been shown to be protected by nectar foraging ants (e.g. Koptur, 1984, Rico-Gray & Thien 1989a, Costa et al. 1992, Koptur et al. 1998, Oliveira et al. 1999); however, protection by ants is not universal (e.g. Rico-Gray & Thien 1989b). The effect of ants on the outcome of an interaction with plants, or the number of ant-plant interactiones can vary among sites (Bentley 1976, Barton 1986, Rico-Gray et al. 1998), between seasons (Rico-Gray & Sternberg 1991, Rico-Gray 1993), or will depend on weather conditions (Rico-Gray & Castro 1996, Rico-Gray et al. 1998). Thus, in order to assess the effects of ants on plant defense, and eventually on fruit and seed production, the above factors should be considered (Koptur 1992, Whitman 1994). Turnera ulmifolia L. (Turneraceae) is a polymorphic polyploid complex of herbaceous, perennial weeds, bearing extrafloral nectaries, and native throughout much of the neotropics (Gama et al. 1985, Barrett & Shore 1987, Keeler 1989, Baker & Shore 1995).
The results of a previous allelochemical survey (R. Mata, unpubl. data; Torres-Hernández 1995) showed that T. ulmifolia does not exhibit a significant chemical arsenal to deter herbivores. Since there is usually a trade-off in plant defenses, i.e., a lack of redundancy of defenses that act over the same temporal, spatial, and/or herbivore scales (e.g. McKey 1989, Davidson & Fisher 1991, Ågren & Schemske 1993, but see Steward & Keeler 1988) we hypothesized that ants visiting extrafloral nectaries were responsible for plant defense against herbivores. Here we evaluate the effect of different ant species on the reproductive fitness (estimated as end-of-season fruit set per treatment) of T. ulmifolia over two seasons. We included the different ant species visiting the individuals of T. ulmifolia in the study site because an increase in plant fitness may depend on the different components of an ant assemblage; usually larger ants offer better protection against insect herbivores than smaller ants (Schemske 1982, Horvitz & Schemske 1984, Oliveira et al. 1987, Koptur & Lawton 1988, Rico-Gray & Thien 1989a).
Materials and methods
Field observations were made at Centro de Investigaciones Costeras La Mancha, located on the coast of the state of Veracruz, Mexico (19/ 36' N, 96/ 22' W; elevation is < 100 m). Sampling was done in the sand dune matorral, where plant species composition varies depending on sand mobility, and protection to wind and salt spray. Common species are: Caesalpinia crista, Chamaecrista chamaecristoides (Leguminosae), Hibiscus tiliaceus (Malvaceae), Opuntia stricta (Cactaceae), Palafoxia texana (Compositae), Paullinia fuscescens (Sapindaceae), and Turnera ulmifolia (Turneraceae) (Moreno-Casasola et al. 1982, Dubroeucq et al. 1992). The climate is warm and subhumid; mean annual temperature is 24/-26/C, and a rainy season occurs between June and September. Total precipitation varies between years, for example, it was higher in 1992 (1774.5 mm), compared to both 1991 (1503.2 mm) and 1993 (1501.5 mm).
Turnera ulmifolia inhabits a variety of vegetation associations, exhibiting two contrasting patterns of floral morphology, where populations are either dimorphic or monomorphic for a range of floral traits (e.g. style length, stamen height, pollen size) (Barrett & Shore 1987). In the study site T. ulmifolia grows on the semistabilized and stabilized sand dunes, is monomorphic, self-compatible with long styles and a range of stamen heights, they flower and fruit year-around, with a peak during the Summer (rainy season) (Torres-Hernández 1995). Branches grow continuously from an apical meristem, producing leaves regularly, flowers are axilar, and one to three flowers are in anthesis per day; not all leaves are associated with flowers (Gama et al. 1985, Torres-Hernández 1995). Flowers remain in anthesis less than a day, and the associated leaf remains throughout fruit development. Extrafloral nectaries (EFN) are located at both sides of the petiole, close to the insertion of the floral pedicel in leaves with flowers; the nectar produced is a balanced solution of sucrose, glucose and fructuose (Elias et al. 1975, Elias 1983). Ants (Camponotus planatus, C. abdominalis, Conomyrma sp., Crematogaster brevispinosa, Forelius sp., Pseudomyrmex sp.), wasps (Polistes sp. and an undetermined species), and honey bees (Apis mellifera) forage for the nectar produced by the EFN's. The main leaf herbivore is a caterpillar (Euptoieta claudia, Lepidoptera: Nymphalidae), which is highly active between June and August. A previous survey determined that the experimental removal of >50% of leaf area significantly reduces fruit production (Torres-Hernández 1995).
The effect of ant presence on fruit production by Turnera ulmifolia was evaluated using ant-exclusion experiments. In August 1991 we selected five sets of T. ulmifolia individuals (3-4 plants/set) and marked 30 branches per set. We favored branches over individuals as sampling units because of the difficulty to find more than 4-5 plants visited by the same ant species (which is influenced by nest location) under similar microenvironmental conditions. The use of branches as sampling unit is considered appropiate in descriptive systems because plants are basically modular organisms (Harper 1977), they are somewhat physiologically autonomous (Casper & Niesenbaum 1993, Niesenbaum 1993), and site-specific effects can be reduced (Herrera, 1995). The T. ulmifolia individuals selected were in the sun and on the top part of sand dune slopes. The first three sets were each visited by a different ant species (set 1 Camponotus planatus, set 2 C. abdominalis, set 3 Conomyrma sp.), set 4 was visited by a mixed group of species (Conomyrma sp., Crematogaster brevispinosa, Forelius sp.), and we eliminated and blocked future ant access from plants in set 5. Plants were censused biweekly between August 1991 and July 1992 to make sure that ants had not gained access to the experimental plants, and to count fruits on all marked branches (an individual fruit was counted only once); fruit counts per branch were pooled per set and per month. Ant access was blocked from branches of set 5, by applying a band of Tree Tanglefoot (The Tanglefoot Co., Jackson, MS) on the older tissues at the base of each stem, and by pruning all plant parts of other species that could be used by ants as bridges (Rico-Gray & Thien 1989a).
In June 1993, a drier year, we established the second exclusion experiment; however, ants behaved differently, no one species dominated all branches of an individual plant. Consequently, we selected 40 plants which were divided into two sets (20 with ants and 20 with ants excluded), and randomly chose three branches per plant. Every two weeks during four months (June-September, peak flowering and fruiting for T. ulmifolia) we identified ant species (Camponotus planatus, Conomyrma sp., Crematogaster brevispinosa, Forelius sp., Pseudomyrmex sp.), counted fruits per branch, and, to estimate natural herbivory, we assigned a category of herbivore damage per plant (0%, 1-10%, 10-20%, 20-40%, 40-60%, >60%, of leaf tissue per plant). Unfortunately, due to the harsh conditions prevalent in 1993, we lost 14 plants, statistical analyses were done with data obtained from the remaining individuals (18 for plants with ants, 8 for plants without ants); fruit counts per branch were not pooled for the analyses.
Results
The number of fruits produced in the first year by the marked individuals of Turnera ulmifolia under different treatments are shown in Table 1. Fruits were produced throughout the year, however production was not uniform. Plants whose branches were visited by C. abdominalis, the largest ant visiting T. ulmifolia, produced significantly more fruits, than plants visited by any other ant species or those without ants (Kruskal-Wallis, H= 22.158, df= 4, P< 0.001; SigmaStat, 1995). The plants where ants had been experimentally removed produced significantly more fruits, than plants with the ant species mix, Camponotus planatus or Conomyrma sp. (Student-Newman-Keuls, P< 0.05; SigmaStat, 1995). We did not find significant differences in fruit production between the plants visited by C. planatus, Conomyrma sp., or the ant species mix (Student-Newman-Keuls, P > 0.05; SigmaStat, 1995).

The percent of leaf tissue removed by herbivores (herbivory level) and the number of fruits produced per month during the second year by the marked individuals of T. ulmifolia with and without ants, are shown in Table 2.
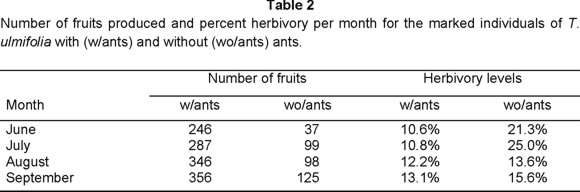
The percent of leaf tissue removed was significantly lower in plants with ants than in plants with ants excluded (Kruskal-Wallis H= 37.272, df= 1, P< 0.001; SigmaStat, 1995). Also, plants with ants produced significantly more fruits than plants with ants excluded (Kruskal-Wallis H= 12.835, df= 1, P< 0.001; SigmaStat, 1995). Although not statistically significant (R2= 0.358, F= 3.342, P= 0.117; SigmaStat 1995), there is a tendency showing more fruits (X) when herbivory (Y) is lower (SigmaStat 1995).
Discussion
Fruit production by Turnera ulmifolia was related to which ant species was present. Plants produced significantly more fruits when the larger ant species was present (Camponotus abdominalis), and significantly less fruits when the smaller ant species were present. In general, small ants have been associated to poor plant defense (Schemske 1982, Horvitz & Schemske 1984, Oliveira et al. 1987, Koptur & Lawton 1988, Rico-Gray & Thien 1989a). Interestingly, however, fruit production was higher in plants where ants had been excluded, than in plants with ants (except for plants with C. abdominalis). Ant exclusion allowed for the nectaries of these plants to be actively visited by wasps and bees. Most adult predatory or parasitoid wasps feed on floral or extrafloral nectar, thus the plants to which they are attracted are often their principal hunting grounds, creating the potential for a competitive interaction for nectaries between ants and wasps (Beattie 1985, Domínguez et al. 1989, Koptur 1992, Whitman 1994, Cardel et al. 1997). Since ants tending extrafloral nectaries may limit the presence of predatory and/or parasitoid wasps and flies (Koptur & Lawton 1988, Pemberton & Lee 1996), the interaction of these organisms with ants could change the level of defense and the overall outcome for the plant. Our observations indicate that wasps visited the plants of T. ulmifolia to hunt and to forage for nectar. However, nectar consumption by wasps was lower relative to consumption by ants, consequently there was an excess of nectar which runned down the surface of the branch, allowing for the colonization by fungus (Capnodium sp. Dothiliales: Ascomycotina). Finally, butterflies visually recognize potential egg predators, such as ants, and actively choose those sites that are better for egg-laying, thus diminishing the risk of death of their offspring (Freitas & Oliveira 1996). It is quite possible that the adults of Euptoieta claudia also recognize the wasps and thus only oviposit on T. ulmifolia plants without wasps.
In summary, our results show that plants associated with the larger ant species produced more fruits than plants associated with the smaller ant species, and that plants associated with ants were subject to lower levels of herbivory. Consequently, ant presence is not synonymous of plant protection, and the level of protection by ants will depend on the size of the worker ants in a guild of ant visitors. Moreover, when ants were excluded, wasps and bees increased their visits, exerting a higher level of protection (i.e. more fruits produced) than that offered by the smaller ant species. In general, ant-plant associations are largely fortuitous, diffuse, and facultative, an selective benefits should accrue to plants that attract a broad array of ants (and other insects for that matter). The greater the diversity of ants, the greater the variety of plant enemies they are likely to remove, and the greater the probability that in any given habitat, season, or time of day, some ant species will forage on the plant.
Acknowledgments
We thank Mónica Palacios-Rios for caterpillar determination, Gloria Carrión for fungus determination, Rachel Mata-Essayang for performing the allelochemical survey, and Paulo S. Oliveira and two anonymous reviewers for comments and suggestions to the manuscript. Field work was supported by CONACYT No. 903579, 1259-N9204, and 0137-N9506 to VRG, and Instituto de Ecología, A.C. No. 902-16.
Literature cited
Ågren, J. & D.W. Schemske. 1993. The cost of defense against herbivores: an experimental study of trichome production in Brassica rapa. Am. Nat. 141:338-350. [ Links ]
Baker, A.M. & J.S. Shore. 1995. Pollen competition in Turnera ulmifolia (Turneraceae). Am. J. Bot. 82:717-725. [ Links ]
Barrett, S.C.H. & J.S. Shore. 1987. Variation and evolution of breeding systems in the Turnera ulmifolia L. complex (Turneraceae). Evolution 41:340-354. [ Links ]
Barton, A.M. 1986. Spatial variation in the effect of ants on an extrafloral nectary plant. Ecology 67:495-504. [ Links ]
Beattie, A.J. 1985. The Evolutionary Ecology of Ant-Plant Mutualisms. Cambridge University Press, Cambridge. 182 pp. [ Links ]
Bentley, B.L. 1976. Plants bearing extrafloral nectaries and the associated ant community: interhabitat differences in the reduction of herbivore damage. Ecology 57:815-820. [ Links ]
Cardel, Y., V. Rico-Gray, J.G. García-Franco & L.B. Thien. 1997. Ecological status of Beaucarnea gracilis, an endemic species of the semiarid Tehuacan Valley, Mexico. Cons. Biol. 11:367-374. [ Links ]
Casper, B.B. & R.A. Niesenbaum. 1993. Pollen versus resource limitation of seed production: a reconsideration. Cur. Sci. 65:210-214. [ Links ]
Costa, F.M.C.B., A.T. Oliveira-Filho & P.S. Oliveira. 1992. The role of extrafloral nectaries in Qualea grandiflora (Vochysiaceae) in limiting herbivory: an experiment of ant protection in cerrado vegetation. Ecol. Entomol. 17:363-365. [ Links ]
Davidson, D.W. & B.L. Fisher. 1991. Symbiosis of ants with Cecropia as a function of light regime. In: C.R. Huxley & D.F. Cutler (Eds.). Ant-Plant Interactions. Oxford University Press, Oxford. pp. 289-309. [ Links ]
Domínguez, C.A., R. Dirzo & S.H. Bullock. 1989. On the function of floral nectar in Croton suberosus (Euphorbiaceae). Oikos 56:109-114. [ Links ]
Dubroeucq, D., D. Geissert, P. Moreno-Casasola & G. Millot. 1992. Soil evolution and plant communities in coastal dunes near Veracruz, Mexico. Cah. Orstom. Sér. Pédol. 27:237-250. [ Links ]
Elias, T.S. 1983. Extrafloral nectaries: their structure and functions. In: B. Bentley & T.S. Elias T.S. (Ed.). The Biology of Nectaries. Columbia University Press, New York. pp. 174-203. [ Links ]
Elias, T.S., W.R. Rozich & L. Newcombe. 1975. The foliar and floral nectaries of Turnera ulmifolia L. Am. J. Bot. 62:570-576. [ Links ]
Freitas, A.V.L. & P.S. Oliveira. 1996. Ants as selective agents on herbivore biology: effects on the behaviour of a non-myrmecophilous butterfly. J. An. Ecol. 65:205-210. [ Links ]
Gama, L., H. Narave & N.P. Moreno. 1985. Turneraceae. In: A. Gómez-Pompa & N. P. Moreno (Eds.). Flora de Veracruz (fasc. 47). INIREB, México. [ Links ]
Harper, J.L. 1977. Population Biology of Plants. Academic Press, New York. 892 pp. [ Links ]
Herrera, C.M. 1995. Microclimate and individual variation in pollinators: flowering plants are more than their flowers. Ecology 76:1516-1524. [ Links ]
Horvitz, C.C. & D.W. Schemske. 1984. Effects of ants and an ant-tended herbivore on seed production of a neotropical herb. Ecology 65: 1369-1378. [ Links ]
Keeler, K.H. 1989. Ant-plant interactions. In: W.G. Abrahamson (Ed.). Plant-Animal Interactions. McGraw-Hill, New York. pp 207-242. [ Links ]
Koptur, S. 1984. Experimental evidence for defense of Inga saplings (Mimosoideae) by ants. Ecology 65:1787-1793. [ Links ]
––––––––––. 1992. Extrafloral nectary-mediated interactions between insects and plants. In: E. Bernays (Ed.). Insect-Plant Interactions (Volume IV). CRC Press, Boca Raton. pp. 81-129. [ Links ]
Koptur, S. & J.H. Lawton. 1988. Interactions among vetches bearing extrafloral nectaries, their biotic protective agents, and herbivores. Ecology 69:278-283. [ Links ]
Koptur, S., V. Rico-Gray & M. Palacios-Rios. 1998. Ant protection of the nectaried fern Polypodium plebeium in central México. Am. J. Bot. 85:736-739. [ Links ]
McKey, D. 1989. Interactions between ants and leguminous plants. In: C.H. Stirton & J.L. Zarucchi (Eds.). Advances in Legume Biology. Monographs in Systematic Botany, 29. Missouri Botanical Garden, St. Louis, Missouri. pp. 673-718. [ Links ]
Moreno-Casasola, P., E. van der Maarel, S. Castillo, M.G. Huesca & I. Pisanty. 1982. Ecologia de la vegetacion de dunas costeras; estructuras y composicion en el Morro de la Mancha, Ver. Biotica 7:491-526. [ Links ]
Niesenbaum, R.A. 1993. Light or pollen-seasonal limitations on female reproductive success in the understory shrub Lindera benzoin. J. Ecol. 81:315-323. [ Links ]
Oliveira, P.S., A.F. da Silva & A.B. Martins. 1987. Ant foraging on extrafloral nectaries of Qualea grandiflora (Vochysiaceae) in cerrado vegetation: ants as potential antiherbivore agents. Oecologia 74:228-230. [ Links ]
Oliveira, P.S., V. Rico-Gray, C. Díaz-Castelazo & C. Castillo-Guevara. 1999. Interaction between ants, extrafloral nectaries and insect herbivores in Neotropical coastal sand dunes: herbivore deterrence by visiting ants increases fruit set in Opuntia stricta (Cactaceae). Fun. Ecol. 13:623-631. [ Links ]
Pemberton, R.W. & J.H. Lee. 1996. The influence of extrafloral nectaries on parasitism of an insect herbivore. Am. J. Bot. 83:1187-1194. [ Links ]
Rico-Gray, V. 1993. Use of plant-derived food resources by ants in the dry tropical lowland of coastal Veracruz, Mexico. Biotropica 25:301-315. [ Links ]
Rico-Gray, V. & G. Castro. 1996. Effect of an ant-aphid-plant interaction on the reproductive fitness of Paullinia fuscescens (Sapindaceae). Southwest. Nat. 41:434-440. [ Links ]
Rico-Gray, V. & L. da S.L. Sternberg. 1991. Carbon isotopic evidence for seasonal change in feeding habits of Camponotus planatus Roger (Formicidae) in Yucatan, Mexico. Biotropica 23:93-95. [ Links ]
Rico-Gray, V. & L.B. Thien. 1989a. Effect of different ant species on the reproductive fitness of Schomburgkia tibicinis (Orchidaceae). Oecologia 81:487-489. [ Links ]
––––––––––. 1989b. Ant-mealybug interaction decreases reproductive fitness of Schomburgkia tibicinis Bateman (Orchidaceae) in Mexico. J. Trop. Ecol. 5:109-112. [ Links ]
Rico-Gray, V., J.G. García-Franco, M. Palacios-Rios, C. Díaz-Castelazo, V. Parra-Tabla & J.A. Navarro. 1998. Geographical and seasonal variation in the richness of ant-plant interactions in Mexico. Biotropica 30:190-200. [ Links ]
Schemske, D.W. 1982. Ecological correlates of a neotropical mutualism: ant assemblages at Costus extrafloral nectaries. Ecology 63:932-941. [ Links ]
SigmaStat. 1995. SigmaStat, statistical software v. 2. Jandel Scientific Software, San Rafael, California. [ Links ]
Steward, J.L. & K.H. Keeler. 1988. Are there trade-offs among antiherbivore defenses in Ipomoea (Convolvulaceae)? Oikos 53:79-86. [ Links ]
Torres-Hernández, L. 1995. Efecto de diferentes especies de hormiga sobre el exito reproductivo de Turnera ulmifolia L. (Turneraceae). Unpublished M.Sc. thesis, Universidad Nacional Autonoma de Mexico, México. [ Links ]
Whitman, D. 1994. Plant bodygards: mutualistic interactions between plants and the third trophic level. In: T.N. Ananthakrishnan (Ed.). Functional Dynamics of Phytophagous Insects. Oxford & IBH Publishing Co. PVT. Ltd., New Delhi, India. Pp. 207-248. [ Links ]














