Introduction
Vegetable oils are an essential part of virtually all human diets. The primary lipids groups present in oils are triglycerides, which are found in the cells responsible for storing and maintaining components of biological membranes (González et al., 2006). Vegetable lipids are usually more unsaturated than those of animal origin, which results in a higher rate of oxidation reactions and faster consumption of the antioxidants present. Moreover, there is a notorious variability in the relative composition of saturated, polyunsaturated, and monounsaturated fatty acids in different vegetable oils (Xing et al., 2019). Specifically, sunflower oil is characterized by a high proportion of polyunsaturated fats, which, although sought for their effects and health benefits, are also more prone to lipid peroxidation, which is why it was selected as the object of study.
Oxidation of vegetable oil is a natural and irreversible reaction related to the oxidation of unsaturated fatty acids by atmospheric oxygen, which increases significantly at high temperatures. It has been documented that the formation and appearance of peroxides is a good indicator of the oxidative rancidity of the oil, since they are one of the most relevant initial formation compounds in the lipid oxidation process (Vanhanen & Savage, 2006), generating unpleasant tastes and odors.
Based on the above, one of the interesting challenges in the field of biotechnology is to achieve the stabilization of edible vegetable oils through the use of effective antioxidant agents (Jan et al., 2001). Traditionally, synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tert-butylhydroquinone (TBHQ), have been used to retard the oxidative degradation of lipids during storage and food preparation. However, their use has been associated with health problems (Engin et al., 2011). Thus, the interest of the population worldwide in consuming natural products that promote preservation and health improvement is increasing. It has been reported that some herbs and spices possess properties that are antioxidants (Katalinic., et al 2006; Gutiérrez-del-Río et al., 2021) and retard the rancidity of oils through the rosemary oleoresins and extracts of hyssop, lemon balm, sage, thyme, and Spanish oregano, among other plants of the Labiatae family (Pokorny & Trojakova, 2004). However, it is worth mentioning that the efficacy of this type of compound is highly dependent on the species used, the type and polarity of the solvent used, the extraction procedure, and the purity and identity of the active compounds present in the extract obtained from natural products, which has led to the application of antioxidants in natural processes have been limited, mainly due to the high variability in their usefulness and high price (Mustafa et al., 2021).
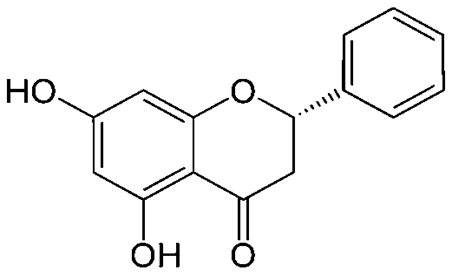
Mexican oregano (Lippia graveolens Kunth) is highly valued in the marketplace due to the presence of volatile, fat-soluble compounds such as thymol and carvacrol in the essential oil. During the oil extraction process, the leaves of this plant produce a large amount of solid waste (bagasse), which represents approximately 95 % of the dry weight with respect to the initial weight (Martínez-Nataren et al., 2012). These residues are rich in flavonoids, which have been shown to have antioxidant properties. However, their use is currently minimal, as they are usually only used as animal feed or compost, or are improperly disposed of, causing potential environmental problems. Among the flavonoids present in both Mexican oregano (L. graveolens) and the residual bagasse, pinocembrin, a flavanone with important benefits, stands out for its nutritional and natural antioxidant properties (Lin et al., 2007). Despite its potential, there is still a lack of studies that explore its extraction from Mexican oregano and residual bagasse, as well as the important application that it could have in the food sector. Studies by Lin et al. (2007) and Cortés-Chitala et al. (2017), have demonstrated the presence of the flavonoid pinocembrin (Figure 1) in Mexican oregano, which has been reported as an important antimicrobial, anti-inflammatory, antioxidant, anticarcinogenic agent, as well as a protective compound against cerebral ischemia and neurodegenerative diseases such as Alzheimer's and Parkinson's, hence, it is considered a compound of special interest in the pharmaceutical industry (Chung et al., 2011; Liu et al., 2014; Zheng et al., 2018). In the food industry, pinocembrin has also shown significant potential for application in food products due to its antimicrobial and antioxidant properties, as reported by Vargas-Sánchez et al. (2014) to extend the shelf life of beef patties using propolis extracts as a natural preservative.
The search for trends and approaches in the utilization and separation of flavonoid compounds, such as pinocembrin, has been carried out in previous studies. Osti et al. (2010) isolated the compound from Sphaeralcea angustifolia, a Mexican plant commonly used in traditional medicine, using column chromatography and achieved the separation of pinocembrin (0.066 mg/mg Ex). On the other hand, Nyotia et al. (2016) isolated pinocembrin from Artocarpus odoratissimus, and the crude extracts were fractionated using vacuum liquid chromatography (VLC) on silica gel using the same separation method. Granados et al. (2018) isolated different flavonoids from three Mexican propolis, including pinocembrin (19, 33, and 55 mg/g Ex), in contrast to the fact that these authors used a gradient mixture as a mobile phase.
Based on the background information highlighting the significant effects of pinocembrin in several applications, in the present study, a method for the concentration of pinocembrin was developed by our research group (Cuevas-González et al., 2021). This method was used to obtain pinocembrin-enriched fractions from hydroethanolic extracts obtained from the solid waste generated during the extraction process of Mexican oregano (L. graveolens) essential oil, with the aim of evaluating the antioxidant potential of pinocembrin in sunflower oil, an oil that is widely marketed and consumed worldwide.
Material and Methods
Raw materials
Mexican oregano (Lippia graveolens Kunth) was obtained from the locality of Huejuquilla, Jalisco, located at 22° 450' N latitude, 103° 450' W longitude, and at an altitude of 1,450 meters above sea level; the sample of Mexican oregano was obtained after the traditional process of shaking and sun drying for 3 days after the cut at the flowering stage at 10 %, maintaining a moisture content of 10 % w/w.
The hulled sunflower seeds were purchased in a municipal market in Zapopan, Jalisco, maintained at room temperature (20 °C), and a moisture content of less than 8 %.
Reagents and standards
The flavonoid standards galanin (99.3 %) and naringenin (99.4 %) were purchased from Sigma-Aldrich (Darmstadt, Germany); whereas pinocembrin (99.8 %), hispidulin (98.9 %), and taxifolin (99.1 %) were purchased from PhytoLab (Vestenbergsgreuth, Germany). Folin-Ciocalteau's reagent, gallic acid (GA), and Di (phenyl)-(2, 4, 6-trinitrophenyl) iminoazanium (DPPH) were purchased from Sigma-Aldrich. Other reagents and chemical
Extraction of essential oil from Mexican oregano (Lippia graveolens) by steam entrainment
The extraction of the essential oil of Mexican oregano was carried out by hydrodistillation method (Hernández et al., 2008), using only the aerial part (leaves and flowers). Fifty g of plant material was placed in a flask 1000 mL balloon with 500 mL of distilled water, maintaining a constant extraction time of 4 h. After the distillation time had expired, the essential oil was recovered and quantified. The aqueous extract obtained was characterized in terms of its pinocembrin content. The solid wastes generated in the extraction of essential oil (oregano bagasse) were filtered through a medium pore filter paper, weighed, and subjected to a drying process at a temperature of 40 °C until constant weight in an oven (GL-70 A - Fab, Nacional). With the dry solid residues (humidity 8-10 % w/w), a hydroethanolic extract was obtained.
Obtaining the hydroethanolic extract from the solid waste generated during the extraction of Mexican oregano (Lippia graveolens) essential oil
The hydroethanolic extract was obtained by the method of maceration with agitation and controlled temperature, optimized by Flores-Martínez et al. (2016). The working system has a reflux to avoid losses due to evaporation. The solid residue (Mexican oregano without essential oil) was subjected to grinding and sieving to obtain the desired particle size (0.423 mm) to facilitate the extraction process. The extraction was carried out in a balloon flask of 500 mL, with a solute-solvent ratio of 1:20, using 58 % ethanol as solvent. The extraction time was 1 h at a temperature of 68 °C with controlled stirring at 300 rpm. After this operation, the extract obtained was filtered and concentrated in 10 volumes in a vertical vacuum rotary evaporator (IKA RV 10) (45 °C and 500 mmHg).
Chromatographic column and silica gel fractionation
Chromatographic column separation was performed in duplicate using an 80 cm column packed with 178 g of silica gel 60 A (0.075-0.030 mm mesh), previously rehydrated with acetonitrile (Cuevas-Gonzalez et al., 2021). The mobile phase was acetonitrile:water in different concentrations (100:0, 90:10, 70:30, and 50:50 %), with the initial elution being 100 % acetonitrile. The chromatographic column was loaded with 8 mL of concentrated extract (2 g dry extract and 6 mL 58 % ethanol). The fractions were collected with an automatic sample collector connected to the chromatographic column at a 1 mL/min flow rate.
At the end of the sample elution process, the fractions obtained were dried in an oven (GL-70A - Fab, Nacional) at a temperature of 40 °C until total evaporation of the solvent. After evaporation of the solvent, the dry fractions were placed in microtubes and kept refrigerated until they were analyzed by thin-layer chromatography (TLC) to identify the flavonoids present.
Identification of flavonoids in the fractions by Thin-Layer Chromatography (TLC)
Identification of the flavonoids present in the fractions obtained in the process purification was performed by thin-layer chromatography using a TLC aluminum plate silica gel 60 F254, 10 cm x 10 cm; toluene, ethyl ether, and acetic acid (10:9:1, v/v/v/v) were used as mobile phase (Cuevas-Gonzalez et al., 2021). The standards used were pinocembrin, naringenin, hispidulin, galanin, and taxifolin at a concentration of 1000 ppm to compare the retention factor (RF). Extracts and fractions were resuspended in 2 mL of 58 % ethanol and applied to the plate above the origin line drawn at 0.7 cm from the bottom edge, with 1 application of extract concentrate and 5 applications for each fraction; the plate was then placed in the chromatographic chamber with a volume of 20 mL of mobile phase, allowing the solvent to elute to the solvent front mark (9 cm). After drying, the plate was developed with a UV lamp at a wavelength of 302 nm. Sixty fractions were obtained, which were grouped into five fractions (1-5) according to their TLC similarity.
Quantification of pinocembrin by densitometry
Once the flavonoids of the obtained fractions were identified, the concentration of pinocembrin was evaluated in the fractions where its presence was detected. The quantification of pinocembrin was performed with ImageJ software (Image Processing & Analysis in Java), measuring the density generated by the samples seeded on silica gel TLC plates (Cuevas-González et al., 2021). The calibration curve was performed with a silica gel TLC plate 60 F254, 10 cm x 20 cm, using pinocembrin in 15 concentrations of 500, 1000, 1500, 2000, 2500, 3000, 3500, 4000, 4500, 5000, 5500, 6000, 6500, 7000, and 7500 mg/L as reference standard. The samples to be analyzed were added to the same plate. The plate was then placed in the chromatographic chamber containing a volume of 20 mL of mobile phase (toluene, ethyl ether, and acetic acid 10:9:1, v/v/v).
After the run, the plate was removed from the chamber and dried using a fume hood, the plate was developed using a UV lamp at a wavelength of 302 nm. Then, the plates were digitized for processing using the software.
Inhibition kinetics in vegetable oils
The procedure begins with the extraction of the crude oil sample, for which the sunflower seeds were ground in a knife mill to an average particle diameter of 2.5 mm. Oil extraction was performed with a Soxhlet device using 4 refills of solvent (petroleum ether) until the sample was exhausted. Solvent was evaporated using a rotary evaporator (40°C, 470 mmHg) to obtain pure vegetable oil. The oil samples were placed in aluminum trays in a drying oven maintained at a constant temperature of 45 °C. In addition, an air circulation pump with a capacity of 500 mL/min was adapted to maintain a constant oxygen concentration inside the drying chamber.
The treatments corresponded to samples of oil with added oil extract and extract enriched with pinocembrin, dosed at 0.1 % based on its total phenolic content, including a positive control (BHT, 100 ppm) and a negative or control test without the use of the extract. To carry out the kinetics of oxidative rancidity inhibition, 7 periodic samples of each treatment, were taken to complete the study in 21 days, each sample was tested for peroxide index determination by quadruplicate.
To determine the effect of the dosage of the pinocembrin-enriched fraction, samples of sunflower oil were subjected to doses of 50, 100, and 150 % (with respect to the initial dose tested) at the same accelerated storage conditions (45 °C) by performing peroxide value tests at 0, 5, 9, 14, and 18 days of storage with 6 replicates. To determine the treatment with the best activity, the average rate of formation of peroxides was determined for each of them, given by the slope of the corresponding linear equation. The ratio between the oxidation rate (meq peroxides formed/kg day) of the control with respect to the oxidation rate of each treatment was used to determine the Antioxidant Capacity Index (ACI), with the highest value corresponding to the best treatment (Peñaloza et al., 2017).
Determination of total phenols
The quantification of total phenols was performed using the Folin-Ciocalteu reagent (Cortés-Chitala et al., 2021). The extracts were diluted to 10 % and the reaction was carried out by mixing 0.5 mL of 0.67 N Folin reagent and 0.5 mL of 1.9 M Na2CO3, after 1 h of rest, the samples were read at 760 nm in a spectrophotometer UV/Visible using gallic acid (GA) as a reference standard.
Antioxidant capacity by DPPH
The antioxidant capacity of the extracts was determined spectrophotometrically in the presence of 1,1-diphenyl-2-picrylhydrazine (DPPH) radical at 518 nm on a UV-visible spectrophotometer. Two mL of 80 % methanol (blank) and 2 mL of extracts diluted to 0.1 % each were taken. Then 2 mL of freshly 2.5 mM DPPH solution was added to the prepared extracts. The blank and samples were read after 30 min. The percentage inhibition was calculated using the equation described by Cortés-Chítala et al. (2021).
2 mL of 80 % methanol (blank) and 2 mL of 0.1 % diluted extracts were taken. 2 mL of freshly prepared 2.5 mM DPPH was added to each. The reading of the blank and the samples were carried out after 30 min.
Determination of the peroxide index by volumetric method
The peroxide index was evaluated according to the method reported by Ruiz (2015), based on the oxidation of potassium iodide. A small amount of the sample is dissolved in a solution of acetic acid/dichloromethane (3:2) and reacted with potassium for 60 seconds, the iodine released forms a dark blue color with starch, which can be titrated with a sodium thiosulfate solution. The result is expressed in milliequivalents of active oxygen per kg of oil.
Statistical analysis
All experiments were performed in triplicate and data were expressed as mean ± standard deviation. The data obtained were subjected to analysis of variance (ANOVA) and comparisons of means between treatments were made by LSD, differences were considered significant at p < 0.05 (STATGRAPHICS Centurion XVI version 16.1.18.).
Results and Discussion
Characterization of Mexican oregano extracts
The extracts of Mexican oregano (L. graveolens) obtained were analyzed and compared for phenolic content, antioxidant activity (DPPH), and pinocembrin content (Table 1). The Folin-Ciocalteu analysis showed that the content of polyphenolic compounds for the oregano extract without essential oil obtained a value of 3.09 mg GA/mL, a value higher than that reported by Cid et al. (2019), who prepared an ethanolic extract of the solid residues of oregano (Poliomintha longiflora) with a total phenol content of 1.32 mg GA/mL.
The antioxidant capacity (DPPH) of the oregano extract without essential oil showed a value of 6.97 mg EqTx/mL. It is worth mentioning that there are no reports of phenolic content or antioxidant activity from the characterization of Mexican oregano residual bagasse other than those obtained by our research group (Cuevas-González et al., 2021).
Although oregano without essential oil is considered a waste product, this by-product presented a value similar to that reported in a previous study (Flores-Martinez et al., 2016) for the extract obtained from Mexican oregano without treatment. This variation is attributed to the difference in the chemical composition of oregano after the essential oil extraction process since it is known that the essential oil of oregano is rich in antioxidant compounds insoluble in polar solvents (Loeza et al., 2020). However, the pinocembrin content, a compound of interest for the present study, the oregano extract without essential oil was the one that presented the best results concentration of this compound with a value of 0.766 mg/mL Ex, while the untreated oregano extract presented a value of 0.659 mg/mL Ex, slightly higher than that obtained by Flores-Martínez et al. (2016), where they report a value of 0.574 mg/mL Ex determined by High-performance liquid chromatography coupled with electrospray ionization-quadrupole-time of flight-mass spectrometry (LC-ESI-QTOF-MS/MS). The difference obtained could be due to specific characteristics in the management of the raw material. It is worth mentioning that, as with the solid waste, we studied the presence of pinocembrin in the aqueous residue obtained after the extraction process of the essential oil, which was discarded because it did not contain pinocembrin, the compound of interest.
Table 1 Determination of total phenols, DPPH, and pinocembrin of Mexican oregano extracts.
| Sample | Total phenols (mg GA/mL Ex) | DPPH (mg EqTx/mL Ex) | Pinocembrin (mg/mL Ex) |
|---|---|---|---|
| Oregano extract without essential oil | 2.99 ± 0.015 a | 5.48 ± 0.033 a | 0.71 ± 0.026 b |
| Pinocembrin-enriched fraction | 3.09 ± 0.005 a | 4.36 ± 0.033 b | 0.029 a |
*GA, gallic acid; EqTx, trolox equivalent. Different letters in superscript indicated significant statistical differences between samples according to LSD test (p < 0.05).
Identification of flavonoids present in oregano extract without essential oil
To identify the main flavonoids of the oregano extract without essential oil, it was fractionated through a silica gel column, and 60 fractions were collected. These fractions were injected for a chromatographic run in TLC in conjunction with the available standards, under the aforementioned conditions.
From fractions 27 to 41, the flavonoid compounds present were detected by comparing their RF with those of the pure standards, and the following components were identified pinocembrin (RF= 0.77), galangin (RF= 0.71), naringenin (RF= 0.64), hispidulin (RF= 0.50), eriodctiol (RF= 0.47), without finding the compound taxifolin (Table 2).
From fractions 27 to 41, the flavonoid compounds present were detected by comparing their RF with those of the pure standards, and the following components were identified pinocembrin (RF= 0.77), galangin (RF= 0.71), naringenin (RF= 0.64), hispidulin (RF= 0.50), eriodctiol (RF= 0.47), without finding the compound taxifolin (Table 2).
Table 2 RF values obtained in the TLC analysis of fractions 1, 2, 3, 4, and 5.
| Sample | RF | ||||
|---|---|---|---|---|---|
| Extract | 0.47 | 0.5 | 0.64 | 0.71 | 0.77 |
| Pinocembrine | - | - | - | - | 0.77 |
| Galangin | - | - | - | 0.71 | - |
| Naringenin | - | - | 0.64 | - | - |
| Hispidulin | - | 0.5 | - | - | - |
| Eriodictyol | 0.47 | - | - | - | - |
| Fraction 1 | - | - | 0.64 | 0.71 | 0.77 |
| Fraction 2 | - | 0.5 | 0.64 | 0.71 | 0.77 |
| Fraction 3 | - | 0.5 | 0.64 | 0.71 | 0.77 |
| Fraction 4 | - | 0.5 | 0.64 | 0.71 | 0.77 |
| Fraction 5 | - | 0.5 | 0.64 | 0.71 | 0.77 |
Quantification of pinocembrin from oregano extract without essential oil fractions
The pinocembrin quantification was carried out considering the calibration curve constructed for this purpose, represented by the equation of the straight line (y = 1.9315x + 985.93) with R² = 0.9922, where y = optical density and x = concentration of pinocembrin (mg/mL). Table 3 shows the results obtained from the quantification of pinocembrin in the selected fractions (named from 1 to 5 according to their TLC similarity), in addition to their corresponding recovery yields, obtaining a total recovery yield of 56.6 %.
| Samples | Pinocembrin (mg/mL) | Yield (%) |
|---|---|---|
| 1 | 8.56 ± 0.000 c | 6.14 ± 0.010 c |
| 2 | 25.73 ± 0.000 b | 18.51 ± 0.098 b |
| 3 | 35.15 ± 0.000 a | 25.24 ± 0.030 a |
| 4 | 7.37 ± 0.029 d | 5.29 ± 0.070 d |
| 5 | 1.99 ± 0.012 e | 1.42 ± 0.018 e |
| Total | 56.6 % |
Table 3. Fractions of Mexican oregano extract without essential oil obtained with chromatography in column of silica gel.
Different letters in superscript indicated significant statistical differences between samples according to LSD's test (p < 0.05).
The separation by hydrophilic silica chromatographic column tested allowed to fractionate the extract to obtain concentrations of pinocembrin close to those obtained by Granados et al. (2018), who reported total concentrations of pinocembrin of 19, 33, and 55 mg/g Ex in Mexican propolis, the main source of pinocembrin (Salatino et al., 2011; Przybyłek & Karpiński, 2019).
It is worth mentioning that our results obtained with the separation method developed here for Mexican oregano were superior, considering the results obtained by Osti et al. (2010), who obtained concentrations of 0.066 mg/mg Ex of pinocembrin in Sphaeralcea angustifolia. These differences may be due to the efficiency and operating conditions of the methods used and the nature of the extraction material.
Sunflower oil oxidation kinetics
To facilitate the visualization of the results obtained from the kinetics of oxidation of sunflower oil with the different treatments is shown only in Figure 2, the arithmetic average corresponding to the 4 repetitions for each treatment.
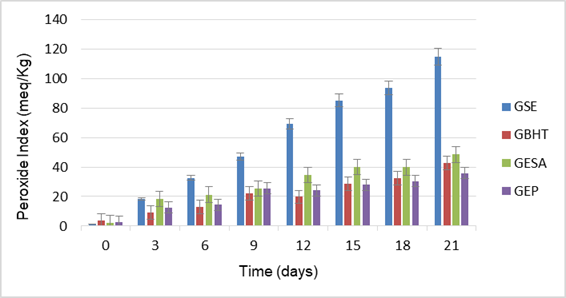
Figure 2 Oxidation kinetics of sunflower oil with the different treatments. GSE (sunflower oil pure), GBHT (with added BHT), GESA (with extract without essential oil), and GEP (with pinocembrin enriched extract).
Generally, it can be observed that sunflower oil without oregano extract (GSE), from 6 day already shows oxidative alteration indexes considerably 32.6 ± 0.98 meq/Kg, obtaining Peroxide Indices (PI) of 114.6 ± 4.01 meq/Kg at the end of the test (21 days), reflecting a clear deterioration of the oil at this time and under the storage conditions tested, it is which confirms the high lability of sunflower oil to oxidative processes because of its high concentration of unsaturated fatty acids (69 %) and its high ratio of unsaturated to saturated fats much higher than one (6.27).
Likewise, the results indicate that from day 9 onwards, the treatments generate a significant protective effect (p < 0.05) considering the lipid oxidation process of the untreated sunflower oil (47.1 ± 1.65 meq/Kg), being the BHT the best protective agent (PI= 21.9±0.66 meq/Kg). At the end of the trial (day 21), a significant difference was found between all the treatments (p < 0.05), with the pinocembrin-enriched extract (GEP) being the best (p < 0.05) protective agent (PI= 35.9±1.07 meq/Kg) by reducing the formation of peroxides by values of close to 69 % with respect to the degree of oxidation found at the end of treatment GSE, a fact that indicates that the antioxidant polyphenols from Lippia graveolens are capable of interacting in the oxidative process with the species reactive to the oxygen in formation, thereby potentially extending the life span of the sunflower oil.
These results show the important protective effect of oregano extract and the effect of the extract enriched in pinocembrin, showing even a better antioxidant effect than that shown by BHT at standard concentrations of commercial application for this purpose (100 ppm).
Table 4 shows the equations of the linear modeling corresponding to the hydroperoxide production as a function of time, as well as the values of the antioxidant capacity corresponding to each treatment. With these results, we obtained the Antioxidant Capacity Index (ACI), with the highest value corresponding to the best treatment.
Table 4 Model equations of peroxide formation in sunflower oil and capacity indexes antioxidant for the different treatments.
| Treatment | Equation | R2* | ACI |
|---|---|---|---|
| GSE (Control) | PI = 5.3649 t + 2.2706 | 0.9805 | 1.000 |
| GBHT | PI = 1.7448 t + 0.1293 | 0.9463 | 3.075 |
| GESA | PI = 1.8459 t + 7.4923 | 0.9168** | 2.906 |
| GEP | PI = 1.3967 t + 4.4842 | 0.9339** | 3.841 |
R2* linear correlation coefficient. ** The asterisks indicate that the values show a difference compared to the control (p < 0.05).
As can be seen, the oxidation curves can be represented by linear models, because the degrees of oxidation obtained correspond to the first stages of the oxidative rancidity process of the oil.
The data obtained suggest that the treatment corresponding to the oil enriched with extract pinocembrin presents a higher degree of protection for the sunflower oil, with oxidation rates 3.8 times lower than those obtained for the untreated sunflower oil. The treatments with extract without essential oil and BHT present oxidation rates up to 3 times slower than the oxidation rate of the original untreated sunflower oil. It is remarkable that the values of improvement in oxidative stability obtained here are higher than those reported by Peñaloza et al. (2017), who used Rosmarinus officinalis extract as an antioxidant in the oxidation process of olive oil palm during the frying process.
To determine the optimum concentration of the pinocembrin-enriched extract, considering the results by sextuplicate of peroxide formation at 0, 5, 9, 14, and 18 days for each concentration tested, the time required (in days) was determined for each concentration to achieve a peroxide value of 30 meq/kg in the sunflower oil for each antioxidant concentration used according to the linear equation (PI vs t). In addition, the ratio P was calculated between the corresponding times required for each concentration tested (tm) with respect to that required for the control treatment (tc), corresponding the highest P value obtained to the concentration of extract to be used. A P value >1 is an indication of an antioxidant protective effect for the assay (Peñaloza et al., 2017).
Table 5 shows the modeling equations for the production of hydroperoxides as a function of time, as well as the P values that correspond to each concentration tested.
Table 5 Model equations of peroxides formation in sunflower oil and P indices for different concentrations.
| Concentration | Equation | R2* | Time (days) for PI=30 meq/kg | P(tm/tc) |
|---|---|---|---|---|
| Zero (Control) | PI = 5.2329 t + 1.3665 | 0.9958 | 5.47 | 1.000 |
| 0.5 | PI = 2.1076 t + 3.5467 | 0.9651 | 12.55 | 2.294 |
| 0.1 | PI = 2.0323 t + 1.8060 | 0.9682 | 13.87 | 2.535 |
| 1.5 | PI = 1.2782 t + 4.4842 | 0.9789 | 21.69 | 3.965 |
R2* linear correlation coefficient. The values do not show a significant difference compared to the control (p < 0.05).
The results shown indicate that all the tested concentrations have a good degree of protection in sunflower oil (p > 2). Likewise, the rate of formation of peroxides (given by the slope of the linear equation shown) decreases as the concentration of the extract increases, this implies a greater antioxidant protective effect. The inverse behavior is observed for the time required to achieve the stipulated concentration of peroxides (30 meq/Kg), i.e., the higher the concentration of extract, the less time required.
The above results also indicate that the concentration with a significantly higher degree of protection for sunflower oil corresponds to the highest concentration tested (0.15 %) of the extract enriched in pinocembrin with protection indexes close to 4 units, i.e., increasing the shelf life of the oil by approximately 4 times with respect to untreated oil. Medium and low concentrations treatments show similar P values and peroxide formation times which generates an increase in shelf life of close to 100 %.
Considering the above, the best concentration to be used for sunflower oil protection under the accelerated storage conditions corresponding to the pinocembrin extract with 0.15 % w/w, which showed a dose-dependent antioxidant response by increasing the stability of sunflower oil by up to 400 % through its application.
Conclusions
The residual bagasse of Mexican oregano obtained after having extracted its essential oil is a good source of antioxidant compounds of potential interest in edible fats and oils industry for its high content of phenolic compounds and associated antioxidant capacity. Hydroalcoholic extracts from this bagasse, as well as the extract enriched in pinocembrin generated, have a good protective effect against the process of lipid oxidation of sunflower oil under accelerated storage conditions, with the extract being pinocembrin-enriched product showed the strongest antioxidant effects, including on the synthetic antioxidant tested (BHT). The present study demonstrated the efficacy of the extracts without essential oil and pinocembrin-enriched Mexican oregano in the stabilization of oxidative activity of sunflower vegetable oil, positioning itself as a good alternative for stabilizing lipid matrices, with even greater protective effects than some of the other synthetic antioxidants, so these extracts constitute an antioxidant product with a significant potential impact on the food sector at the commercial level.











 nova página do texto(beta)
nova página do texto(beta)