INTRODUCTION
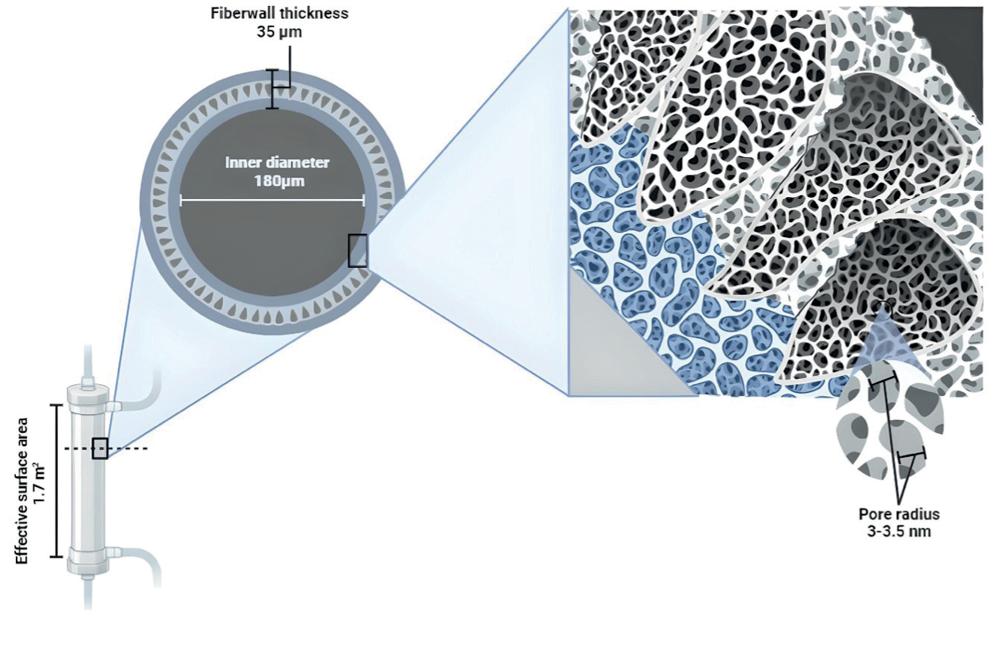
Medium cut-off (MCO) membranes are a novel generation of dialyzers manufactured with polyarylethersulfone/polyvinylpyrrolidone, and their mean pore radius is 5 nm, a value between high-flux (HF) and high cut-off (HCO) membranes1. Their pore size and distribution are similar to the glomerular basement membrane, with an effective radius between 3 and 3.5 nm, a cut-off that is close to the molecular weight of albumin and a high retention onset, so in summary, they allow better removal of medium-sized molecules without increasing albumin losses2,3. Hemodialysis (HD) treatment with MCO membranes has also been referred to as expanded HD (HDx®) given its broader range of solute removal4,5. This modality seems attractive given its enhanced permeability, selective solute retention, and superior internal retrofiltration (back filtration), resulting from combined diffusive and convective clearance within the same dialyzer, without replacement fluid, as with online hemodiafiltration (OL-HDF) (Fig. 1).
Herein, we describe their effects on the clearance of uremic toxins (UT), the damping of inflammation and cardiovascular risk, as well as on improved body composition, quality of life (QOL), and the decrease in maintenance HD costs. Finally, a summary of their use in patients with COVID-19 is presented.
THE EFFECT OF MCO MEMBRANES ON UREMIC TOXIN REMOVAL
Removal of β2-microglobulin (β2M)
(β2M, 11.8 kDa) is a UT, the prototype of medium molecular weight molecules; it is a marker of membrane efficiency in the removal of this class of solutes6,7. From the initial clinical study in which the performance of different MCO filters was evaluated, increased β2M removal was demonstrated. The reduction ratio (RR) with MCO surpassed HF (78.5 vs. 73.5%, p < 0.001), but did not significantly differ from OL-HDF (78.5 vs. 80.6%)3. However, Maduell et al. did not detect RR differences between the MCO and 8 HF dialyzers used in OL-HDF, thus reinforcing the non-inferiority of MCO filters in comparison with OL-HDF in β2M removal8. The established benefits of MCO filters over HF are constant, even in studies with a greater number of treatment options, such as that by Belmouaz et al. Patients were treated for 3 months with MCO, followed by 3 months with HF, and vice versa; MCO was found to be superior in terms of the β2M RR when compared with HF (73% vs. 68%, p = 0.04)9. The largest randomized clinical trial that has evaluated the efficacy and safety of MCO dialyzers included 172 randomized patients that were either treated with MCO or HF for 24 weeks. The group of patients treated with MCO had greater β2M RR after 4 weeks in comparison with those on HF (75.7% vs. 64.9% p < 0.001). This pattern persisted until week 2410.
A prospective study compared the MCO filters with 6 HF dialyzers, including 3 super HF dialyzers (SHF), in HF and OL-HDF. No significant differences were found in the β2M RR between the HF, SHF, OL-HDF, and the MCO dialyzers. MCO was only when compared with an HF dialyzer (p < 0.001)11. Finally, in the first randomized, controlled, crossover trial that compared chronic therapy with HF versus MCO versus OL-HDF for 4 weeks, the β2M RR was statistically greater in OL-HDF and MCO when compared with HF (62% vs. 73% vs. 27% respectively, p < 0.0001)12. In conclusion, based on the currently available evidence, we can claim that MCO filters are superior to HF while yielding the same efficacy as OL-HDF in the removal of medium molecular weight UT such as β2M.
Removal of free light chains
Increased serum levels of free light chains have been shown to be directly associated with greater mortality in patients with end-stage renal disease13. Therefore, free kappa (κFLC, 22.5 kDa) and lambda (λFLC, 45 kDa) light chains have been used as the prototype of medium-middle and large-middle UT, respectively6,7. Kirsch et al. reported that the RR of λFLC was greater with MCO, 42.5% in comparison with HF 12.9% (p < 0.001) and HDF 37.9% (p < 0.001); the RR of κFLC were MCO 72.9% versus HF 36.4% (p < 0.001), and 71.6% with HDF (p = 0.3), thus demonstrating better UT removal with MCO3.
In a clinical trial with 172 patients randomized to treatment to either MCO or HF for 24 weeks, the RR of λFLC was the primary efficacy outcome. MCO proved to be superior in terms of the RR of these UT at 24 weeks, 33% versus 17% in HF (p < 0.001), and UT removal improved within the first 4 weeks (p < 0.001)10. MCO performance in the clearance of these UT was also evaluated in comparison with OL-HDF, HF, and SHF HD (SHF) in a prospective trial with 8 patients. Belmouaz et al. found a greater κFLC RR with MCO, OL-HDF, and SHF dialyzers in comparison with HF dialyzers. There were no significant differences between MCO and OL-HDF. As to λFLC, OL-HDF was found to be superior to all other dialyzers (p < 0.01). This study emphasizes the non-inferiority of MCO versus OL-HDF in the elimination of UT in the medium molecular weight range11.
Evidence is limited as to the clearance of other middle molecular weight molecules. YKL-40 is a 38 kDa glycoprotein expressed on macrophages of early atherosclerotic plaque, and that has been independently associated with cardiovascular mortality in patients with renal failure14. In a clinical trial that evaluated the clinical efficiency of MCO3, the RR of YKL-40 was greater with MCO 60.5% versus 19.2% in HF (p < 0.001), and 44.8% in HDF, demonstrating MCO superiority3. Likewise, in a study by Hadad-Arrascue et al., MCO yielded better results when compared with OL-HDF, in terms of YKL-40 removal, but interestingly, this did not occur with toxins of lower molecular weight such as β2M, κFLC, and FGF-23 (32 kDa)14.
We can, therefore, ascertain that in the removal of UT with a medium molecular weight between 11.8 kDa and 45 kDa, MCO filters are superior to HF, and just as effective as treatment with OL-HDF.
Removal of protein-bound UT
Protein-bound UT (PBUT) are a group of low molecular weight substances (<500 Da) that are mostly a byproduct of intestinal metabolism; their affinity to plasma proteins is variable, which prevents their removal with conventional dialysis therapies. Indoxyl sulfate and p-cresol sulfate have been the most closely linked to increased cardiovascular risk, and their clearance is the best marker when analyzing the removal of this group of toxins6,15.
Few studies have evaluated the ability of MCO to remove PBUT. The REMOVAL-HD study was a non-randomized, multicenter trial that included 89 patients treated with MCO for 24 weeks, with two 4-week washout periods with HF, before and after the intervention. The primary aim was to evaluate changes in serum albumin during the treatment period, and among the secondary outcomes, the authors analyzed changes in pre-dialysis levels of different UT. An exploratory analysis of REMOVAL-HD studied the effects of MCO on the removal of PBUT such as indoxyl sulfate and p-cresol. The pre-dialysis serum levels of total indoxyl sulfate did not differ between groups at week 12 or 24. Likewise, total baseline p-cresol did not differ at weeks 12 or 24. On comparison of the concentrations of free indoxyl sulfate and p-cresol, no significant differences were detected either after treatment with MCO16. In another prospective, crossover study, 22 patients on chronic OL-HDF were randomized to treatment with HF, MCO, and OL-HDF for 3 consecutive weeks, and serum concentrations were measured pre-and post-dialysis. The RR of indoxyl sulfate and p-cresol showed no significant differences between the various modalities15. Finally, the results of a randomized, controlled, crossover study conducted in a single center in Mexico agree with previously described findings in the literature. After 4 weeks of treatment-wash out with each evaluated modality (HF, OL-HDF, and MCO), no significant differences were detected in the removal rates of indoxyl sulfate or p-cresol with MCO when compared with OL-HDF and HF12.
Based on current evidence, one can assert that the elimination of PBUT is completely dependent on residual kidney function and that despite all efforts to further increase the removal of larger-sized toxins, treatment with MCO has been unable to efficiently clear this type of solutes. Table 1 summarizes the main clinical trials that have explored the removal of middle molecular weight UT with MCO in comparison with other dialysis modalities.
Table 1. Effect of the different dialysis modalities on uremic toxins removal
| Characteristic | Kirsch et al. 20163 |
Belmouaz et al. 20209 |
Weiner et al. 202010 |
Belmouaz et al. 202211 |
Maduell et al. 202252 |
Vega et al. 202312 |
Kim et al. 202215 |
|---|---|---|---|---|---|---|---|
| Study desing | Prospective, open-label, controlled, randomized, crossover pilot study | Cross-over prospective study | Open label, multicenter RCT | Single center, prospective study | Prospective single-cohort study | Single center, cross-over, RCT | Prospective, randomized, cross-over study |
| Modalities | MCO versus HF versus OL-HDF | HF versus MCO | HF versus MCO | HF versus SHF versus HDx versus OL-HDF | OL-HDF versus MCO versus HF | HF versus MCO versus OL-HDF | HF versus MCO versus OL-HDF |
| Time intervention | Single session | 12 weeks each modality | 24 weeks | Single session | Single session | 4 weeks each modality | 3 weeks each modality |
| Patients | 39 | 40 | 172 | 8 | 23 | 22 | 22 |
| Age (mean, ± SD) | 55 ± 13 | 75 ± 9 | 59 ± 13 | 68 | 68 ± 12 | 36 | 62 ± 11 |
| Residual diuresis 500 mL/day | Not reported | NA 95% < 200 mL |
Not reported | NA 100% < 300 mL |
NA 100% < 50 mL |
NA 100% < 200 mL |
NA 100% < 100 mL |
| β2M RR | HF 73%*
MCO 78% OL-HDF 80%+ *p < 0.001 +NS |
HF 68% MCO 73% p = 0.04 |
MCO 73% HF 65% p < 0.001 |
HF 65% SHF 73% MCO 79% OL-HDF 79% NS |
HF 74% MCO 77% OL-HDF 83%* *OL-HDF versus all p < 0.001 |
HF 27% MCO 73% OL-HDF 62% p < 0.0001 |
– |
| κFree light chains | HF 36% MCO 72% OL-HDF 71%+ + p = 0.3 *p < 0.001 |
– | HF 50% MCO 63% p < 0.001 |
HF 46% SHF 56% MCO 66% OL-HDF 75%* * OL-HDF versus HF p < 0.001 |
HF 66% MCO 77%+ OL-HDF 84%* *OL-HDF versus all p < 0.001 +MCO versus HF p < 0.001 |
– | – |
| λFree light chains | HF 12%* MCO 42% OL-HDF 37%+ + *p < 0.001 |
– | HD 17% MCO 33% p < 0.001 |
HF 17% SHF 33% MCO 46% OL-HDF 60%* *OL-HDF versus HF, SHF, MCO p < 0.01 |
HF 24% MCO 48%+ OL-HDF 59%* *OL-HDF versus all p < 0.001+ MCO versus HF p < 0.001 |
– | – |
| pIndoxyl sulfate | – | – | – | – | – | HF -16% MCO -90% OL-HDF -50% p = 0.3 |
HF 33% MCO 36% OL-HDF 40% NS |
| p-cresol | – | – | – | – | – | HF -3% MCO -3% OL-HDF -5% p = 0.6 |
HF 27% MCO 29% OL-HDF 34% NS |
*Significant: p < 0.05.
+There was only a difference in the experimental group (MCO) versus control (HF) in TNF-α mRNA and IL-6 mRNA. ‘RR corrected for hemoconcentration (Bergstrom and Wehle formula).
HD: hemodialysis; RR: reduction ratio (pre-HD concentration-post-HD concentration/pre-HD concentration ×100); HF: high flow hemodialysis; MCO: medium cut-off membranes; OL-HDF: online hemodiafiltration; NA: not available; SHF: super high flow.
THE EFFECT OF MEDIUM CUT-OFF MEMBRANES ON INFLAMMATION, MINERAL METABOLISM, AND CARDIOVASCULAR OUTCOMES
Effect of medium cut-off membranes on inflammation
Patients with CKD are in a persistent inflammatory state characterized by elevated concentrations of inflammatory markers that may contribute to an increased cardiovascular risk17-19. Since MCO were designed, the generated hypothesis suggested that a larger pore size could potentially increase the elimination of cytokines, and thus contribute to the regulation of the imbalance between inflammation and antioxidant capacity20-22. Several clinical trials have focused on proving the reduction of various inflammatory cytokines with different dialysis modalities, as shown in table 2. Zickler et al.22 found that the use of MCO was significantly associated with a decrease in the expression of tumor necrosis factor alfa (TNF-α) and interleukin-6 (IL-6) messenger RNA in comparison with HF, but there were no differences in the plasma concentration of these and other cytokines. The largest clinical trial included 86 patients with MCO versus. HF, and revealed that the RR was greater with MCO for TNF-α but not IL-6; the latter increased by 50% in comparison with the baseline value in the group with HF10. Lim et al.23, compared MCO versus. HF for 12 weeks, and at the end of the study, they detected an RR for TNF-α with MCO of 41% versus 37% with HF, which was associated with an improvement in iron metabolism and resistance to erythropoietin-stimulating factors. However, in this and all studies conducted to date, the long-term impact of decreasing the levels of inflammatory cytokines remains unknown9,24. Subsequently, MCO versus OL-HDF were compared, demonstrating that the ability to eliminate inflammatory cytokines was similar with both modalities. As expected, on comparison of MCO versus OL-HDF versus HF, the latter yielded a lower RR for cytokines14,12.
Table 2. Effect of the different dialysis modalities on the inflammatory state
| Characteristic | Zickler et al. 2017‡22 | Weiner et al. 202010 | Lim et al. 202032 | Belmouaz et al. 20209 | Cozzolino et al. 202124 | Hadad et al. 202214 | Vega et al. 202312 |
|---|---|---|---|---|---|---|---|
| Age (mean ± SD) | 59 ± 17 | 59 ± 13 | 63 ± 14 | 76 ± 10 | 71 ± 13 | 61 ± 12 | 41 ± 17 |
| Residual diuresis > 500 mL/day | 18 (38) | NA | 10 (20), > 100 mL/day | 2 (5), > 300 mL/day | NA | 10 (23) | Anuria |
| Time intervention | 4-week and 8-week extension | 24 weeks | 12 weeks | 24 weeks | 24 weeks | 12 weeks | 12 weeks |
| Study desing | 23 patients MCO versus 25 patients HF | 86 patients MCO versus 86 patients HF | 24 patients MCO versus 25 patients HF | 20 patients MCO and cross-over HF versus 20 patients HF and cross-over MCO | 10 patients MCO and cross-over versus 11 patients HF and cross-over MCO | 21 patients MCO versus 21 patients OL-HDF | 27 patients cross-over for HF versus MCO versus OL-HDF |
| TNF-α, RR | 15% MCO 5% HF | 49% MCO* 35% HF | 41% MCO* 37% HF | 37% MCO 26% HF | NA | NA | 37% MCO 16% HF 2% OL-HDF |
| IL-6, RR | 33% MCO 44% HF | 15% MCO ↑50% HF | NA | 9% MCO 11% HF | 39% MCO ↑32% HF | 14% MCO 17% OL-HDF | 3% MCO ↑14% HF ↑4% OL-HDF |
| C-reactive protein, RR | 39% MCO 28% HF | 11% MCO 10% HF | ↑18% MCO ↑22% HF | NA | NA | 7% MCO 9% OL-HDF | 1% MCO 2% HF 1% OL-HDF |
*Significant: p < 0.05.
‡There was only a difference in the experimental group (MCO) versus control (HF) in TNF-α mRNA and IL-6 mRNA. RR corrected for hemoconcentration (Bergstrom and Wehle formula).
HD: hemodialysis; RR: reduction ratio (pre-HD concentration - post-HD concentration/pre-HD concentration ×100); HF: high flow hemodialysis; MCO: medium cut-off membranes; OL-HDF: online hemodiafiltration; NA: not available.
Effect of medium cut-off membranes on mineral metabolism and cardiovascular outcomes
Like inflammation, vascular calcification is a common complication that contributes to the increase in cardiovascular risk in patients with CKD, in addition to the classical risk factors25. The increase in the concentration of organic and inorganic molecules circulating in plasma and the homeostatic abnormalities in mineral metabolism further advances vascular injury and worsens outcomes in this population16. Different toxins and mineral metabolism markers, such as indoxyl sulfate, sulfated p-cresol, fibroblast growth factor-23, fetuin-A, and calciprotein particles, among others, correlate with this vascular calcification1,16. These are low- and medium-molecular weight toxins, but some are tightly protein-bound which hinders their elimination with conventional dialysis techniques16,26.
MCO membranes have been studied in this context, with promising results1. Ciceri et al. conducted a crossover study that included 20 patients that were managed for 3 months with HF and 3 months with MCO, to analyze various pathogenic mechanisms of vascular calcification; they established that the serum of patients treated with MOC had a lesser degree of procalcification potential26, as previously described in 27. The REMOVAL-HD trial detected a greater RR for FGF-23 with MCO at 12 weeks in comparison with baseline values, and this reduction was sustained even by week 2416.
Information is scarce on the clinical impact of MCO. Lee et al.28 conducted a clinical trial comparing cardiovascular parameters in patients with MCO versus OL-HDF. The studied outcomes were changes in the brachial-ankle pulse wave velocity, echocardiographic parameters (left ventricular ejection fraction and left ventricular mass), coronary artery calcium scores (CAC), and cardiovascular mortality over 1 year; there were no between-group differences. The CAC scores remained stable in the OL-HDF group, while the MCO group showed a growing tendency in the score (p = 0.012). This is the first study designed to investigate the clinical impact of MCO on cardiovascular clinical outcomes, but further evidence is necessary to reach solid conclusions on the usefulness of this new dialyzer in the modification of these outcomes.
EFFECT OF MEDIUM CUT-OFF MEMBRANES ON BODY COMPOSITION
As kidney disease progresses, worsening of renal function and the chronic uremic inflammatory status lead to nutritional and metabolic abnormalities that negatively impact the energy and protein balance, thus resulting in the loss of body proteins and energy reserves; this has been attributed to all the above-mentioned factors (uremic toxin accumulation, inflammation, the dialysis treatment 29. Although protein and energy depletion are considered multifactorial, dialysis techniques with the ability to eliminate toxins of greater molecular weight could maybe positively impact body composition and nutritional state. In this regard, Belmouaz et al. conducted a post hoc study of a previous clinical trial that had included eight patients with HF and eight with MCO; all patients completed at least 12 months with the assigned dialyzer, and all had a baseline and at 1-year bioimpedance. Lean body mass and lean tissue index improved significantly with MCO (both, p < 0.05); these parameters are good biomarkers of the protein energy-wasting syndrome in this population30.
EFFECT OF MEDIUM CUT-OFF MEMBRANES ON QUALITY OF LIFE
The fact that CKD decreases patients' QOL is well-established. Many studies have associated some symptoms with the poor removal of medium-middle and medium-large molecules. This generated the hypothesis that the increased removal of these UT with MCO could lead to a better QOL in patients. One of the most relevant studies in this area is the COREXH study, which included 992 patients and compared the effect of HF versus MCO for 12 months. QOL was evaluated with the KDQoL-SF36 questionnaire and the authors detected symptoms such as restless leg syndrome. Part of the results included improvement in three of the five domains of the KDQoL-SF36 questionnaire (symptoms, effects of kidney disease, and disease burden); they also observed a decrease in the severity of symptoms associated with HD. Restless leg syndrome manifestations decreased from a 22% baseline value to 10% after 12 months with MCO31.
The relationship between medium molecule concentrations and the changes in symptoms and QoL have been evaluated in some studies. A study by Lim et al. randomized 49 patients to MCO versus OL-HDF. Baseline QoL was evaluated at baseline, and again 12 months later with the KDQoL-SF36 questionnaire; they also collected information on symptoms such as pruritus with another questionnaire and a visual analog scale. They also analyzed changes in the previously mentioned parameters and their relation with the RR of different medium molecules. At 12 weeks, the scores in patients with MCO improved in the physical functioning, physical role, morning pruritus distribution, and frequency of scratching during sleep domains. All these changes correlated with better RR of κFLC and λFLC, suggesting that part of the improvement could be attributed to better clearance of this type of UT32 Similar results have been found in other studies with other instruments that measure QOL33.
Finally, in another study that compared MCO versus OL-HDF, questionnaires were applied every 3 months for a year to evaluate the time to recovery after HD, and although no changes were detected in the levels of hemoglobin, C-reactive protein, or albumin, the time to recovery decreased in the MCO group, whereby the percentage of patients that took over 360 min to recover, decreased34. Despite this data, some studies have reported contradicting results. In two different studies that compared MCO versus OL-HDF, no differences were detected in QoL10,35.
We can hence conclude that the increased removal of UT with MCO could apparently contribute to an improvement in QoL, a decrease in recovery time after HD, and fewer treatment-related symptoms. However, studies encompassing larger patient groups and longer follow-ups are necessary to identify which patients will benefit most from this approach.
EFFECT OF MEDIUM CUT-OFF MEMBRANES ON HEALTH ECONOMICS
Hospitalization rates and costs
The hospitalization and mortality rates in patients on dialysis are higher in comparison with the general population25; some reports have stated that these patients are hospitalized an average of 2 times/year36. The effect of MCO on the number of hospitalizations has been examined in some populations (Table 3).
Table 3. Comparison of hospitalization rates reported for patients in MCO membranes
| Study, country | Study design | Population (n), time to outcome | No. Hospitalization events | Hospitalization rate (patient/year) | Hospital days patient-year |
|---|---|---|---|---|---|
| Bunch et al.53 (COREXH) Colombia | Prospective cohort | n = 638 1 year | 673 | 0.79 (IC 95% 0.73-0.85) | 6.91 (IC 95% 6.74-7.09) |
| Molano et al.37 Colombia | Retrospective cohort | MCO n = 546 versus HF n = 534 2 years | MCO 727 HF 854 |
MCO 0.93 (IC 95% 0.82-1.03) HF 1.13 (IC 95% 0.68-0.99) |
MCO 6.45 (IC 95% 6.29-6.62) HF 10.18 (IC 95% 9.96-10.4) |
| Sanabria et al.38 Colombia | Observational Cohort before-after design | n = 81 1 year | MCO 61 HF 57 |
MCO 0.71 (IC 95% 0.55-0.92) HF 0.77 (IC 95% 0.6-0.98) |
MCO 4.41 (IC 95% 3.97–4.90) HF 5.94 (IC 95% 5.41–6.50) |
| Blackowicz et al.39 USA | Randomized controlled open-label | MCO n = 86 versus HF n = 85 4.5 months | MCO 18 HF 31 |
MCO 0.56 (IC 95% 0.3-0.81) HF 1.02 (IC 95% 0.57-1.24) |
MCO 4.6 (IC 95% 3.9-5.5) HF 4.1 (IC 95% 3.3-5.2) |
| Cho et al.44 Korea | Ambispective cohort | MCO n = 76 versus HF n = 38 3 years | MCO 22 HF 48 |
NA | NA |
MCO: medium cut-off membranes; HF: high-flux hemodialysis.
In one of the most important studies on the subject, Molano et al. reported a lower hospitalization rate (−20%) in patients with MCO versus HF (0.93 vs. 1.13 patients/year, p = 0.04), and a decrease in the rate of non-fatal cardiovascular events, although there was no difference in mortality at 20 months37. Later, in a "before/after" observational cohort study in Colombia that included 81 patients whose treatment was changed from HF to MCO, and a follow-up of 1 year, the authors reported a decrease in the hospitalization rate from 0.77 to 0.71 (NS) patients/year and a decrease in hospitalization days from 5.94 to 4.41 (days/patient/year) (p = 0.0001)38. Likewise, Blackowicz et al. evaluated the effect of MCO versus HF on the hospitalization rates and treatment costs; they reported a decrease of 46% in the rate of hospital admissions (0.56 vs. 1.02 patients/year, p = 0.042), and an average hospital stay of 4.6 versus 4.1 days (NS), reflected in a decrease in hospital costs of US$ 6091.0039.
Although the cost of an MCO dialyzer may be up to twice that of a conventional filter40, when the decrease in hospitalization rates and hospital stay in the MCO group are taken into account, dialysis-associated total costs do decrease38,39.
Erythropoietin-stimulating agents
UT and chronic inflammation compromise iron metabolism in dialysis patients and interfere with the response to ESA41. As previously stated, MCO increases the clearance of medium molecular weight molecules and inflammatory factors, which could improve the response to erythropoietin-stimulating agents (ESA). A randomized study that compared HF versus MCO showed a decrease in the median ESA dose (−49.8 vs. 8.1 U/Kg/wk., p = 0.023), and an increase in serum iron and transferrin saturation, and hence, a significant decrease in the ESA resistance index24. Furthermore, another observational study demonstrated a decrease in the ESA dose 6 months after initiating MCO, and the lower ESA requirement persisted when compared with conventional HD42.
However, not all studies reached favorable conclusions on this subject. After a 3-month crossover study of HF and MCO, Belmouaz et al. found no differences in the iron profiles, nor in the ESA dosage or resistance9 Likewise, Cho et al. did not detect a decrease in the median ESA dose nor in iron profiles at 12 months43, or at 3 years44. We can conclude that to date, it appears that the use of MCO decreases the inflammatory profile and tends to foster lower ESA doses, but evidence remains limited in terms of the effectiveness of MCO on ESA use. Table 4 summarizes the studies published on the subject. Figure 2 summarizes the beneficial effects that have been demonstrated with the use of MCO membranes.
Table 4. Change in ESA dose and erythropoietin resistance index for patients with MCO membranes
| Study, country | Study design | Population (n), time to outcome | Median dose ESA (Baseline) | Change in ESA dose ∆
[U/kg/wk] |
ERI |
|---|---|---|---|---|---|
| Lim et al.23 Korea | Randomized controlled open-label | MCO n = 24 HF n = 25 12 weeks |
MCO: 133.9 ± 91.5a
HF: 126.9 ± 125.8a |
MCO: −49.8 ±
81.6b
HF: 8.1 ± 90.2b |
∆ − 5.2 ± 7.8 versus 0.1 ± 9.1c |
| Yeter et al.42 Turkey | Non-randomized, controlled | MCO n = 16 HF n = 16 LF n = 15 6 months |
Baseline (U × 103) per session
MCO 4 (2.6-4)d HF 5.4 (3-10)d LF 7 (3.3-10.3)d |
6th month: (U ×
103)
MCO 3.6 (2.9–4.6)d HF 6 (4.6–8.6)d LF 5.4 (1.4-8)d |
NA |
| Belmouaz9 France | Randomized, controlled cross-over | 40 patients 3 months |
NA | After treatment (UI ×
103)
MCO: 3.12 (2-5.3)d HF 3.44 |
MCO: 12 (7-18)e
HF: 15 (8-22)e (NS, p = 0.14) |
aWeight-adjusted ESA (U/kg/wk) ± SD.
bChange in median dose ESA (∆ U/kg/wk) ± SD.
cChange in median ERI after intervention.
dMedian ESA dose per HD session [U×103] (IQR).
eERI after follow-up period.
MCO: medium cut-off membranes; HF: high-flux hemodialysis; LF: low-flux hemodialysis; ERI: erythropoietin resistance index (U/kg/wk/g/dL); ESA: erythropoietin-stimulating agents.
Environmental impact
The environmental impact of dialytic modalities is high, particularly HD, which is the most used treatment for CKD, consumes a considerable quantity of water and energy, and produces a large amount of waste45. The amount of water used depends mainly on its treatment and the modality used. With HF, less is consumed (0.5 m3/session) compared to HDF, which can even go up to 35 l depending on the type of replacement used46. The use of MCO membranes provides benefits similar to those obtained with HDF and with lower water requirements given their filtration-retrofiltration properties1,2. However, it is urgent to establish public policies for the management of all waste caused by the health-care system in an effort to be more sustainable.
EFFECT OF MEDIUM CUT-OFF MEMBRANES ON COVID-19
COVID-19 triggers an uncontrolled inflammatory process that leads to organic injury, and its accentuated magnitude is associated with unfavorable clinical outcomes47. In this context and given the correlation between inflammatory cytokines and COVID-19 severity, the use of extracorporeal treatments with MCO or HCO membranes was posited as a possible immune modulator, capable of removing inflammatory cytokines in patients on chronic HD or with AKI and requiring replacement therapy48.
Two prospective, randomized trials have evaluated the impact of MCO on COVID-19 and chronic HD. Yalın et al. failed to demonstrate a benefit from the use of MCO membranes, although the MCO group had greater COVID-19 severity and warranted a more prolonged hospitalization (21.9 vs. 11.5, p = 0.022); there were no differences in death rates nor admissions to the intensive care unit49. Esposito et al. evaluated the inflammatory cytokine profile and detected no differences in the removal of circulating cytokines or clinical outcomes at 14 days50. Finally, Salazar et al. compared OL-HDF versus MCO in patients with Covid-19, revealing increased TNFα clearance in comparison with OL-HDF, as well as a decrease in deaths in the MCO group (18.2% vs. 57.1%, NS)51.
Despite the evidence on the removal of pro-inflammatory cytokines in COVID-19, MCO has not proven effective in terms of clinical outcomes, and is therefore not recommended as immune modulation therapy that would sufficiently limit COVID-19 severity; further evidence is necessary to establish the role of MCO in the context of acute disease.
CONCLUSIONS
Given the reviewed and summarized evidence presented in this article, we believe that at least in the medium-term, the use of MCO membranes increases the removal of medium-sized UT, that in turn, is reflected in clinical and paraclinical benefits such as improved QOL, less hospitalizations in the 1st year, and decreased ESA dosing, and improved outcomes when compared with HF. Questions that remain to be answered in the future include whether these results will persist in the long-term and whether they will be reflected in decreased morbidity and mortality in patients on chronic HD, the crux in the management of these patients.











 nueva página del texto (beta)
nueva página del texto (beta)