INTRODUCTION
High low-density lipoprotein-cholesterol (LDL-C) level is among the most common forms of dyslipidemia in Mexicans, being present in nearly half of the adult population. This strong risk factor for cardiovascular (CV) events remains largely untreated and underdiagnosed1. Management of dyslipidemia is centered in lowering the concentration of atherogenic particles, estimated with LDL-C levels. Lipid-lowering therapy (LLT) results in CV benefits and reduced rates of both CV events and mortality2. Statins are the first drug of choice, as recommended by most guidelines3-5. Despite the proven benefits of LLT, under treatment is a major area of concern in the management of dyslipidemia. Additional LLTs include ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, which have been shown to provide a clinical benefit in combination therapies, but their use is limited and often not considered in clinical practice by attending physicians.
Undertreatment is a multifactorial challenge, in which conditions related to patients, physicians, and health services mutually interact. Some of these factors could be overcome by the implementation of patient-centered strategies or with educational programs for physicians. Most studies reporting these factors have been conducted in Western Europe and North America6,7. The International ChoLesterol management Practice Study (ICLPS) was designed to provide the corresponding data in countries outside of Western Europe, areas in which growing trends in CV mortality have been reported during the past decade. Physician practices of 18 countries, including Mexico, were evaluated to measure achievement of LDL-C targets in patients who were already receiving stable LLT to identify factors independently linked to undertreatment. Here, we aim to provide an in-depth characterization of ICPLS data obtained from Mexican patients. This is, to our best knowledge, the first evaluation specifically dedicated to the identification of factors related to LDL-C goal non-achievement in our population.
METHODS
Study Population and Patient Selection
This report contains data collected for the ICLPS in Mexican participants8. ICLPS is a cross-sectional observational study which included adult patients treated in Mexican facilities who had been receiving a stable type and dose of LLT for ≥3 months before enrollment and had their LDL-C value measured while receiving stable LLT in the previous 12 months. Patients participating in a clinical trial or who had received a PCSK9 inhibitor in the preceding 6 months were excluded from the study. To ensure that the results adequately reflected the management of dyslipidemic patients in real-life practice, the contribution of each medical specialty made in the management of such patients was provided by a national expert. To limit bias in the selection of the study sites, potential centers/physicians were identified because of their high demand of services, included in a list and selected using a randomization process controlled to ensure a balanced representation of each specialty. To limit patient selection bias, sites were instructed to recruit a minimum of five consecutive patients per site. A predefined 2-week interval was selected during which all consecutive consenting patients who attended the visit for any reason were enrolled. As not all sites could start recruitment at the same time, a timeframe of 3-6 months was given for recruitment depending on the total number of sites/patients.
Data collection
Physicians completed a questionnaire that collected both patient and physician information. From physicians, we obtained demographic data, medical specialty, years of practice, type and location of practice, main workplace, mean number of patients consulted per day, choice of and adherence to practice guidelines for lipid disorders (i.e., European Society of Cardiology and European Atherosclerosis Society ESC/EAS, American College of Cardiology/American Heart Association [ACC/AHA], and other international/local/national guidelines), and the definition that he or she used to diagnose statin intolerance (i.e., intolerance to 1, 2, or ≥3 statins). A case report form was completed for each patient during a single visit. The data collected included: demographic information; results of physical examination, CV risk factors, type of hypercholesterolemia (primary, secondary, or unknown), LDL-C values (calculated or measured directly; on current treatment and untreated if available) and other lipid variables, current use of LLTs and/or antithrombotic drugs, socioeconomic profile, and the investigators assessment of the patients CV risk level. Data quality control was performed by trained personnel at more than 10% of randomly chosen sites. Risk factors were defined as proposed by 2011 ESC/EAS guidelines; familial hypercholesterolemia was defined according to the Dutch Lipid Clinic Network9.
Statistical Analysis
Baseline characteristics are presented using mean (±SD) or median (interquartile range) values for continuous variables and as frequencies for categorical data. The primary outcome measure for this study was the proportion of patients taking LLT who did not achieve LDL-C targets as defined by 2011 ESC/EAS guidelines: <1.8 mmol/L (70 mg/dL) for very-high risk, <2.5 mmol/L (100 mg/dL) for high risk, and <3.0 mmol/L (115 mg/dL) for moderate-risk patients. The Systematic Coronary Risk Estimation (SCORE) chart for high-risk countries was used to stratify patients by their CV risk10. A series of logistic regression models were developed to test the relationship between non-achievement of LDL-C targets and demographic, clinical, and treatment characteristics. All analyses were conducted using SAS version 9.2.
RESULTS
Physician Characteristics
We included patients recruited by 18 physicians across Mexico (71.2% men, mean age of 48.3 ± 10.9 years, and 21.9 ± 9.9 years of practice) including nine general practitioners, two cardiologists, three internal medicine specialists, three endocrinologists, and one lipidologist. Most attended patients were from urban regions (94.4%) and private practice settings (61.1%). All but two physicians reported following specific guidelines or recommendations for the management of lipid disorders; the majority reported following the ACC/AHA Guidelines on the treatment of blood cholesterol (56.3%), followed by the ESC/EAS Guidelines for the management of dyslipidemia (43.8%). In general, physicians reported seeing a median of 5.0 (3.0-7.0) patients with dyslipidemia and/or with lipid-modifying treatments per day, representing 40.6% (26.7-50.0%) of their daily practice.
Patients characteristics and CV risk
From a total of 653 patients who were screened, 23 were ineligible for inclusion in the study, and four had incomplete information. Our final study sample was composed of 626 patients. The mean age of the participants was 59.3 ± 12.7 years; 55.6% were women; 60.1% had native Latin American ancestry; 572 (91.4%) were evaluated in urban areas; and 444 (70.9%) completed secondary education or higher. Hypertension was present in 367 cases (58.6%) and 413 (66.0%) reported not doing regular physical activity. Type 2 diabetes (T2D) was present in 367 patients (58.6%) with a median of 9.0 (4.0-16.0) years from diagnosis; 91 patients (24.9%) reported having diabetes-related microvascular complications, and 56 (15.3%) reported experiencing at least one episode of symptomatic hypoglycemia in their lifetime. Median body mass index was 28.8 kg/m2 (26.2-32); close to 40% of the study subjects were obese. A total of 145 patients were former smokers and 412 (65.8%) were current smokers. Metabolic syndrome as defined according to Adult Treatment Panel -III criteria was present in 342 patients (54.7%), and in 407 (65.2%) according to IDF criteria.
Overall, 90 patients (14.4%) had documented coronary artery disease, defined as a previous acute coronary syndrome (77/90, 85.6%), previous percutaneous coronary intervention (61/90, 67.8%), or previous coronary artery bypass graft (15/90, 16.7%). Twenty-four familial hypercholesterolemia cases were included in the study. The median (interquartile range) time since a diagnosis of dyslipidemia was 4.0 (1.0-7.0) years. Of 498 (79.6%) patients in whom the SCORE CV risk could be calculated, 210 (42.2%) were at very high risk, 235 (47.2%) were at high risk, 42 (8.4%) were at moderate risk, and 11 (2.2%) were at low risk.
Physician-estimated CV risk correlated poorly with the calculated risk. Over half of the patients at high/very-high calculated risk were estimated by physicians to be at a lower-risk level (Fig. 1). Conversely, 47.2% of calculated low and moderate risk patients were estimated by physicians to be at a higher level of risk. The LDL-C value at the time of the first diagnosis before starting LLT was available in 175 (27.9%) patients. The mean value was 141.8 ± 56.1 mg/dL (3.7 ± 1.5 mmol/L); and 62/175 (35.4%) of patients had an LDL-C value >3.4 mmol/L (130 mg/dL). The distributions of LDL-C levels according to CV risk levels are presented in figure 2. Mean high-density lipoprotein-cholesterol (HDL-C) concentration was 44.9 ± 15.3 mg/dL. The corresponding value for triglycerides was 254 ± 333 mg/dL. The prevalence of mixed dyslipidemia was 60.6%.

Figure 1 LDL-C value according to calculated cardiovascular risk level (calculated using SCORE) at enrollment before (A) and after (B) starting on lipid-lowering therapy. LDL-C: low-density lipoprotein cholesterol; SCORE: Systemic Coronary Risk Estimation.
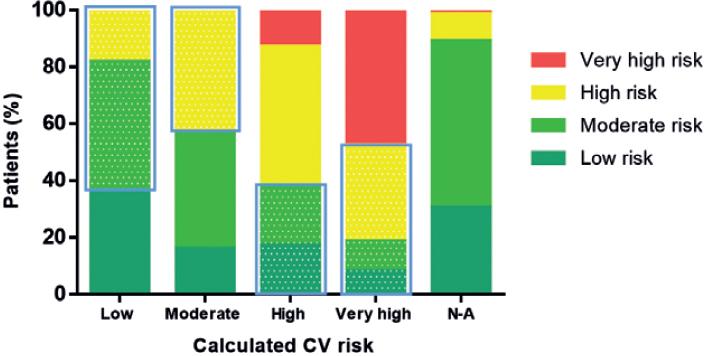
Figure 2 Concordance between estimated cardiovascular (CV) risk by attending physicians compared to calculated CV risk using SCORE. Shaded areas represent over and underestimation of risk per calculated CV risk category. Weighted Kappa = 0.260; 95%CI 0.206-0.314. SCORE: Systemic Coronary Risk Estimation.
Effect of LLT and LDL-C Goals
At study enrollment, 97.4% were receiving a statin (62.8% on statin monotherapy, 26.7% statin+fibrate, and 5.6% statin+ezetimibe (Table 1). About 28% of statin-treated patients were receiving high-intensity statin therapy (atorvastatin 40/80 mg or rosuvastatin 20/40 mg), and 11.3% were on the highest dose regimen available in Mexico. Overall, patients had a median LDL-C decrease of −23.1% (−43.7-−2.4%) from diagnosis to the inclusion in the study. Close to half (58%) of the cases had LDL-C change lower than 30%; a 30-50% reduction occurred in 24%; and a change greater than 50% occurred in merely 17.7% of the participants. Only 33% of the cases that had a 50% LDL-C change had a high or very high risk. On the other hand, only 240 cases (38.4%) had LDL-C below 100 mg/dL (the LDL-C goal accepted in the majority of the lipid guidelines). Furthermore, the goal of the intensive treatment (<70 mg/dL) was found only in 80 cases (12.8%); 80% of them had high or very high CV risk. Other secondary lipid goals were not met in the study subjects. More than half (58.5%) had HDL cholesterol below target value (<40 mg/dL in men and <50 mg/dL in women); this rate was not affected by the CV risk stratification. The same trend was observed in the triglyceride concentrations, as triglyceride concentrations >150mg/dL were found in 52.2% of the participants.
Table 1 Use of lipid-lowering therapies (LTT) by patients from the evaluated physicians, overall, and stratified according to cardiovascular risk level as evaluated by EUROSCORE
| Lipid-lowering therapy | Total (n = 626) (%) | Low risk (n = 11) (%) | Moderate risk (n = 42) (%) | High risk (n = 235) (%) | Very-high risk (n = 210) (%) | Not assessable (n = 128) (%) |
|---|---|---|---|---|---|---|
| Any statin | 610 (97.4) | 9 (81.8) | 42 (100.0) | 227 (96.6) | 208 (99.0) | 124 (96.4) |
| High-statin dosage | 173 (28.4) | 4 (44.4) | 12 (28.6) | 62 (27.3) | 70 (33.7) | 25 (20.2) |
| On highest statin dose | 69 (11.3) | 1 (11.1) | 5 (11.9) | 16 (7.0) | 36 (17.3) | 11 (8.9) |
| Statin monotherapy | 393 (62.8) | 3 (27.3) | 23 (54.8) | 148 (63.0) | 129 (61.4) | 90 (70.3) |
| Statin+fibrate+other LLT | 165 (26.3) | 6 (54.6) | 11 (26.2) | 66 (28.1) | 62 (29.6) | 20 (15.7) |
| Statin+cholesterol absorption inhibitor+other LLT | 35 (5.6) | 0 (0.0) | 3 (7.1) | 11 (4.7) | 12 (5.7) | 10 (7.8) |
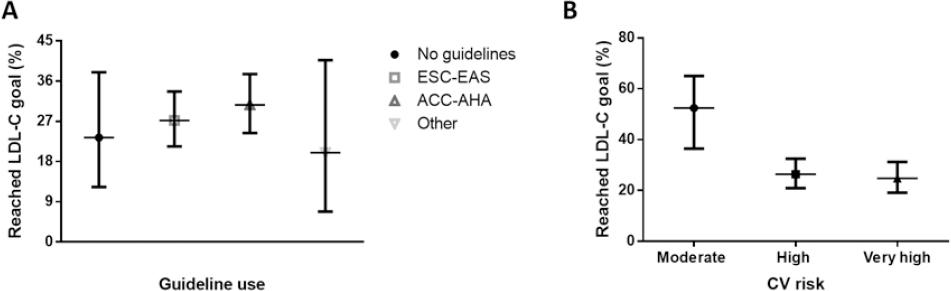
LDL-C Goal Achievement and CV Risk
Patients at low CV risk had the highest percentage reduction of LDL-C followed by those at very-high-risk and moderate risk. Most patients did not achieve their target LDL-C reduction according to the expected CV risk category (Table 2). The proportion of patients who achieved the LDL-C targets was higher in the moderate risk group and the lowest in the very-high-risk group (24.8% for very high risk, vs. 26.4% for high-risk and 52.4% for moderate risk, Fig. 3). Among patients at high and very high CV risk, who were those with suboptimal LDL-C goals, 54.5% and 68.6%, respectively, were either retired or unemployed and 13 patients at those CV risk levels had work disability due to CV disease, suggesting a potential financial reason contributing to LDL-C goal non-achievement. The percentage of patients who achieved the target goals according to CV risk category when estimated by physicians was 35.9% versus 26.3% when CV risk was assessed based on the ESC/EAS recommendations (p < 0.001). The concordance between physician-estimated CV risk and guideline-estimated risk was moderate to strong (κ = 0.721, 95%CI 0.652-0.791), indicating that goal non-achievement could also stem from inadequate CV risk calculation.
Table 2 Factors associated with decreased likelihood to achieve LDL-C goals according to ESC/EAS guidelines under statin therapy in Mexican population
| Parameter | ƒÀ-coefficient | OR | 95%CI | p-value |
|---|---|---|---|---|
| High risk versus Moderate CV risk | 1.121 | 3.0694 | 1.5683-6.0070 | 0.0011 |
| Very high versus Moderate CV risk | 1.207 | 3.3423 | 1.6902-6.6094 | 0.0005 |
| Statin intolerance | 1.598 | 4.9431 | 1.9279-12.6738 | 0.0009 |
| Diabetes versus No diabetes | 0.599 | 1.8198 | 1.1732-2.8228 | 0.0075 |
| Secondary or higher versus Lower education | -0.603 | 0.5473 | 0.3466-0.8641 | 0.0097 |
| NHS versus Private | 0.905 | 2.4723 | 1.5243-4.0093 | 0.0002 |
| Female versus Male | 0.594 | 1.8111 | 1.2141-2.7018 | 0.0036 |
| BMI >30kg/m2 versus BMI <25 kg/m2 | 0.686 | 1.9869 | 1.1483-3.4382 | 0.0141 |
| BMI 25-29.99 kg/m2 versus BMI <25 kg/m2 | 0.641 | 1.8994 | 1.1003-3.2786 | 0.0213 |
| Abdominal obesity IDF | 0.904 | 2.4698 | 1.5301-3.9867 | 0.0002 |
| Metabolic syndrome IDF | 0.623 | 1.8654 | 1.2252-2.8402 | 0.0036 |
| Triglycerides .150 mg/dL versus <150 mg/dL or unknown | 0.428 | 1.5349 | 1.0296-2.2882 | 0.0355 |
CV: cardiovascular; NHS: National Health Service; BMI: body-mass index; IDF: International Diabetes Federation.
Factors Associated with Non-Achievement of LDL-C Goals in the Study Sample
Patients at high or very high CV risk were approximately three-fold less likely to achieve their LDL-C goals compared to patients at moderate CV risk (Table 2). Overweight and obesity, abdominal obesity, and metabolic syndrome defined using International Diabetes Federation criteria, statin intolerance, female sex, treatment in public versus private settings, diabetes, and hypertriglyceridemia were also associated with failure to achieve targets, whereas higher levels of education were associated with a lower risk of non-achievement of LDL-C goal. In relation to the etiology of dyslipidemia, we identified 426 patients with secondary dyslipidemia among whom only 110 (38.4%) reached LDL-C goals. A similar scenario was observed for primary or familial hypercholesterolemia: among the identified 15 patients, merely 7 (46.7%) reached LDL-C goals.
Finally, we explored the reasons for not prescribing the highest dose of the statin according to their baseline LDL-C level and CV risk. The most common reason was the physicians perception that an acceptable LDL-C change was achieved (55%) followed by economic reasons (23.5%) and lack of tolerability by the patient (13%). The most common treatment-related complaint was muscle pain (24%); in the majority of these cases (72%), no assessment of the plasma creatine kinase levels was requested. A quarter of the statin-treated patients received different statin in the past. The most common reason for changing the statin was the physicians decision based on the LDL-C achieved (70%) followed by the cost of treatment (11.1%).
DISCUSSION
ICLPS provides a real-life assessment of the effectiveness of the LLT in non-European countries. Here, we described the results of Mexican participants. Our results reveal major gaps in the implementation of stable LLT in a representative set of patients treated by experienced physicians; however, this issue is not limited to Mexico given that the same problem was observed in all countries included in ICLPS8. The percentage of cases that attained the LDL-C treatment goals was low, especially among very high and high CV-risk groups. As reported by the ICLPS study, only a quarter of very high and high CV-risk patients achieved their risk-based LDL-C targets as recommended by current guidelines. Our data identify areas of opportunity to improve effectiveness of the LLT prescription and CV risk estimation in practice. Guidelines should consider the inclusion of actions to avoid clinical inertia and identify the cases with the highest likelihood of abandoning LLT or being undertreated11-14.
The ICPLS methodology explores both patient-related and physician-related factors that may limit the impact of LLT. Our results reveal significant disparities in LLT and LDL-C goal achievement, which impacts adequate CV risk management, treatment access, and might influence the effectiveness of LLT. Previous reports consistently showed lower LDL-C goal achievement rates in women, associated with the physicians perception of lesser CV risk and a higher probability to discontinue therapy13-15. Our results agree with the REGARDS study, which showed that older age, sex, race, poverty, and insurance type influence access to LLT and LDL-C goal achievement. Disparities in health-care access in Mexico have also shown to decrease the likelihood of cholesterol screening, thus affecting disease identification and prompt initiation of treatment16-18. Data reported here may be useful for the Mexican health-care system to adapt approaches to reduce CV-risk burden and decrease gaps limiting adherence, reducing intrinsic inequalities in the Mexican health-care system which restrain adequate management of CV risk.
The high prevalence of T2D is a peculiarity of the Mexican population1,19. Data from ENSANUT 2006 reported that only 28.6% of individuals with T2D had LDL-C <100 mg/dL and 10.5% had LDL-C <70 mg/dL and projected that over two-thirds of patients with T2D in Mexico were not at ESC/EAS goal levels but were nonetheless, eligible for LLT. Furthermore, ENSANUT 2012 showed that <3% of patients with T2D were under statin therapy20. T2D increases the risk of hypertriglyceridemia, which leads to an underestimation of LDL-C using the Friedewald equation21. Recent data showed that LDL-C estimation using Martins formula is more accurate in Mexican patients with familial combined hyperlipidemia, suggesting it may be a useful tool to address LDL-C undertreatment in patients with comorbid hypertriglyceridemia22. Furthermore, abdominal obesity, polypharmacy, and depression are diabetes-related comorbid conditions, which may also interfere with the adherence to LLT. These comorbidities interfere with LDL-C management in T2D and might explain the adverse association observed in relation to goal non-achievement in T2D in our cohort, contrasting with pooled data from ICLPS.
LDL-C goal non-achievement is reliant both on accurate CV risk estimation and the proper selection of statin dosage by the practitioner. In our study, physician-estimated risk influenced statin treatment intensification, indicating the necessity to improve CV risk estimation with population-specific data, such as those provided by the Globorisk collaboration23. Our data strongly suggest that clinical inertia and non-adherence to guidelines are common and should be considered a target for public health policies24,25. Increasing LLT adherence thus relies on overcoming many factors which affect access to treatment and treatment adherence itself; therefore, it is possible that the therapeutic gap for goal non-achievement in very-high and high CV risk patients may not be fully mitigated by use of statin therapy alone and that local guidelines should work on improving recommendations related to treatment assignment and targeted treatment with more effective LLTs. Complementary actions to be considered are combination therapies with ezetimibe and/or PCSK9 inhibitors which have shown that decreasing LDL-C to lower levels than recommended by guidelines may provide additional CV benefit26-28. Studies of LLT combinations in T2D and metabolic syndrome may shed light on the added benefits of such therapies in settings like Mexico, where hypercholesterolemia often is the result of comorbid metabolic abnormalities.
Our study had some strengths and limitations. The observational setting provides real-world evidence for benefits of LLT and the barriers of LDL-C goal achievement in everyday clinical practice; nevertheless, it is subject to limitations including lack of data before clinical diagnosis of primary and secondary dyslipidemias, treatment-dosage specifications, and difficulty in assigning causality to our observations. Even though random selection of centers and physicians reduced selection bias, most of our patients were from urban settings, and a large proportion had non-public insurance, which limits extrapolation of our results to a national scale with underrepresentation of non-urban or public-sector settings. Furthermore, the use of SCORE may affect CV risk estimation in our population, where it has been shown to offer questionable risk prediction29. Finally, we did not assess the role of apolipoprotein B as an LLT goal to reduce CV risk; given the role of apolipoprotein B in the pathogenesis of atherosclerosis and CVD, this remains an area of opportunity for future studies.
The achievement of LDL-C goals in Mexico is suboptimal and even lower compared to other countries included in the ICLPS collaboration. Regional differences related to intrinsic metabolic burden, health care, and social determinants of health intervene in proper LDL-C goal achievements and suggest that LLT should be tailored to meet necessities of individual countries. These interventions should be in attendance to country-specific disparities and considering intrinsic risk for conditions which alter atherogenic profiles, including diabetes, metabolic syndrome, and insulin resistance. The development of local guidelines which aim to reduce barriers for LLT access, optimize treatment intensity assignment and use of combination therapies should result in increased goal achievement and a substantial decrease in CV disease rates in Mexico.











 nueva página del texto (beta)
nueva página del texto (beta)