Introduction
Radiofrequency (RF) ablation is an effective and safe technique that has become the standard treatment for various arrhythmias. Since ablation results are improving and the incidence of complex arrhythmias is increasing, the issue of radiation exposure has become increasingly important.1
Fluoroscopy is a standard navigation guide used to perform RF ablation and is associated with exposure to ionizing radiation. The increasing complexity of electrophysiology (EP) procedures requires detailed mapping and extensive ablation therapy. Thus, procedural and, more notably, fluoroscopic times (FT) have progressively lengthened.2
Radiations are odorless and invisible, so becoming complacent about their dangers is easy. Operators are so caught up in performing procedures that they overlook the tools that help reduce radiation exposure. Consequently, patients and operators can be exposed to higher radiation levels than necessary.3
There are two main deleterious effects of radiation: deterministic and stochastic effects. The first ones occur once a threshold of exposure has been exceeded, and their severity increases with increasing doses, becoming evident days to months after exposure. On the other hand, stochastic effects are related to the potential future harm to the tissue and the body. Deterministic effects of most concerns for patients and operators include skin injuries (which may occur when FT exceed 20 min, using high-contrast fluoroscopy mode, or 60 min in low-level fluoroscopy) and cataract (present in one-third to half of the interventional cardiologists, whose dose threshold was 2 Gy for a single dose or 5 Gy for fractionated dose). The stochastic effect of most concern is a carcinogenic effect in exposed patients and physicians. In this effect, the cell is modified by DNA damage but remains viable, the harm expressed through cell proliferation. The cancer risk is highest in children, higher in women than in men, and reduced by one-half in the elderly. The tissues with a higher risk of radiation-induced cancer were the breast, colon, lung, stomach and bone marrow.4,5
The best model to estimate radiation risk is the linear-no-threshold model, which supports the concept that no radiation dose, even the smallest, can be considered completely safe. The risk is dose-related, with higher radiation levels related to higher risks. Based on this rationale, using non-standard fluoroscopy settings or implementing the ALARA principle (as low as reasonably achievable) for EP procedures seem insufficient to provide complete protection for the patient and laboratory staff.6,7
Therefore, significant efforts have been made in the past few years to reduce radiation exposure among patients and operators. For instance, incorporating advanced imaging modalities such as real-time ultrasonography, intracardiac echocardiography (ICE), and three-dimensional electroanatomic mapping (3D-EAM) systems have greatly reduced the requirements for fluoroscopy in EP laboratories without any significant difference observed in the safety and efficacy of the procedures.2
In the last decade, EAM systems have noted a steep development. Currently, they enable the generation of 3D reconstruction of any part of the heart without the need for fluoroscopic navigation. Despite these indisputable advantages, they do not include information obtained by real-time fluoroscopy. This important limitation is tackled by the new CARTO-UNIVU™ module as it seamlessly combines fluoroscopy images with 3D-EAM into a single accurate 3D view. It helps reduce the procedural time (PT) and fluoroscopy dose (FD) to as low as reasonably achievable. It helps reduce radiation exposure for physicians, staff, and patients, allowing navigation, with confidence, from an integrated view with just one fluoroscopic image or cine sequence needed for continuous anatomical orientation, making it possible to perform an equally precise ablation.8-10
This article aims to share our single center first experience with the CARTO-UNIVU™ module.
The CARTO-3® system uses hybrid electromagnetic and current-based navigation to allow precise catheter location. A locator sensor in the distal end of the ablation catheter interacts with three electromagnetic fields generated by a location pad positioned underneath the patient Table, providing a map of any heart chamber. Six electrode patches positioned at the patient’s front and back screen a unique current emitted from different catheter electrodes, providing additional information for catheter electrode localization.
In addition, 3D-EAM displays the voltage of the recorded electrograms, low voltage or scar region defined by the voltage map is known to be correlated with the arrhythmogenic substrate. The anatomic shell thereby constructed is, in some cases, integrated with the 3D anatomic dataset or generated by previous computed tomography or magnetic resonance imaging (MRI) scans to optimize the use of such a system.
The CARTO-UNIVU™ module integrates the CARTO-3® system with the fluoroscopic image or cine. This system consists of a registration plate mounted onto the location pad positioned under the patient Table, which aligns the CARTO-3® system to the conventional fluoroscopy, and a software module. It has a very simple and efficient workflow consisting of a small registration step at the beginning of the procedure with a single snapshot of the fluoroscopic incidences of interest.
In all procedures, a quadripolar diagnostic catheter was positioned into the right ventricular apex or right atrium and a decapolar catheter into the coronary sinus under fluoroscopic guidance. The locator sensor determined catheter position, then the CARTO-UNIVU™ module was registered, and cine loops were recorded in anteroposterior, right, and left anterior oblique projections. Subsequently, the EAM was created under active catheter tracking in pre-recorded cine loops. Consequently, the operator can handle the catheters, mimicking the use of fluoroscopy but without the use of further radiation exposure. Lesion formation parameters such as ablation time, contact force, impedance, and RF energy are disclosed to illustrate the lesions. Catheter ablation (CA) procedures were performed with the EAM using the pre-recorded cine loops and only, if necessary, with active fluoroscopy.
Without this technology, operators must rely on 3D-EAM and fluoroscopic images for catheter positioning.1,11,12
Here, we report four cases of our single center first experience of the CARTO-UNIVU™ module.
Patient 1. An 86-year-old female was admitted to the emergency room with signs and symptoms of acute heart failure (HF). She had type 2 diabetes, Parkinson’s disease, and atrial fibrillation (AF).
The ECG revealed a pre-excited AF with a rapid ventricular response. Transesophageal echocardiography (TEE) showed a thrombus in the left atrial appendage. After clinical stabilization, the patient was discharged with warfarin for four weeks. Shortly before the end of the four-week treatment, the patient was again hospitalized for acute HF. During hospitalization, a TEE was performed that excluded thrombus. The patient was then submitted to electrical cardioversion with reversion to sinus rhythm. Later, an electrophysiological study (EPS) was performed. An electrical programmed ventricular stimulation showed concentric and decremental retrograde conduction. The tricuspid annulus was mapped during atrial pacing, locating the accessory pathway (AP) in the right posteroseptal region. An application of RF energy was performed with the immediate disappearance of ventricular preexcitation.
The PT was 50 minutes, fluoroscopy time (FT) was 2.3 minutes, and FD was seven mSv (Figures 1 and 2).

Figure 1: Left anterior oblique projection. His bundle signal (orange dot). An application of RF energy was delivered with the immediate disappearance of ventricular pre-excitation.

Figure 2: Anteroposterior and left anterior oblique projection. The activation mapping identified the hotspot on the right posteroseptal region of the tricuspid annulus.
Patient 2. A 33-year-old woman with a medical history of lymphocytic colitis and symptoms of fatigue and palpitations underwent an EPS in 2018 due to premature ventricular complexes (PVC) arising from the right ventricular outflow tract (RVOT). A cardiac MRI showed no structural cardiac abnormality. An ablation was successfully performed without complications.
In 2020, the patient presented with similar symptoms and underwent a 24-hour Holter monitoring that revealed > 22,500 (19.4%) interpolated PVCs with a predominant focus on the RVOT. Transthoracic echocardiography (TTE) showed a left ventricular ejection fraction (LVEF) in the lower limit of normal (54%).
A second EPS was performed, and activation mapping revealed the earlier activation focus on the upper and lateral region of the RVOT, just below the pulmonary valve. Pace-mapping found a 12/12 correlation with the PVCs. Eight RF applications were performed in that location with the complete cessation of extrasystoles. Isoprenaline was administered, and extrasystoles were not induced.
The PT was 90 minutes, FT was 1.8 minutes, and FD was 21 mSv (Figures 3 and 4).

Figure 3: Right anterior oblique and antero-posterior projection. The activation map revealed the earlier activation focus on the upper and lateral region of the RVOT, just below the pulmonary valve.
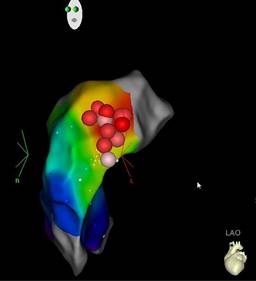
Figure 4: Left anterior oblique projection. Eight ablation lesions on the upper and lateral region of the RVOT, which corresponds to the hotspot (red dots).
Patient 3. A 59-year-old female with a past medical history of obesity, arterial hypertension, and dyslipidemia was admitted to the emergency room with a 1-month history of irregular and intermittent palpitations associated with dyspnea for progressively lesser efforts, orthopnea, and peripheral edema. Lab results were unremarkable. The ECG revealed supraventricular tachycardia with a heart rate of 170 bpm. TTE revealed biventricular systolic dysfunction. Adenosine bolus was administered (6 + 12 mg), resulting in auriculoventricular node slowing. Atrial tachycardia was revealed as the supraventricular rhythm.
A bolus dose of amiodarone followed by infusion was administered with reversion to sinus rhythm. Amiodarone was discontinued due to QT interval prolongation. During hospitalization, atrial tachycardia recurred frequently. The patient also presented with typical atrial flutter (AFL).
The patient was submitted to an EPS. At the beginning of the procedure, the ECG showed typical AFL. An activation map of the cavotricuspid isthmus (CTI) was performed, and a point-by-point ablation line at the medium CTI was carried out with reversion to sinus rhythm. Programmed ventricular electrical stimulation showed concentric and decremental conduction through the AV node. Programmed atrial electrical stimulation was performed with rapid induction of focal atrial tachycardia.
An activation mapping was performed with a multipolar catheter. An area of highest precocity at the level of the interatrial septum was located. A patent foramen oval (PFO) was detected, a mapping-ablation catheter progressed through the PFO, and the left atrium was mapped. In the right anteroseptal region, the highest precocity was located. An RF application was performed at this level, with immediate interruption of the tachycardia. Additional ablation lesions were delivered.
Programmed electrical stimulation was performed again under isoprenaline without tachycardia induction. Bidirectional conduction block in CTI was confirmed by pacing maneuvers.
The PT was 110 minutes, FT was 3 minutes, and FD was 17.1 mSv (Figure 5).
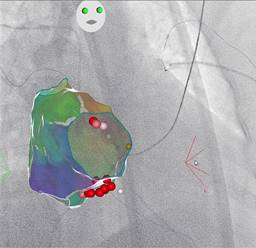
Figure 5: Right anterior oblique projection. His bundle signal (orange dot). A point-by-point ablation line at the medium CTI was carried out, and RF applications were performed in the right anteroseptal region.
Patient 4. A 54-year-old woman, with a medical history of arthropathic psoriasis and some episodes of paroxysmal supraventricular tachycardia (PST) was submitted to an EPS. The programmed ventricular electrical stimulation documented a concentric and decremental retrograde conduction through the AV node. Then, programmed atrial electrical stimulation revealed a dual physiology nodal conduction without tachycardia induction. The programmed atrial stimulation was repeated, under isoprenaline, with an AV nodal re-entry tachycardia induction. An RF pulse was applied in the slow conduction pathway in sinus rhythm. Programmed electrical stimulation was performed again, under isoprenaline, without evidence of dual nodal conduction and, consequently, without induction of AV nodal re-entry tachycardia.
The PT was 60 minutes, FT was 2.5 minutes, and FD was ten mSv (Figure 6).
Discussion
3D-EAM was developed to support the mapping and ablation of arrhythmic substrates by providing a more useful image of heart chamber size and catheter positioning than fluoroscopy alone. Long-standing concerns about the effects of ionizing radiation exposure on laboratory staff and patients led researchers to explore the ability of these systems to effectively and safely minimize radiation exposure. Currently, the goal is to attempt the elimination of fluoroscopy, even for more complex CA procedures. Operators rely on this traditional imaging modality and usually generate several fluoroscopic images during a CA procedure. The integrated view provided by the CARTO-UNIVU™ module in conjunction with the CARTO-3® system, enables operators to obtain several sources of visual information using different imaging modalities, helping navigation during CA procedures. This approach increases confidence and decreases the necessity of repeated fluoroscopic images.11-13
CA procedures can be subdivided into four stages: vascular access, positioning the catheter inside the heart chambers, EPS, and ablation. The EAM can markedly decrease radiation exposure in the last two stages and lesser in the previous two, mostly if the operator is less skilled. «Near-zero» or «zero» fluoroscopic procedures can be achieved only after appropriate training and experience.11-13
Specifically, a diagnostic EPS is associated with a mean effective dose of 3.2 mSv, comparable to 160 chest radiographs (each one has approximately 0.02 mSv) or 1.2 years of background radiation. In contrast, a CA procedure is associated with 15.2 mSv, equivalent to 760 chest radiographs or 5.7 years of background radiation.5 The overall risk of fatal malignancy caused by radiation increases by 0.05% for every ten mSv of exposure. Therefore, a CA procedure with mean radiation exposure of 15 mSv is related to a higher cancer risk of 1 in 750 men aged 50. There is strong evidence linking radiation exposure and cancer for doses > 50 mSv. This cumulative radiation exposure can be reached with a single examination but more frequently by repeated diagnostic or interventional procedures.14
Several studies have examined FD in patients during CA procedures and estimated excess fatal malignancies of 0.3 and 2.3 per 1,000 patients per hour of fluoroscopy.11
Although the total PT was unaffected, several studies found a statistically significant reduction in FT and FD during CA procedures using the CARTO-UNIVU™ module. Sporton et al. compared 3D-EAM (CARTO) and conventional fluoroscopically guided activation. The authors found that 3D-EAM is viable and associated with a drastic reduction in FD (one-fifth) compared with the conventional approach (6.2 ± 6.1 vs 20.8 ± 32.7 Gray, p = 0.003). The mean FT in the CARTO group was 20 minutes less than that in the conventional group (9.3 ± 7.6 vs 28.8 ± 19.5 min, p < 0.001). The use of EAM was associated with similar PT, success, and complication rates.11 Separate series by Akbulak et al. and Huo et al. demonstrated that the use of the CARTO-UNIVU™ module (compared to the CARTO-3® system) for AF ablation procedures was associated with a significant decrease in FD: by 50% in Akbulak et al. series (883 vs 476 cGy × cm2; p < 0.001) and by 75% in Huo et al. series (2,440 vs 652 cGy × cm2; p < 0.001) without affecting PT, complications, or jeopardizing long-term success.15,16 Expanding on this information, Christoph et al. examined the use of the CARTO-UNIVU™ module for a wide spectrum of arrhythmias. The authors revealed a significant reduction in FD: 60% for AFL ablation (1,641 vs 657 cGy × cm2, p = 0.002), 49% for AF (7,369 vs 3,726 cGy × cm2, p < 0.001), 68% for atrial tachycardia (5,088 vs 1,620 cGy × cm2, p < 0.001), and 41% for VT ablation (12,550 cGy × cm2 vs 7,391 cGy × cm2, p = 0.017).1 Cano et al. demonstrated an 82% reduction in FT and 65% reduction in FD with the CARTO-UNIVU™ module in AF procedures.10 Sakama et al. studied CTI ablation in patients with AFL using ablation under the guidance of the CARTO-UNIVU™ module and demonstrated a shorter FT (0.2 ± 0.4 vs 1.7 ± 2.0 min, p < 0.001) and shorter RF time (4.2 ± 2.4 vs 5.1 ± 2.5 min, p = 0.011).17 Moreover, Sommer et al. demonstrated a significant learning effect associated with using this technology and reported a significant decrease in FT and FD with the operator’s familiarity with the technology. Concretely, when comparing the first 50 cases with the last 50 cases, the FT reduced from 6.0 to 1.1 minutes, and FD reduced from 2,363 to 490 (p < 0.001 for both).18
In our series, in contrast to a CARTO-3® group of Christoph et al. study, there was a lower FT during ablation of AP (case 1) (2.3 vs 7.1 ± 1.2 min), PVC (case 2) (1.8 vs 17.6 ± 2.3 min), AFL (case 3) (3 vs 8.6 ± 0.8 min) and PST (case 4) (2.5 vs 23.4 ± 3.1 min). The FD was also reduced during ablation of AP (case 1) (2,536 [7 mSv] vs 3,823 ± 868 cGy × cm2), PVC (case 2) (7,609 [21 mSv] vs 4,688 ± 838 cGy × cm2) and PST (case 4) (3,623 [10 mSv] vs 5,088 ± 969 cGy × cm2), with no reduction in the AFL ablation (case 3).1
This article describes our first experience with the CARTO-UNIVU™ module and includes our learning curve. As our experience of the system has grown, we have used less fluoroscopy to confirm catheter position, which is already available from the CARTO map. We recently acquired a DecaNav® decapolar catheter that allows its placement in the coronary sinus without fluoroscopy, which will further decrease FD.
When considering implementing new technology, cost-effectiveness is an important consideration. Nonetheless, this increased cost is likely compensated by the decrease over the years of radiation-induced malignancies.11
In our experience, the use of the CARTO-UNIVU™ module promoted a marked reduction in radiation exposure during RF ablation of a wide range of arrhythmias without prolonging PT and compromising patient safety. Achieving a low level of radiation exposition is imperative in clinical practice. «Near-zero» or «zero» fluoroscopic procedures are as safe and effective as the traditional fluoroscopy-guided approach. The possibility of broader access at a reasonable cost, mainly in developing countries, would bring this benefit even closer to a large number of patients.











 nova página do texto(beta)
nova página do texto(beta)




