1. Introduction
Currently, the Biochemical Oxygen Demand (BOD) is being replaced by the Chemical Oxygen Demand (COD), since it presents several operational advantages such as short handling time, practicality and low equipment costs. Nevertheless, its analysis requires the use of chemical reagents such as mercury sulfate (HgSO4), potassium dichromate (K2Cr2O7) and silver sulfate (Ag2SO4), that oxidize the sample and leave as a by-product a liquid waste containing high concentrations of silver, mercury, and chromium; likewise, it presents an acid pH (0.01) [1]. Therefore, it is corrosive and toxic, and for its great pollutant potential, it cannot be discharge to the sewage according to international standards listed in Table 1 [2]-[5].
According to the technical standard NMX-AA-030/1-SCFI-2012, which establishes how to determine COD, 10 mL of the sample are required for the analysis, generating 40 ml of hazardous waste [6]. As well, this standard stablishes that each laboratory must contemplate, within its quality control program, the final destination of the waste generated during the measurement.
The waste generated by the determination of the COD presents a concentration of 1133 mg/L of Ag, 2252 mg/L of Hg and 950 mg/L of Cr.
Silver exposure can cause brain damage, argiria, and cardiac anomalies. Also, silver compounds are extremely toxic to animals, causing lung inflammation and reaction cytotoxic [7].
Mercury concentration over 150 mg/L is lethal to human, causing damage to the nervous system, DNA and chromosomes; it also affects the nervous and cardiovascular system and can cause brain damage [8]. Some effects of mercury in animals are kidneys and intestines damage, failure to reproduce, DNA alteration, as well as abortions and blood pressure and heart rate alteration [9].
Furthermore, chromium (VI) concentration over 800 mg/L causes weakening of the immune system and kidneys, damages the liver, cancer, respiratory diseases, stomach ulcers and alters genetic material [10]. Chromium (VI) is considered a cytotoxic and genotoxic element and can cause soil acidity and damage to water bodies [11].
Previous research carried out on the treatment of liquid waste with Ag, Hg and Cr, point to the ion exchange as one of the options, which consist of the replacement of the ions present in an exchange material by ions of a different species that the ones present in the dissolution. This method has the great advantage that, unlike chemical precipitation, it does not generate sludge. However, the ion exchange makes use of a material called resin, which has a high cost of acquisition and maintenance. In addition, the resin generates an effluent with high concentrations of metals that must be adequately managed as hazardous waste if the recovery of metals is not possible. As well, this method is very expensive to treat large water flows [12].
On the other hand, the electrochemical treatments also arise as an option for the removal of heavy metals and consist of oxidation-reduction reactions induced by electrical energy. Compared to chemical precipitation, this technique has the advantage of possible obtaining of the metal with better characteristics for its reuse. However, if there are several metals in the solution, it is difficult to obtain a usable metal product. Likewise, the efficiency of the operation decreases when the concentration of the metal is less than 10 mg / L [13].
Membrane processes, which consist of physical separation of the solutes from the waste water passing through a selective membrane at a certain time of ions, also represent an option for the removal of heavy metals in liquid. These processes are divided into microfiltration, ultrafiltration, nanofiltration, reverse osmosis, dialysis and electrodialysis. Although this technique presents high removal efficiencies, it generates a large amount of sludge containing heavy metals. In addition, the cost of the technique is high due to the acquisition of the membranes, which require continuous maintenance and pretreatment of the sample to avoid dirtiness and lengthen its useful life [14].
This paper is focused on the optimization and application of chemical precipitation as a method of treatment of liquid waste containing Ag, Hg and Cr.
Chemical precipitation, which consists of the separation of metal ions from a solution as a consequence of the decrease in the solubility of metals, presents many advantages over other treatment methods, such as short handling time, it does not require highly qualified personnel, high removal efficiencies of heavy metals, easy application and low operating costs [15].
The aim of this research is to optimize and apply a technique for the treatment of hazardous liquid waste generated by the determination of COD, through a practical, economic, and efficient physicochemical process, which consist of chemical precipitation.
2. Materials and Equipment
2.1. Materials
In order to prepare the sample, 1.5 µm fiberglass filter Whatman 934-AH, a Beaker Pyrex 1L and a Büchner flask Schott Duran 1L was used.
For Ag precipitation, sodium chloride J.T. Baker 99 % purity was needed. Whereas, for Hg precipitation Iron sulfide Meyer 99 % purity was used and for Cr precipitation the precipitating reagent was Sodium hydroxide J.T. Baker 98 % purity.
Nitric acid J.T. Baker 66.5 % purity, Silver standard Perkin Elmer 1000 ppm/HNO3 500 mL, Mercury standard Perkin Elmer 1000 ppm/HNO3 500 mL and Chromium standard Perkin Elmer 1000 ppm/HNO3 500 mL were used to perform the sample characterization.
3. Experimental Methods
3.1. Sampling
The representative sampling of hazardous waste with Ag, Hg and Cr from the analysis of the COD of the laboratories of CONAGUA (Water National Commission), which serves industries and other laboratories generating a diverse sample, was carried out, following the standards NOM-011-SCT2/2012 and PROY-NMX-AA-003/3-SCFI-2008 [16]-[17].
According to the previous standards, 5 samples of 2 L of COD waste were taken during 2 years, from 2016 to 2018 and stored separately in a 4-liter amber flask, kept in the dark at a 2°C temperature, according to the standard ISO 5667-3:2018 [18]. A 1 L aliquot was taken from each sample to carry out the experiments.
3.2. Characterization
A 100 ml sample of the COD liquid waste was taken and the pH was measured following the standard NMX-AA-008-SCFI-2000 with a pH meter, with which the temperature of the sample was also measured according to the standard NMX-AA-007-SCFI-2000 [19]-[20].
Subsequently, the quantitative determination of Ag, Hg and Cr was carried out. The analysis of the previous elements was carried out by the technique of Atomic Absorption and Flame Emission in Perkin Elmer Atomic Absorption Spectrophotometer AA200 following the standard NMX-AA-051-SCFI-2016 [21].
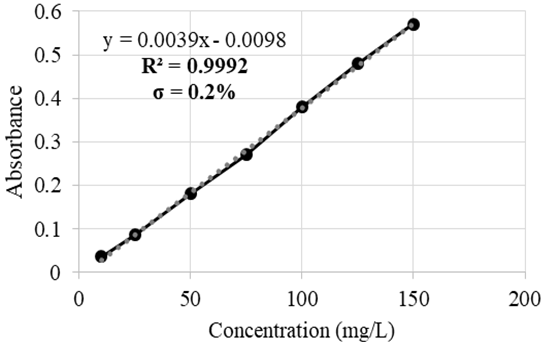
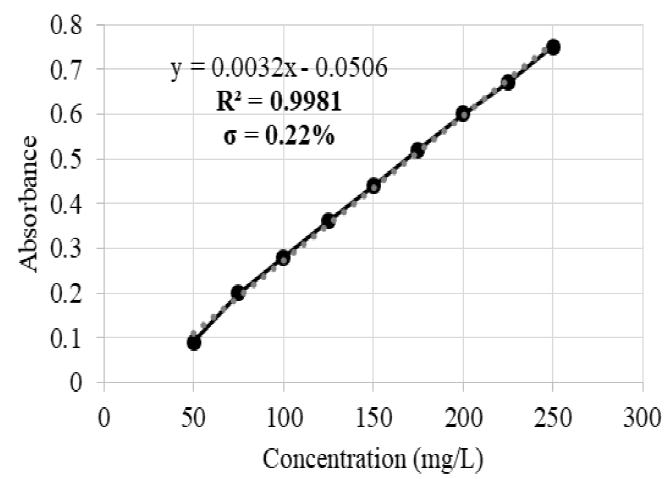
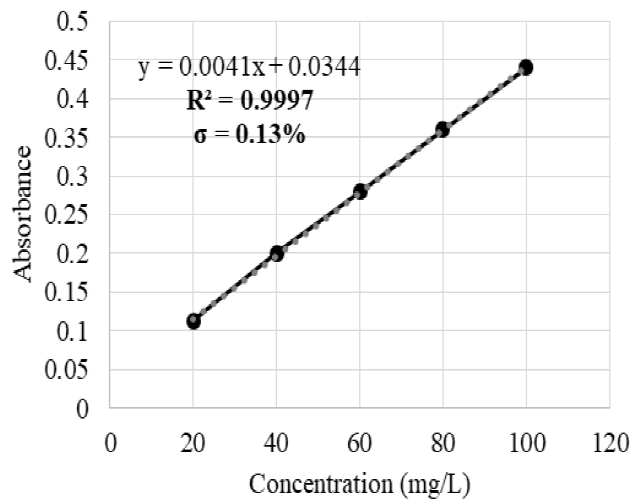
The calibration curves covered the following ranges: 10-150 mg/L for Ag with a coefficient of determination (R2) of 0.9992 and a dilution factor of 1:10 (Figure 1); 50-250 mg/L for Hg with R2=0.9981 and 1:10 dilution factor (Figure 2); and 10-100 mg/L for Cr with R2=0.9997 and 1:10 dilution factor (Figure 3). For each of the samples, 3 replicas were carried out with a maximum 5 % deviation.
3.3. Reproduction
According to Gutiérrez et al. (2013) [1], 2 g of NaCl were added to a 1 L sample and kept it in constant agitation for 10 minutes. Then, it was vacuum filtered using 1.5 µm and the process was repeated carrying out a second precipitation.
A sulphidation device was installed in the fume hood and the remaining liquid of the Ag precipitation was placed in a water bath in constant agitation. 50 g of FeS and 300 mL of HCl were placed in a beaker. Both beakers were connected to a gas scrubber containing distilled water. The solutions were kept in contact for 15 minutes and processed with vacuum filtration.
The remaining liquid was diluted to a 1:2 concentration with distilled water and 30 mg/L Na3PO4 were added. Subsequently, a 50 % NaOH solution was added until obtaining a pH 8.5. The solution was centrifuged for 60 minutes at 7500 rpm.
3.4. Optimization
The chemical reactions were performed in the following order considering the solubility product constant of the compounds to be precipitated listed in Table 2, and the solution pH since Ag and Hg precipitates need acid pH to be formed and Cr precipitation need neutral pH to occur.
Table 2. Solubility constant of the precipitate products and the compounds present in the COD waste.
| Compound present in the COD waste | Solubility product constant (kps) | Precipitate product | Solubility product constant (kps) |
| Silver sulfate (Ag2SO4) |
1.6x10-5 | Silver chloride (AgCl) | 1.6x10-10 |
| Mercury sulfate (I) (Hg2SO4) |
4.8x10-4 | Mercury sulfide (HgS) | 4.0x10-54 |
| Chromium sulfate (III)
Cr2(SO4)3 |
7.5x10-7 | Chrome hydroxide (III) Cr(OH)3 | 7x10-31 |
Based on stoichiometric calculations, experiments of the treatment method were conducted in laboratory for its optimization considering the variables contact time, amount of reagent and temperature. First, an aliquot of 1L was taken and prepared by vacuum filtration using 1.5 µm fiberglass filter to remove any suspended solids that could interfere with subsequent reactions.
In the method proposed by Gutiérrez et al. (2013) [1], NaCl is used as a reagent to precipitate silver as AgCl. According to this, 2 g of NaCl were added to the sample and kept in constant agitation for 5 minutes. The aliquot was then centrifuged for 5 minutes at 3600 rpm to separate the phases and recover the silver chloride. Subsequently, the procedure was repeated with a second precipitation. Eq. (1) shows the process of Ag precipitation.
For Hg precipitation, and in order to recover Hg as HgS, in this phase 0.5 g of FeS were added to the remaining liquid of the previous stage and it was kept in agitation for 5 minutes at room temperature. Then, it was centrifuged at 3600 rpm for 10 minutes and the process was repeated for a second precipitation according to Eq. (2).
In order to precipitate Cr (VI) as Cr(OH)3, the pH of the remaining liquid from the previous phase was adjusted to obtain 8.5 ± 0.5 following Eq. (3).
In order to do so, the sample was diluted with distilled water to a 1:2 concentration, which was placed in constant agitation and in contact with a pH meter. Then, a 20 % solution of NaOH was added until reaching a pH of 8.5 ± 0.5. Subsequently, the sample was centrifuged for 10 minutes at 3600 rpm to recover Cr(OH)3.
3.5. Statistical analysis
The obtained results from the optimization stage were submitted to a statistical analysis using a 23 factorial design to evaluate the effect of the variables time, reagent concentration and temperature in the final concentration of heavy metals, and in this way, determine which is the optimal value of the variables.
The following experiments were conducted in which different reagent concentrations (FeS for Hg and NaCl for Ag), temperature and contact time were evaluated to precipitate Ag and Hg. To obtain the optimal value of the previous variables, the final concentration of Ag and Hg was measured as response; Table 3 shows the decoded values for each analyzed variable.
Table 3. Levels of the variables to be optimized for Ag and Hg precipitation.
| Variable | Level (Ag precipitation) | Level (Hg precipitation) | ||
| - | + | + | - | |
| Reagent (g/L) (A) | 4 | 8 | 1 | 2 |
| Contact time (min) (B) | 10 | 40 | 10 | 20 |
| Temperature (°C) (C) | 25 | 60 | 25 | 60 |
A 95 % Tukey test was carried out to establish the optimal combination of variables in the precipitation of Ag and Hg.
The quadratic equation model for predicting the optimal conditions for Ag and Hg precipitation can be expressed according to Eq (4).
Where (i) is the linear coefficient, (ii) the quadratic coefficient, (β) the regression coefficient, (ij) the interaction coefficient, (k) the number of factors studied and optimized in the experiment and (ɛ) the random error.
3.6. Application and characterization of the sample and by-products
At the end of the treatment, the remaining liquid from the process was characterized by Atomic Absorption for Ag and Cr and the hydride generator for Hg in the Atomic Absorption Spectrophotometer. The calibration curves covered the following ranges: 0.1-10 mg/L for Ag with a coefficient of determination (R2) of 0.9976; 0.007-0.02 mg/L for Hg with R2=0.9987; y 0.1-10 mg/L for Cr with R2=0.9978.
Likewise, X-ray fluorescence was performed for the salts of the metals recovered during the process to evaluate the purity of the by-products generated, an analysis carried out at the National Institute of Nuclear Research (ININ).
The optimized method was applied. In order to do so, 5 replicas of the method were carried out in different days and by different analysts to guarantee the reproducibility of the technique.
4. Results and discussion
Color changes were observed during the precipitation process, indicating that the reactions were being carried out correctly. A change in color from aquamarine to thick blue-white was observed accompanied by the formation of a white precipitate in the Ag precipitation stage.
At the end of the centrifugation, the liquid returned to its original aquamarine color. In the Hg precipitation stage, the sample changed from an aquamarine color to a grayish black color, presenting the formation of a black precipitate. During the precipitation of Cr, the liquid became light brown, ending in a dark green.
The remaining liquid complies with the national and international standards listed in Table 4 for the three heavy metals and pH, so that the waste is no longer considered hazardous and can be disposed safely to the sewer or can be reused in industrial processes.
Table 4. Concentration of heavy metals before and after treatment.
| Element | Before treatment (mg/L) | After treatment (mg/L) | Allowable limits for discharge | ||
|---|---|---|---|---|---|
| Mexico | Germany | China | |||
| Ag | 1133 | 0.10 | 5 | 0.1 | 0.5 |
| Hg | 2252 | 0.01 | 0.01 | 0.05 | 0.05 |
| Cr | 950 | 0.36 | 0.5 | 0.5 | 1.5 |
| pH units | |||||
| pH | 0.01 | 8.5 | 5.5 ≤ pH ≥ 10 | ||
The treatment method offers removal efficiencies of 99.99 % for Ag and Hg, and 99.96 % for Cr, in addition to a neutral pH.
Table 5 shows a comparison between the method established by Gutiérrez et al. (2013) [1] and the method optimized in this paper.
Table 5. Comparison of liquid waste treatment methods generated by the COD.
| Factor | Gutiérrez et al. (2013) | Optimized method | ||
|---|---|---|---|---|
| Removal efficiency | Ag | 94.31 % | Ag | 99.99 % |
| Hg | 99.99 % | Hg | 99.99 % | |
| Cr | 98.17 % | Cr | 99,96 % | |
| Time | Ag | 20 minutes plus vacuum filtration | Ag | 20 minutes |
| Hg | 15 minutes plus vacuum filtration | Hg | 30 minutes | |
| Cr | 60 minutes plus NaOH addition | Cr | 10 minutes plus NaOH addition | |
| Total time | 95 minutes plus vacuum filtration | 60 minutes | ||
| Amount of reagent for 1L | Ag | 4 g of NaCl | Ag | 4 g of NaCl |
| Hg | 50 g of FeS of 300 mL of HCl | Hg | 1 g of FeS | |
| Cr | 35 ml of NaOH | Cr | 36 ml of NaOH | |
The treatment of hazardous waste was carried out in 60 minutes, compared to the 95 minutes used by Gutiérrez et al. (2013) [1], due to the optimization made in the amount of reagents, the use of centrifugation to separate the phases and the adding of FeS directly instead of using a sulphidation device. Likewise, the concentration used of the precipitating reagents was optimized based on stoichiometric calculations, allowing to optimize the reaction time.
According to the same authors, a sulphidation device must be installed and HCl added to precipitate Hg; However, in the optimization presented in this paper, high efficiencies of Hg removal were obtained by adding FeS directly and keeping in contact for 5 minutes. For the precipitation of Cr, high removal efficiencies were obtained using a 20 % NaOH solution and a 10 minute centrifuge time.
It should be mentioned that, unlike the authors cited, in the present paper centrifugation is used as a method of phase separation and not vacuum filtration, since the latter required up to 2 hours to filter only 1 L of sample due to the large amount of solids recovered in Ag and Hg precipitation; whereas, centrifuging the sample, a good separation of the phases is achieved, as well as high removal efficiencies and a reduction of the time used up to 92 %. The chemical reactions carried out in the present work did not require the addition of thermal energy; therefore, they were carried out at room temperature.
Also, it has been carried out the optimization of the removal efficiency of Ag, Hg and Cr and the costs of the method. It is also verified, with a 95 % confidence, that the method is reproducible; therefore, it could be implemented in CONAGUA laboratories.
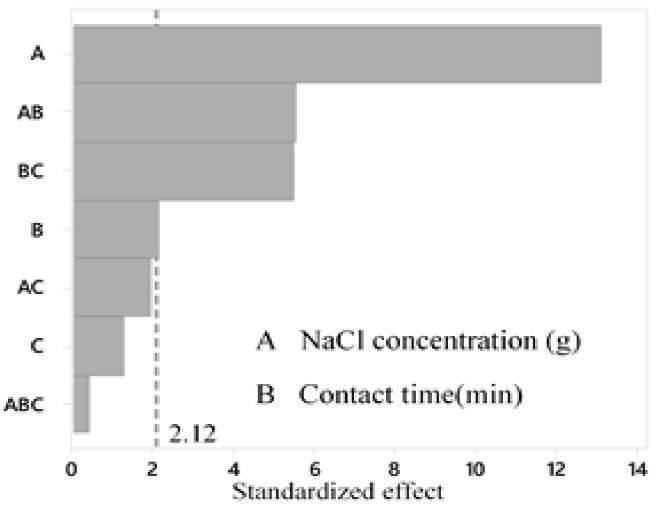
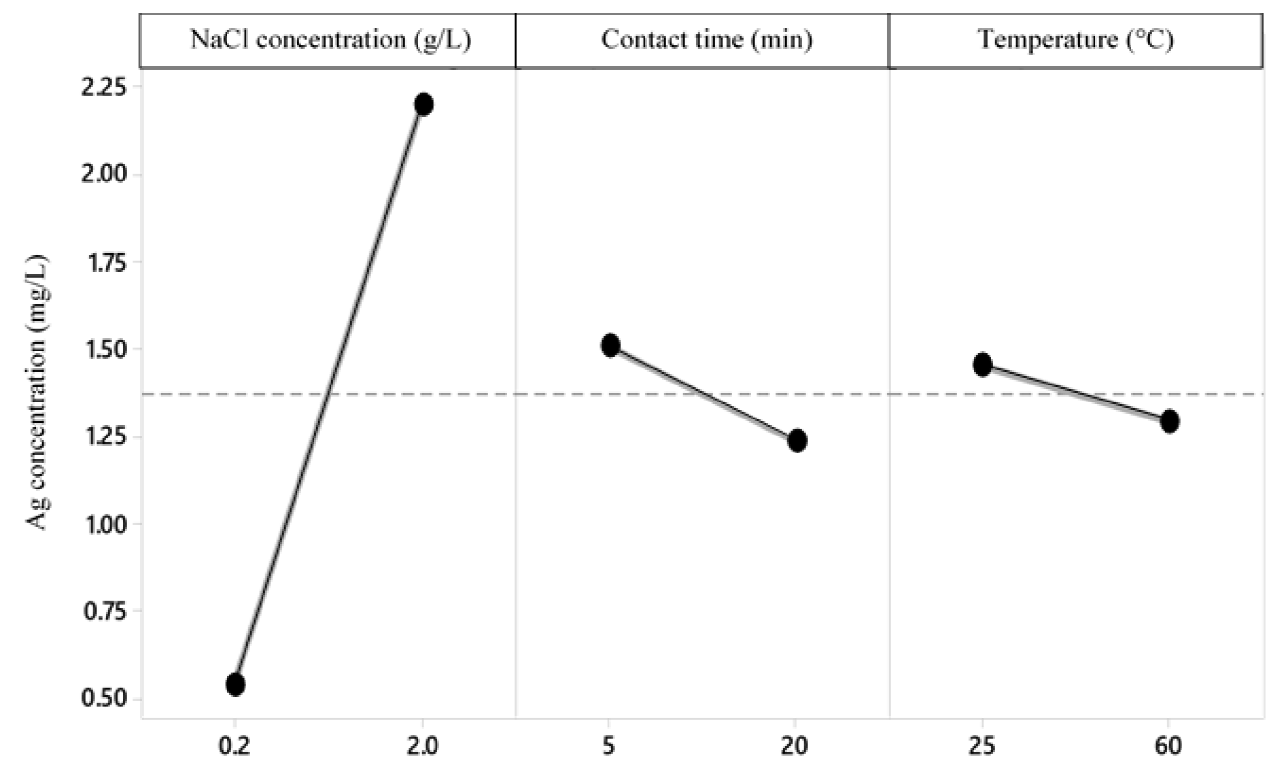
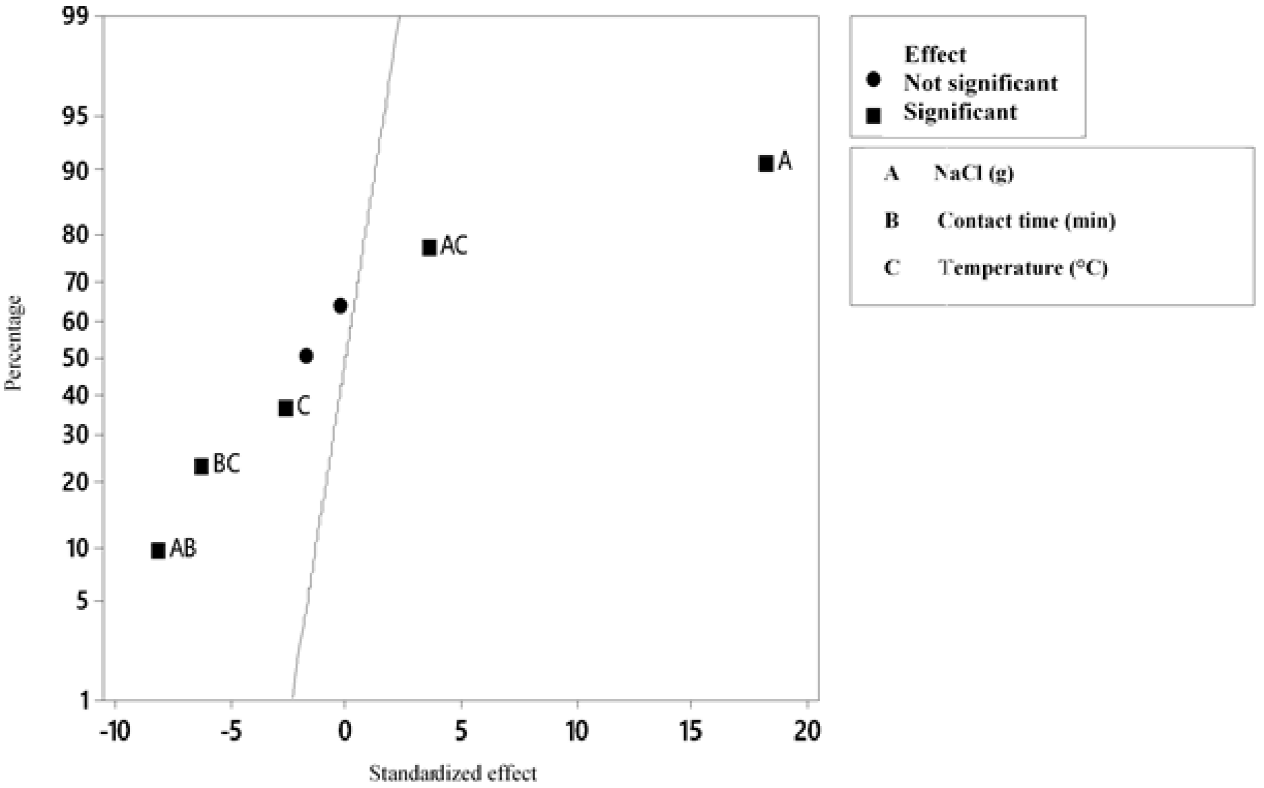
According to the variance analysis (Table 6), with a level of significance of 95 %, for the silver precipitation process, the variables contact time and amount of NaCl added to the sample have a significant effect on the final concentration of Ag; while, the temperature has no effect on it (Figure 4, 5 y 6).
Table 6. Variance Analysis for Ag precipitation.
| Source | df | SS | MS | F Value | P Value |
| C | 1 | 0.1538 | 0.1538 | 1.58 | 0.226 |
| B | 1 | 16.6350 | 16.6350 | 171.42 | 0.000 |
| A | 1 | 0.4453 | 0.4453 | 4.59 | 0.048 |
| B*A | 1 | 2.9786 | 2.9786 | 30.69 | 0.000 |
| C*B | 1 | 0.3604 | 0.3604 | 3.71 | 0.072 |
| A*C | 1 | 2.9295 | 2.9295 | 30.19 | 0.000 |
| A*B*C | 1 | 0.0159 | 0.0159 | 0.16 | 0.691 |
| Error | 16 | 1.5527 | 0.0970 | ||
| Total | 23 | 25.0711 |
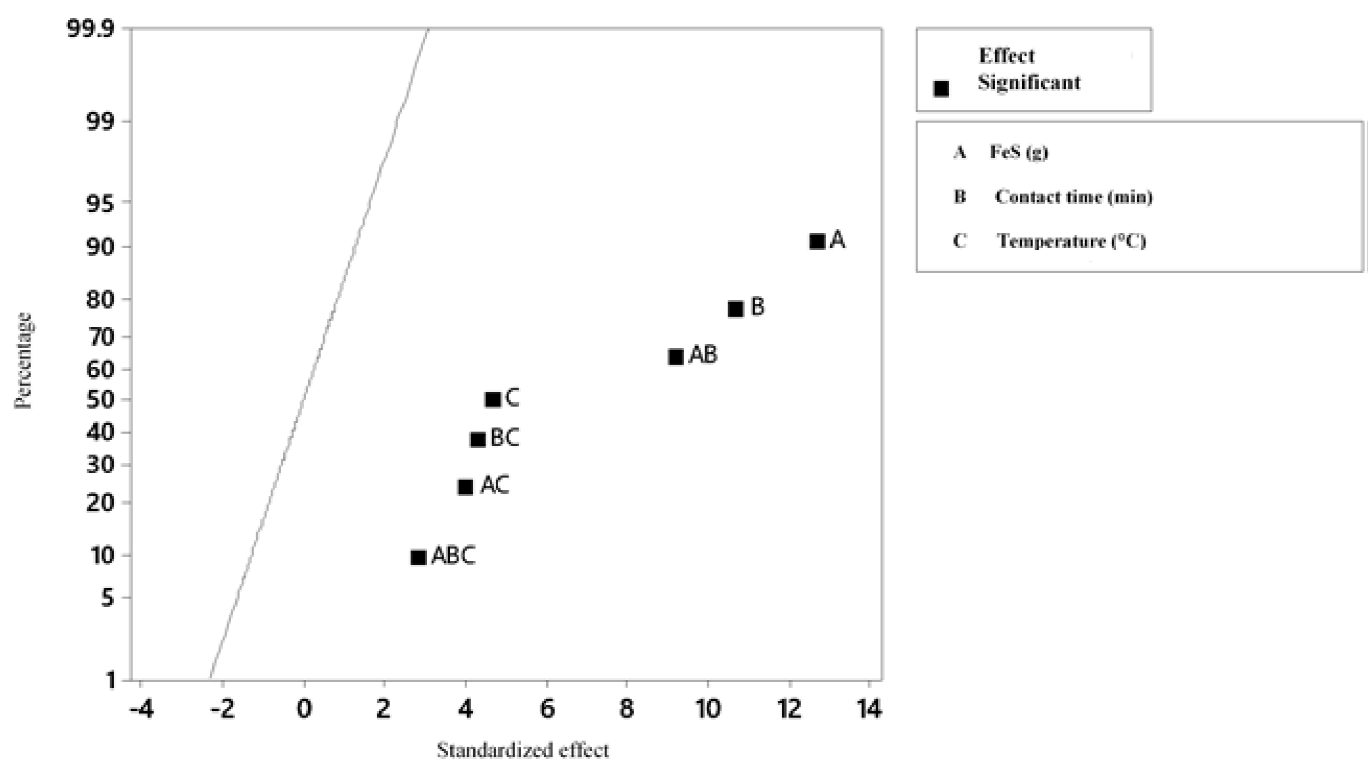
On the other hand, it is proved that there is interaction between the amount of NaCl added and the contact time, the amount of NaCl and the temperature and contact time and temperature. However, there is no interaction between the three factors; the most significant interaction is between the contact time and the NaCl concentration (Figure 4). In addition, it is concluded that the 23 factorial design is suitable for the experiment, since besides being a simple model, it allows explaining the 95.16 % variability of the data. Thus, it is concluded that the variables temperature and amount of NaCl are those that influence the process, the latter being the most influential in the final concentration of Ag.
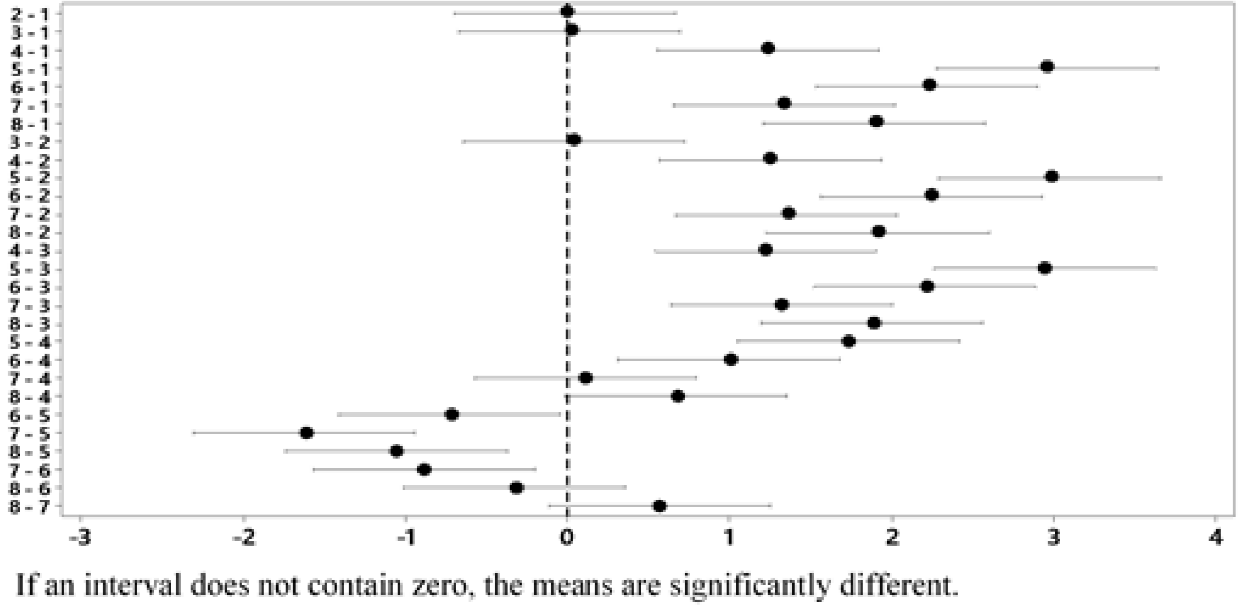
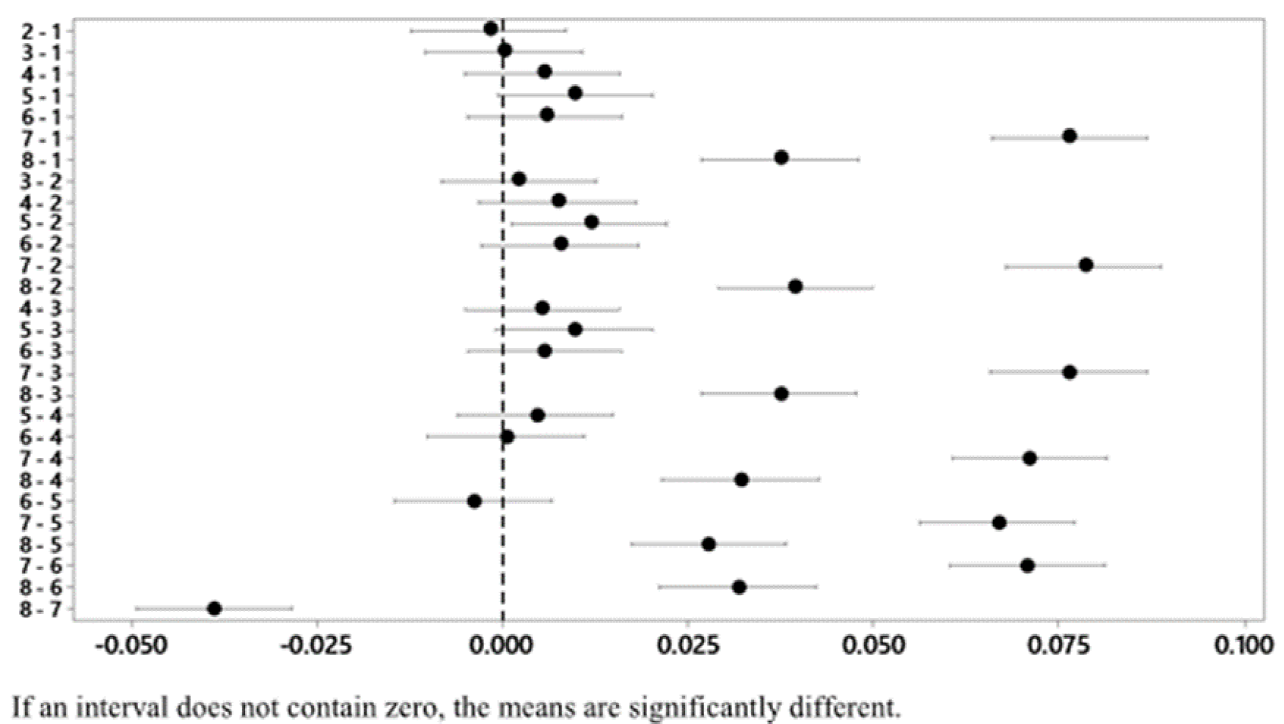
The treatment whose combination is 2 g-5 min-60 °C presents a lower final concentration of Ag. With a confidence of 95 % and according to Tukey's test, it is verified that this treatment does not differ statistically from treatments 1 and 3, which represent the combinations 4 g-20 min-25 °C and 4 g-40 min-60 °C, respectively (Figure 7). Therefore, it is possible to use any of the 3 previously mentioned treatments to obtain an Ag concentration that complies with the standard.
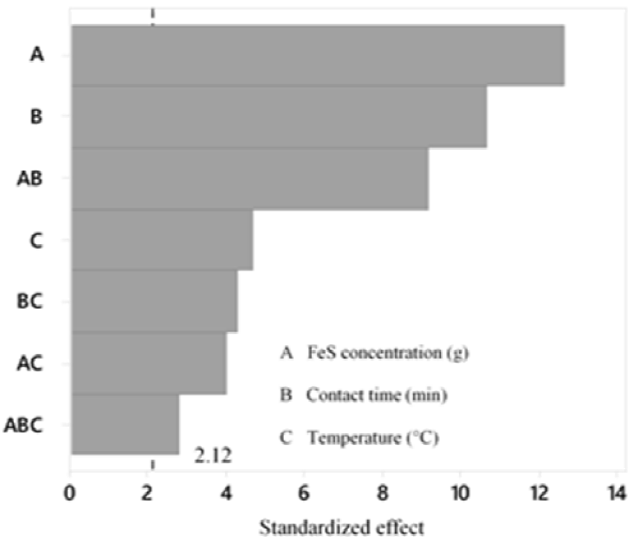
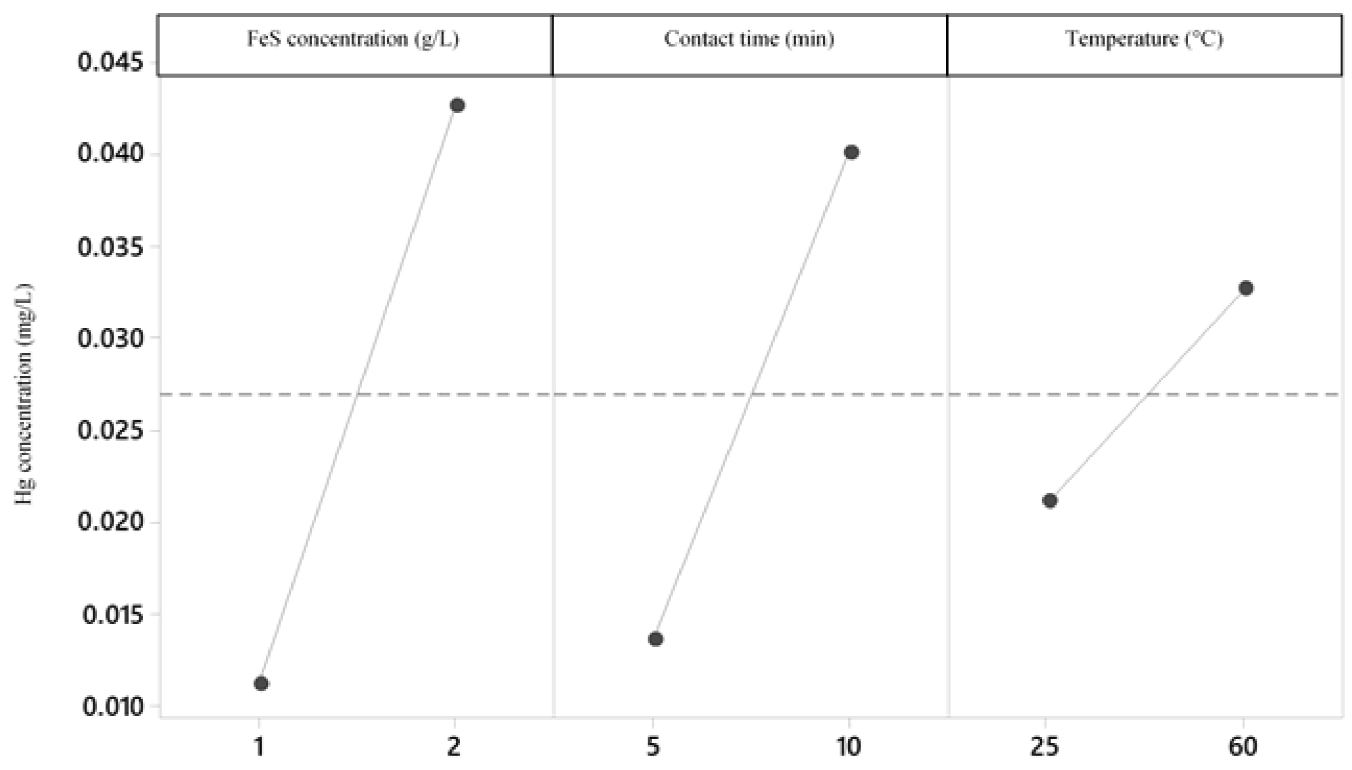
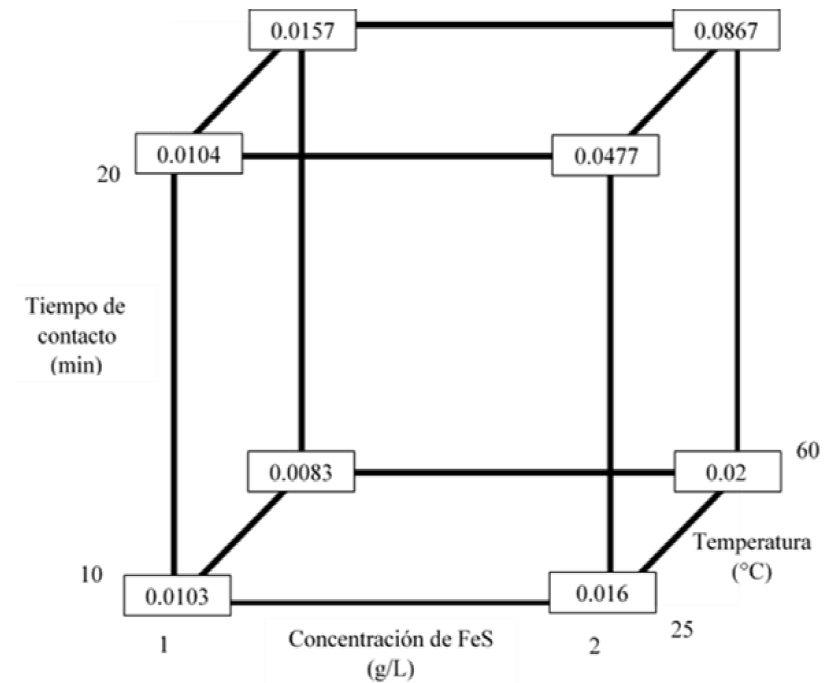
According to the variance analysis performed (Table 7) with a level of significance of 95 %, for the Hg precipitation process, the three variables have a significant effect on the final concentration of Hg, with the concentration of FeS being the most influential (Figure 8-10); Therefore, the three variables must be optimized. Likewise, it is proved that there is interaction between the three variables and that the most significant interaction is between the contact time and FeS concentration (Figure 8); in addition, the 23 factorial design explains 94.77 % of the variability of the data.
Table 7. Variance Analysis for Ag precipitation.
| Source | df | SS | MS | F Value | P-value |
| B | 1 | 0.0059 | 0.0059 | 160.94 | 0.000 |
| C | 1 | 0.0042 | 0.0042 | 114.18 | 0.000 |
| A | 1 | 0.0008 | 0.0008 | 21.82 | 0.000 |
| B*C | 1 | 0.0031 | 0.0031 | 84.34 | 0.000 |
| A*B | 1 | 0.0005 | 0.0005 | 16.14 | 0.001 |
| A*C | 1 | 0.0007 | 0.0007 | 18.21 | 0.001 |
| A*B*C | 1 | 0.0003 | 0.0003 | 7.87 | 0.013 |
| Error | 16 | 0.0006 | 0.0001 | ||
| Total | 23 | 0.0161 |
The treatment whose combination is 1 g-5 min-60 °C presents a lower final concentration of Hg. With a confidence of 95 % and according to Tukey's test, it is verified that this treatment does not differ statistically from the treatment that corresponds to the combination 1 g-5 min-25 °C (Figure 11). Therefore, it is possible to use any of the 2 previously mentioned treatments to obtain an Hg concentration that complies with the standard.
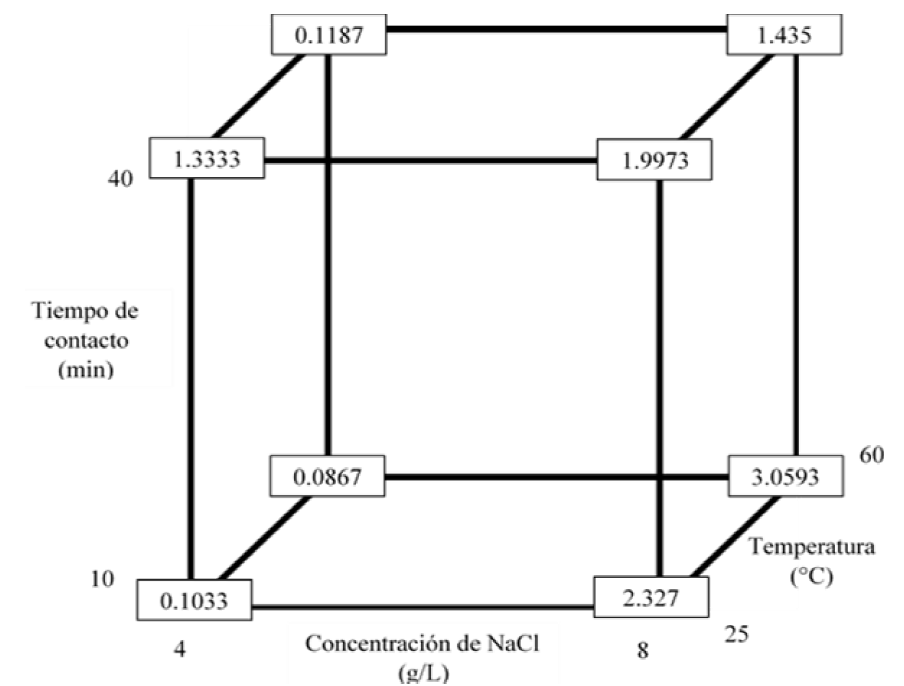
To optimize time, amount of reagent used and save energy, it is concluded that the best treatment to remove Ag is the treatment in which 0.2 g of NaCl are added, a contact time of 5 minutes is needed and carried out at room temperature. This treatment presented an average final Ag concentration of 0.1033 mg/L (Figure 12).
In order to save energy, it is concluded that the best treatment to remove Hg is the treatment in which 1 g of FeS is added, there is a contact time of 5 minutes and it is carried out at room temperature. This treatment presented an average final concentration of Hg of 0.0103 mg/L (Figure 13).
Table 8 shows the results obtained from the 5 replicas that were carried out of the optimized method.
Table 8. Final concentration of Ag, Hg and Cr after the optimized treatment method.
| Replica | Ag (mg/L) |
Hg (mg/L) |
Cr (mg/L) |
| 1 | 0.1 | 0.0110 | 0.360 |
| 2 | 0.101 | 0.0101 | 0.355 |
| 3 | 0.1 | 0.0100 | 0.351 |
| 4 | 0.11 | 0.0102 | 0.360 |
| 5 | 0.1 | 0.0101 | 0.360 |
| Mean ( |
0.1022 | 0.01028 | 0.3572 |
| Standard deviation (S) | 0.0044 | 0.00041 | 0.0040 |
| Coefficient of variation (CV) | 4.29 % | 3.56 % | 1.025 % |
| Horwitz coefficient (% CV) | 22.55 % | 31.87 % | 18.68 % |
According to Horwitz and Albert [22], the precision of the method is considered acceptable when its coefficient of experimental variation is lower than the value calculated with the Horwitz equation following Eq. (5).
Where (C) is the concentration of the analyzed element expressed in g/ml.
Replicas listed in Table 5 present a coefficient of variation much lower than that calculated with the Horwitz equation, which indicates that it is possible to apply the method obtaining an excellent precision.
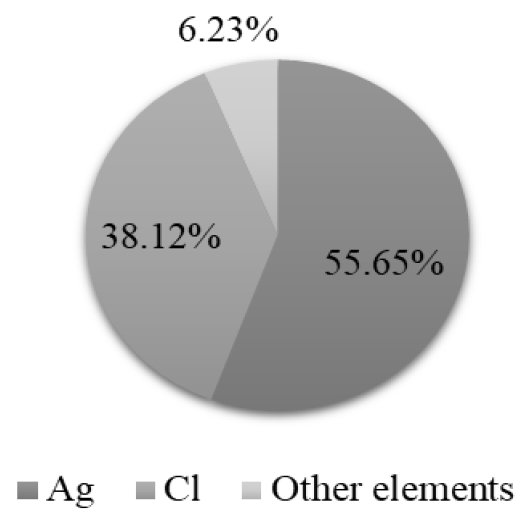
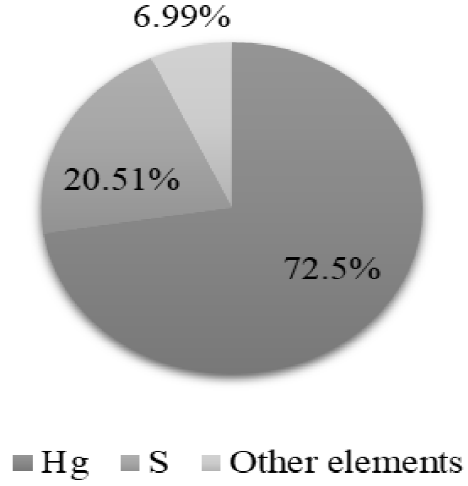
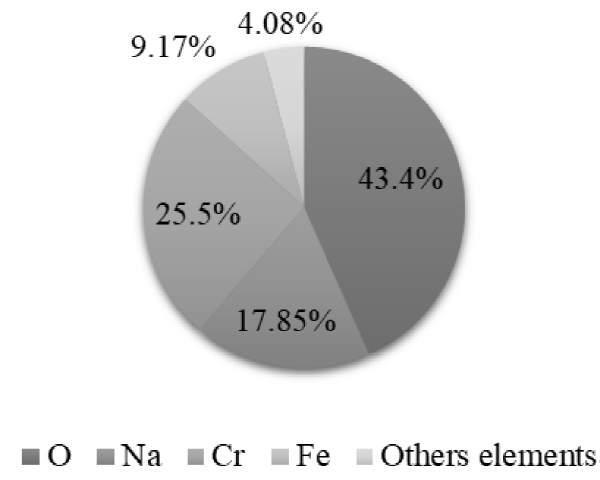
The X-ray fluorescence indicates that the solids recovered during the procedure present high percentages of purity, being 94% for the silver chloride (AgCl) recovered in the Ag precipitation (Figure 14) and 93 % for the mercury sulfide (HgS) recovered in Hg precipitation (Figure 15). Therefore, these compounds can be reincorporated into other productive processes. Chromium hydroxide (Cr(OH)3), obtained in the precipitation of Cr, presents high concentrations of sodium and iron due to the presence of other contaminants in the liquid waste, which did not allow a proportion of the precipitating reagents to react (Figure 16).
The X-ray fluorescence analysis verifies that the precipitation process is being carried out correctly by demonstrating that, in the Ag precipitation stage, Ag and Cl are mostly recovered; in the Hg precipitation stage, Hg and S are recovered; and in the precipitation of Cr, the elements that compose Cr(OH)3 are recovered.
The results obtained show that the treatment method removes the heavy metals at high concentrations in such a way that they comply with the maximum permissible limits established in the standard NOM- 002-SEMANAT-1996, GB 8978-1996 and Ordinance on requirements for the discharge of wastewater into waters. Through the treatment of waste obtained from the laboratory of CONAGUA Puebla, which serves industries and other laboratories generating a diverse sample, it is verified that the technique allows reducing the toxicity of this type of hazardous waste. Nonetheless, it is recommended to perform more trials in order to prove that this treatment method is reproducible and can apply to industrial wastewater.
The optimized process developed in this paper is practical, which uses only typical materials from a chemical laboratory; economic; whose value per treated liter is $11.00 MXN, and efficient physicochemical process, which presents removal efficiencies of Ag, Hg and Cr greater than 99.95 % and complies with the maximum permissible limits established in national and international standards.
4. Conclusions
The method exhibits important characteristics to highlight: 1) high efficiency of removal of heavy metals, 99.99 % for Ag, 99.99 % for Hg and 99.96 % for Cr; 2) pH neutralization (8.5); 3) reduction of the hazardousness of the liquid waste to be reused in industrial processes; 4) recovery of metallic compounds of high purity for their reincorporation to reproductive processes; 5) reduction of the amount of reagent and temperature; 6) the maximum permissible limits established in NOM-002-SEMARNAT-1996 for wastewater discharge to the sewage system were compiled and 7) the use of the treatment technique for other types of waste containing Ag, Hg and Cr.
With the optimization and application of the method there is the innovation to treat the hazardous liquid waste generated by the determination of the COD in an economic, simple, effective and friendly way with the environment, also reducing the impact on society by reducing the hazard that the waste represents for human health. The present project could be an appropriate option for each generating agency to treat its waste, both in academic and industrial laboratories, or for companies responsible for hazardous waste to do more than confine them. With the innovation of the model projected to the user, there is an openness for the technique to be implemented in the laboratories of CONAGUA and, thus, this organism promotes the accredited laboratories to apply the technique of treatment of hazardous liquid residues arising from the determination of the COD or, on the other hand, the method is implemented by the industries that generate liquid waste containing Ag, Hg and Cr.











 nueva página del texto (beta)
nueva página del texto (beta)