Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Tecnología y ciencias del agua
versão On-line ISSN 2007-2422
Tecnol. cienc. agua vol.9 no.4 Jiutepec Jul./Ago. 2018 Epub 24-Nov-2020
https://doi.org/10.24850/j-tyca-2018-04-08
Articles
Use of faujasite-type zeolite for ion adsorption in municipal wastewater
1Grupo de investigación Materiales, Ambiente y Desarrollo-MADE, Programa de Química, Facultad de Ciencias Básicas, Universidad de la Amazonia, Florencia, Caquetá, Colombia, dayanadelavega1@gmail.com
2Grupo de investigación Materiales, Ambiente y Desarrollo-MADE, Programa de Química, Facultad de Ciencias Básicas, Universidad de la Amazonia, Florencia, Caquetá, Colombia, c.gonzalez@udla.edu.co
3Grupo de investigación Materiales, Ambiente y Desarrollo-MADE, Programa de Química, Facultad de Ciencias Básicas, Universidad de la Amazonia, Florencia, Caquetá, Colombia, c.escalante@udla.edu.co
4Química de Recursos Energéticos y Medio Ambiente-QUIREMA, Instituto de Química, Universidad de Antioquia, Medellín, Colombia, andres.gallego@udea.edu.co
5Grupo de investigación Materiales, Ambiente y Desarrollo-MADE, Programa de Química, Facultad de Ciencias Básicas, Universidad de la Amazonia, Florencia, Caquetá, Colombia; Physikalische Chemie I-PCI, Department of Chemistry, Bielefeld University, Germany, maurin.salamanca@uni-bielefeld.de
6Grupo de investigación Materiales, Ambiente y Desarrollo-MADE, Programa de Química, Facultad de Ciencias Básicas, Universidad de la Amazonia, Florencia, Caquetá, Colombia, l.manrique@udla.edu.co
In this work, a faujasite-type zeolite was synthesized from aluminum and silica gel using the sol-gel method. The zeolite was characterized using infrared spectroscopy (FTIR), x-ray diffraction (XRD), thermogravimetric analysis (TGA) and scanning electron microscopy (SEM). The synthesized material was identified as faujasite-type X. The capacity of the zeolite to remove organic matter, ammoniacal nitrogen, water hardness and heavy metals (Fe, Cu, Zn, and Pb) was evaluated. The removal capacity was studied in municipal wastewater and synthetic water. The sequence of effectiveness for the heavy metal removal by the faujasite-type X zeolite was Zn2+ > Cu2+ > Pb2+ > Fe3+ in municipal wastewater containing a high concentration of organic matter. Several factors affected ion removal, such as the concentration and type of the cationic species and the size of the hydrated cations in the adsorption process. The removal percentage obtained for heavy metal ions, water hardness and ammoniacal nitrogen was higher than 70%. A greater removal capacity for Ca2+ and Mg2+ (water hardness) and ammoniacal nitrogen was found. Therefore, the simplicity and the effectiveness of the synthesized zeolite made this a suitable alternative for treating municipal wastewater for ions, water hardness, and ammoniacal nitrogen removal. The results open the possibility for evaluating the implementation of adsorption columns on a large scale for municipal wastewater treatment.
Keywords Zeolite; heavy metals; municipal wastewater; wastewater treatment; cationic exchange; adsorbent
En este trabajo se sintetizó una zeolita tipo faujasita a partir de aluminio y sílica gel, por el método sol-gel. Dicho procedimiento se realizó por medio de espectroscopía de infrarrojo (FTIR por su nombre en inglés), difracción de rayos X (XRD por su nombre en inglés), análisis termogravimétrico (TGA por su nombre en inglés) y microscopía de electrónica de barrido (SEM por su nombre en inglés). Se identificó el material sintetizado como zeolita tipo faujasita X. Se evaluó la capacidad de remoción de materia orgánica, nitrógeno amoniacal, dureza y metales pesados (Fe, Cu, Zn, Pb) utilizando aguas residuales municipales (ARM) y agua sintética. La afinidad de la zeolita tipo faujasita X, por los iones metálicos estudiados presenta el siguiente comportamiento: Zn2+ > Cu2+ > Pb2+ > Fe3+ en ARM, con alta concentración de materia orgánica. Se observó que la dosis de zeolita, el tipo de ion, la concentración de las especies catiónicas y el tamaño del catión hidratado fueron factores determinantes en el proceso de adsorción. Se obtuvo una mayor capacidad de adsorción de la zeolita para iones de Ca2+ y Mg2+ (dureza), y nitrógeno amoniacal en ARM. La sencillez del proceso y la efectividad conseguida por la zeolita para la remoción de distintos iones con porcentajes de remoción mayores al 70%, la convierten en una alternativa viable como adsorbente de iones metálicos, dureza y nitrógeno amoniacal en ARM. Lo anterior conduce a la posibilidad de implementar columnas de adsorción a gran escala para el tratamiento de ARM.
Palabras clave zeolita; metales pesados; aguas residuales municipales; tratamiento de aguas residuales; intercambio catiónico; adsorbentes
Introduction
Colombia's small cities (100 000 to 200 000 inhabitants) are characterized by a lack of adequate wastewater treatment plants (WWTP). These cities are equipped with combined sewer systems that carry municipal wastewater (MWW) to aquatic ecosystems in urban areas. These water sources are visibly impacted by the discharge of MWW. MWWs are usually composed of domestic, agro-industrial and hospital wastewater, as well as waters with organic compounds that are leached or transported by runoff (Rosas & Mesa, 2002).
Previous characterizations of MWWs in Florencia, Colombia showed that they contained organic matter as well as a high ionic composition, consisting mainly of inorganic metal and non-metal ions (Orozco, Triviño, & Manrique, 2014). Ammoniacal nitrogen, for example, comes mainly from domestic wastes as a result of the breakdown of urea, fertilizers, livestock, agricultural and industrial activities. This causes rivers to have excess nutrients and a decrease in dissolved oxygen, leading to an increase in environments that are toxic to aquatic organisms (Wang & Peng, 2010).
Heavy metals are an example of dissolved ions that are also present in rivers, which are highly toxic to ecosystems and human health (Ltaief, Siffert, Fourmentin, & Benzina, 2015). This group of elements is persistent and is not thermo-degradable or biodegradable. Therefore, they tend to rapidly build up to toxic levels (Chung, Song, Park, & Cho, 2011). This accumulation occurs mainly in living organisms and in the surface soil, which results in the contamination of soil and surface water and may even cause air pollution (Shavandi, Haddadian, Ismail, Abdullah, & Abidin, 2012).
The presence of heavy metals in wastewater is primarily caused by human activities ((Chowdhury, Mazumder, Al-Attas, & Husain, 2016). These metals are used mainly in the agricultural sector, in the form of herbicides and pesticides (Rezania, Taib, Md-Din, Dahalan, & Kamyab, 2016), as well as the industrial sector. Heavy metals can also lead to the contamination of drinking water due to poor treatment or leaching of metals into the water supply system. Also, foods that have been irrigated with wastewater may contain traces of heavy metals and also be contaminated by either organic or inorganic compounds (Qureshi, Hussain, Ismail, & Khan, 2016).
Techniques such as chemical precipitation, ion exchange, filtration, electrochemical treatments, membrane technology, reverse osmosis, solvent extraction, evaporation, and coagulation-flocculation, among others, are the most commonly used methods for the treatment of MWWs (El-Nemr, Khaled, Abdelwahab, & El-Sikaily, 2008). Many of these processes are expensive and can cause problems when it comes to waste disposal, making them difficult to apply on a large scale (Ahmaruzzaman, 2011).
Synthetic wastewater (pollutants and deionized water) containing Ni2+, Pb2+, Zn2+, Cr6+, Cd2+ and Co2+ ions have been treated using ion exchange with faujasite-type zeolites, which has shown that zeolite has a particular affinity towards these ions (Mekatel, Amokrane, Benturki, & Nibou, 2012). Zeolites are solid, inorganic and porous materials with a crystalline structure consisting of aluminum atoms in tetrahedral coordination, causing a negative charge in the aluminosilicate framework, which is compensated by the presence of metal cations. Zeolites have channels and cavities with diameters ranging from 0.3 to 1.5 nm. The channels contain water molecules and compensation cations (Na+, K+, Ca2+, Mg2+), which allow ion exchange and dehydration processes to occur (Koshy & Singh, 2016). Both their large surface area and pore volume provide these materials with high adsorption capacity (Martínez & Corma, 2013).
Zeolitic materials are characterized mainly by their ion exchange and adsorption capacities. In addition, they can be produced in the laboratory using low-cost raw materials (Burakov et al., 2018). These materials have been widely used as adsorbents, molecular sieves, and ion exchangers in the treatment of wastewater, air purifiers, catalysts and catalyst supports. Zeolites have also been used for the removal of heavy metals.
The modification of zeolites has been proposed for improving their properties, as is the case of LTA zeolites and silver-modified zeolites, which improve the adsorption and recovery of mercury (Busca, 2014). In addition, partially dealuminated faujasite-type zeolites have been proved effective in removing hydrocarbons, while type X zeolites (NaX, CaX, and H-USY) promote the removal of sulfur compounds found in contaminated water, soil and air (Visa, 2016). Additionally, naturally-occurring faujasite-type zeolites (Na-X and Na-Y), especially the Na-X zeolite, have a high heavy metal adsorption ability. For Cu2 +, Pb2+ and Zn2+, Na-X zeolite was reported to have a higher maximum adsorption capacity than natural clinoptilolite under similar adsorption conditions (Yuna, 2016).
The aim of this study was to evaluate the zeolite's heavy metal removal capacity by using a synthetic faujasite-type zeolite, given its good performance. First, the zeolite's ability to remove Fe3+, Cu2+, Zn2+, Pb2+ from synthetic water was evaluated using synthetic water. Then, the ability to remove heavy metals, organic matter, water hardness and ammoniacal nitrogen from MWW was also evaluated. In this way, it was possible to study the effect of a complex matrix on the removal efficiency for each ion.
Methodology
Synthesis and characterization of zeolites
Zeolite was synthesized using the method described by Dussan Dussan, Otálora, Chica, Bonilla and Otálora (2012). The characterization of the synthesized zeolite was carried out using thermogravimetric analysis (TGA), infrared spectroscopy (IR), x-ray diffraction (XRD) and scanning electron microscopy (SEM). The thermogravimetric analysis was carried out in an air atmosphere at a ramping temperature rate of 10°C.min-1, from room temperature up to 800 °C, followed by a 10-minute isotherm. The analysis was carried out using Q500 thermogravimetric analyzer (TA Instruments). Fourier transform infrared spectroscopy (FTIR) analysis was conducted using the KBr pellet method in a 4 000 cm-1 to 400 cm-1 wavelength range with a Nicolet 6700 spectrometer. XRD analysis of the synthesized zeolite was conducted using a Panalytical X'Pert Pro Multipurpose Diffractometer (MPD), with an accelerating voltage of 45 kV and a 40 mA current intensity, in a 2θ range of 10 to 80°, a step of 0.020º, and a time per step of 8s. The SEM analysis was conducted using a JEOL JSM-6490LV scanning electron microscope. The sample was previously coated with gold using the Denton Vacuum Desk IV coating system. Additionally, a surface chemical analysis was carried out by energy-dispersive x-ray spectroscopy (EDX).
Sampling and characterization of wastewater
A characteristic municipal wastewater discharge of Florencia, Colombia, was used by the present study. The samples were collected according to standard sampling and preservation techniques by the Colombian Institute of Hydrology, Meteorology and Environmental Studies - IDEAM (Sánchez, 2005). The zeolite adsorption response was evaluated with three concentrations of organic matter, measured as chemical oxygen demand (COD), which were characteristic of MWW at different times of the year. The MWW with higher COD values (400 - 500 mg.L-1) were classified as MWW with high organic matter content, while those with medium and low concentrations were obtained using dilutions to reach concentrations ranging from 200 to 300 mg.L-1 and 80 to 120 mg.L-1, respectively. The characteristics of the MWWs were determined using the closed reflux method for COD. The level of ammoniacal nitrogen was determined by the Nessler method, and the total water hardness was determined by titration (calcium and magnesium). Heavy metal content (Fe3+, Cu2+, Zn2+, and Pb2+) was evaluated by atomic absorption spectrophotometry (AAS). All tests were performed according to the Standard Methods for the Examination of Water and Wastewater (APHA-AWWA-WEF) (APHA-AWWA-WEF, 2012). These methods were used to analyze the response variables in synthetic water adsorption tests and determine the adsorption capacity of zeolites.
Preliminary adsorption tests - Synthetic water
All adsorption tests were conducted using lab-made solutions, e.g., synthetic water (SW). Each of the ions to be evaluated (Fe3+, Cu2+, Pb2+, Zn2+, Ca2+, Mg2+, and NH4 +) was dissolved in deionized water. The optimal dosage at which synthetic faujasite-type zeolite reached the highest ion adsorption rate was determined using zeolite doses ranging from 0.01 g.L-1 to 4 g.L-1, with increments of 0.25 g.L-1. For all cases, the response variable was represented by the removal capacity of the ion.
Adsorption tests in synthetic water and municipal wastewater
The ion removal capacity for both SW and MWW was evaluated using assays in reaction vessels, stirring for 1 hour, with 50 mL each of the ammoniacal nitrogen, hardness (Ca2+, and Mg2+), and heavy metal (Fe3+, Cu2+, Zn2+, and Pb2+) solutions used at different concentrations (maximum and minimum for MWW). Zeolite doses were used according to the results obtained in preliminary tests, and a 1-hour adsorption test was conducted at room temperature with a constant stirring speed. Zeolite doses were used according to the results obtained in preliminary tests. The total concentration for each of the ions was calculated using the aforementioned methods. MMW treatability using faujasite-type zeolites was evaluated according to the pollutant removal percentage found for the samples (metals, ammonia nitrogen, hardness, and organic matter as COD).
Variance was analyzed (ANOVA) based on the results of the experimental design. This analysis made it possible to draw conclusions about the influence of each factor and their correlation. The factors considered in the statistical analysis included the maximum adsorption zeolite dose and higher adsorption concentrations. This analysis involved the use of SPSS statistical software.
The results obtained permitted Freundlich and Langmuir isotherms to be determined in order to find the theoretical model that best fit the experimental conditions. A goodness of fit test (χ2) was performed, then the specific surface area was determined. The adsorption process for each of the isotherms was adjusted based on the results obtained from tests conducted on the SW and MWW samples.
Results and discussion
Zeolite characterization
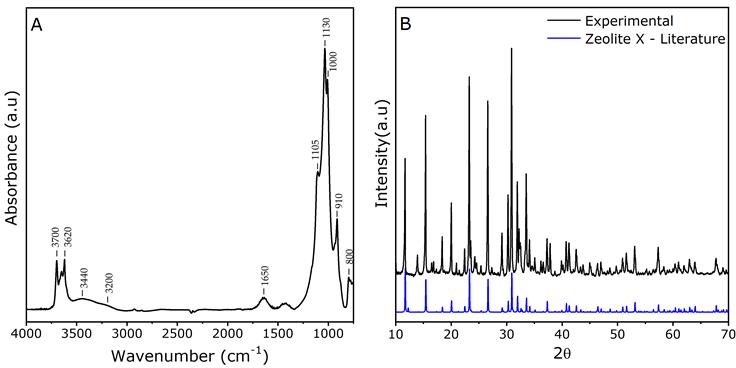
The infrared spectrum obtained with the synthesized zeolite (Figure 1A) had three signals in the OH region, as follows: 3700 cm-1 corresponding to terminal silanols, 3620 cm-1 for Al-OH-Si groups, and a weak band at 3650 cm-1 corresponding to OH extra framework (Bevilacqua, Montanari, Finocchio, & Busca, 2006). The signal shown at 3440 cm-1 corresponds to stretching vibrations of silanol (Si-OH) and O-H (Baur, Héroguel, Spring, Luterbacher, & Kiwi-Minsker, 2016). This is superimposed by bands in the region between 3200 cm-1 and 3400 cm-1 and correspond to O-H vibrations of water. The presence of water in the zeolite is determined by a weak band at wavenumber 1650 cm-1, due to the deformation of the O-H bond found in water molecules (Moulin et al., 2008). The bands in the range 980 cm-1 1200 cm-1 are associated with asymmetric T-O-T stretching (T = Si or Al), and the bands found in 800 cm-1 correspond to symmetrical stretching of the T-O-T bond (Fricke, Kosslick, Lischke, & Richter, 2000).
The diffractogram obtained for the zeolite synthesized in this work (Figure 1B) exhibited diffraction angles 2θ, which was comparable to those previously reported (Treacy & Higgins, 2001) and suggests that the zeolite used was a Na-X faujasite-type zeolite. Additionally, the sample synthesized by the aforementioned technique exhibited high crystallinity.
The thermogram obtained for the zeolite prior to the metal adsorption process (Figure 2A) exhibited mass loss on two occasions. The first mass loss (1.43%) occurred at 110 ° C, which suggests a loss of surface water in the zeolitic material (Ríos, Williams, & Castellanos, 2006). The latter (3.73%) occurred at 200 °C, which suggests the presence of water desorption in the internal zeolite structure (Khaleghian-Moghadam & Seyedeyn-Azad, 2009). The thermogravimetric analysis of the zeolite after the removal process (Figure 2B) showed three mass loss events. An additional event occurring at 90 °C may be related to water molecules adsorbed during the adsorption process. The subsequent losses were the same as those observed in the zeolite before the adsorption process.
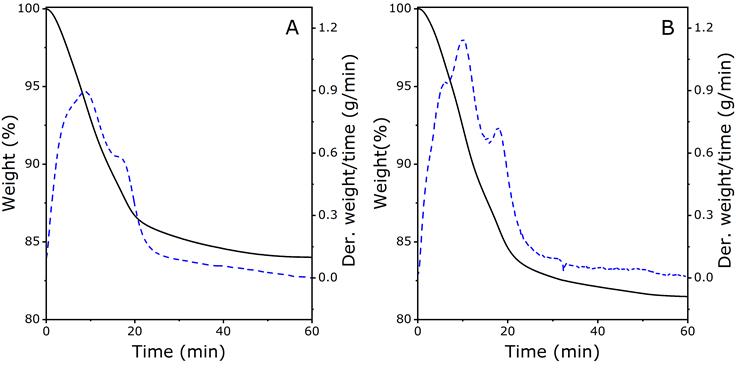
Figure 2 Thermogram for synthetic faujasite zeolite before (A) and after (B) the adsorption process in MWWs. Thermogravimetric analysis (solid line) and the derivative weight loss curve (dash line corresponds).
SEM micrographs prior to adsorption (Figure 3A and 3B) indicated the presence of uniformly-distributed granules that correspond to the zeolite. There were also non-uniform nanocrystal agglomerates on nuclei of a higher constitution, which may be related to the growth of zeolites during synthesis (Dussan, Otálora, Chica, Bonilla & Otálora, 2012). According to the results obtained from x-ray diffraction, these agglomerates were crystalline aggregates. The SEM results showed a sphere-shaped morphology that was characteristic of the zeolites, with sizes ranging from 500 nm to 2 600 nm (Gómez, 2001). Previous studies with commercial zeolites have reported particle sizes between 590 nm to 1 020 nm (Zhang, Yang, Tang, & Yang, 2015) and 2 300 nm crystals (Ansari, Aroujalian, Raisi, Dabir, & Fathizadeh, 2014). Therefore, the morphological characteristics of the synthesized zeolite agreed with those reported by other authors. The ion removal process resulted in changes in the shape, size, and uniformity of the aggregates. In this case, the particles did not have a particular shape and were more agglomerated with each other. In addition, there were particles of much larger sizes, ranging from 700 nm to 6 000 nm, approximately. Usually, the morphology and particle size are not expected to change after ion exchange (Zhang et al., 2015). However, other authors have reported a loss of crystallinity and morphology in crystals, which appear agglomerated without a particular shape after impregnation with metals (Gómez, 2001).
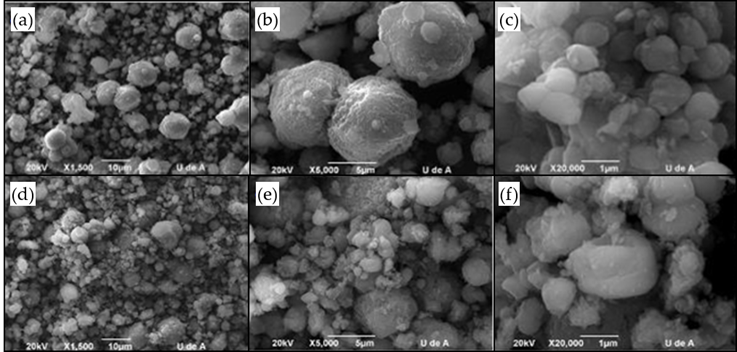
Figure 3 SEM micrographs of synthetized faujasite zeolite before (A, B, C) and after (D, E, F) the adsorption process in MWWs.
The analysis of EDX indicated the presence of oxygen (53.5%), silicon (19.2%), aluminum (15.3%) and sodium (12.0%) on the surface prior to the adsorption process. The chemical composition coincided with that previously reported, where the average formula is defined as Na85Al85Si107O384 (Moulin et al., 2008). After the ion removal, there were traces of magnesium, potassium, and calcium, as well as an increase in oxygen levels. This may be due to the fact that the open structure of the zeolite provides a great ability to incorporate cations such as Mg2+, K+, and Ca2+. The increase in the oxygen mass level suggests that the zeolite underwent hydration (Castaldi, Santona, Enzo, & Melis, 2008).
Removal of ions in synthetic water (SW)
The results obtained from preliminary adsorption tests in SW showed that the optimum zeolite dose for the removal of ions ranged between 0.01 g.L-1 and 1.0 g.L-1. Previous experiments with SW have shown that the ability to remove ammoniacal nitrogen can be improved by increasing the dose of zeolite. The use of a 0.6 g.L-1 dose in the concentrations evaluated (12 mg.L-1 and 25 mg.L-1) can remove ions up to 98%. The initial concentration of ammoniacal nitrogen did not significantly affect the level of adsorption obtained in each experiment. As for water hardness, the removal percentage remained constant (100%) in experiments with higher doses of zeolite, which suggests that the dosage of zeolite and the initial concentration of calcium and magnesium ions do not affect the removal process. This behavior partially coincides with that reported by Manrique, Bonilla, Chica, Otálora and Salamanca (2015), where the dosage of zeolite was shown to affect hardness removal. Also, the use of doses greater than 0.3 g.L-1 resulted in levels of removal greater than 90%.
Figure 4 shows Na-X zeolite's ability to remove heavy metals from synthetic water. For low concentrations of Cu2+ (8 mg.L-1), Fe 3+ (15 mg.L-1) and Zn2+ (10 mg.L-1), the zeolite dose was associated with ion adsorption rates ranging from 90% to 100%. As for high ion concentrations, the use of doses greater than 0.02 mg.L-1 resulted in removal levels above 80%, since higher doses of zeolite leads to an increase in both the surface area and adsorption points, and therefore it promotes ion exchange (Burakov et al., 2018). The Pb2+ ions behaved differently since a higher ion concentration (10 mg.L-1) leads to an increase in removal levels, regardless of the dose of zeolite. For low Pb2+ ion concentrations (3 mg.L-1) the level of removal was drastically reduced by using low doses of zeolite (less than 0.04 mg.L-1), while the use of higher doses of zeolite resulted in removal levels ranging from 50% to 80%. This behavior may be associated with a decrease in the hydrated radius of the ion (0.261 nm) and the ability to diffuse in the zeolite structure. However, a weak interaction between the Pb2+ ion and the zeolite was prevalent, and this was favored by the low ion concentration present in the solution. Faujasite-type zeolite was shown to have greater selectivity for Zn2+ in the absence of other ions. This may be due to the fact that unlike Pb2+ion, Zn2+ has a greater ability to interact with the active zeolite sites (Mekatel et al., 2012). Similar results were obtained by other studies where zeolite exhibited greater selectivity for the Zn2+ ion than for Pb2+ in monocationic solutions Zn2+ > Pb2+> Cd2+ (Castaldi et al., 2008).
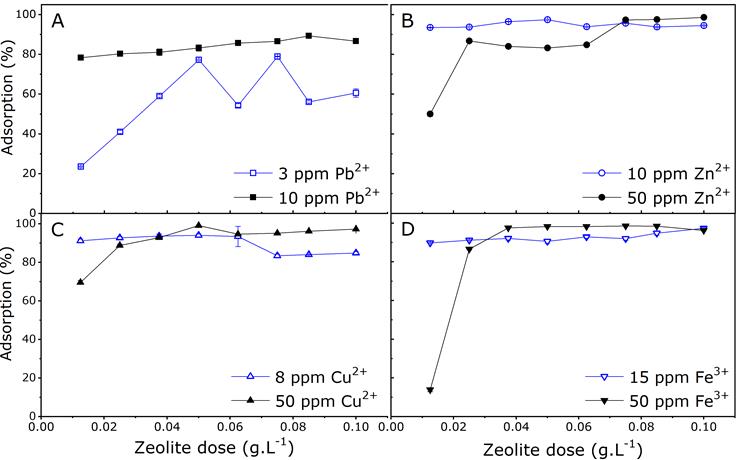
Figure 4 Removal percentage for Pb2+ (A), Zn2+ (B), Cu2+ (C), and Fe3+ (D) in synthetic water at different concentrations, according to the zeolite dose.
Removal levels of around 90% can be obtained for initial concentrations of Cu2+ (8 g.L-1) by using low doses of zeolite (<0.0625 g.L-1). Removal levels of Cu2+ up to 55% by using zeolite doses of 4 g.L-1 and Cu concentrations up to 250 mg.L-1 have been previously reported (Visa, 2016). This shows that the zeolite evaluated in this study was effective for the removal of this type of ion when used in low doses.
Removal of ions in municipal wastewater (MWW)
Figure 5 and Figure 6 show the removal percentages obtained by using different doses of zeolite for each of the ions and the organic matter (quantified as COD) present in MWW, with different initial concentrations of organic matter (high, medium, and low). The competitive effect in such a complex water matrix resulted in an overall decrease in the effectiveness of the adsorption process. Factors such as the concentration, the size and charge of the hydrated cation, the anionic species associated with the cation, and the characteristics of the zeolite, play an important role in the ion exchange results (Ríos, Williams, & Roberts, 2010).
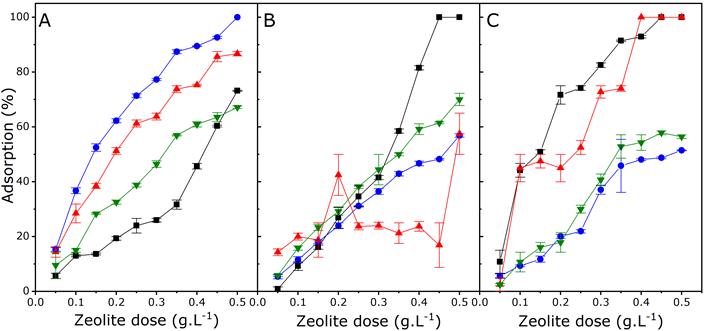
Figure 5 Removal percentage of each of the metal ions in MWWs with
high (A), medium (B), and low (C) initial concentrations of
organic matter. Pb2+ (-



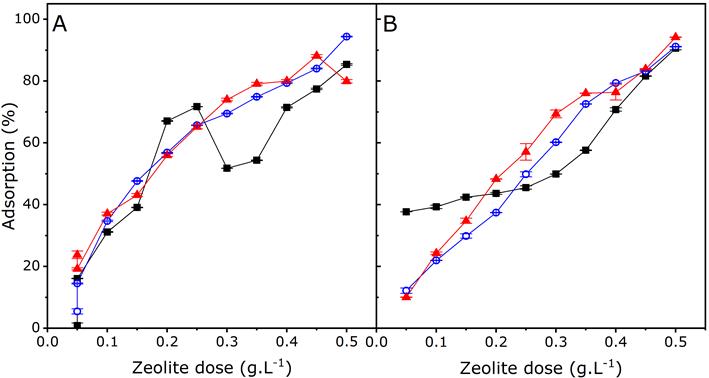
Figure 6 Percentage of adsorption of organic matter as COD (A) and
ammoniacal nitrogen (B) in MWWs with high (-

According to the statistical analysis, the zeolite dose significantly impacts (p < 0.05) ion removal (Pb2+, Fe3+, Cu2+, Zn2+, Mg2+, Ca2+, and ammoniacal nitrogen) as well as organic matter content (COD) in MWWs (Figure 5 and Figure 6). The results show that the higher the zeolite dose, the greater the removal of all the components found in MWWs. COD removal levels between 75% and 95% can be obtained by using zeolite doses greater than 0.4 g.L-1 for the three types of MWWs (high, medium, and low organic matter concentration). This may occur since the higher the initial concentration of the adsorbate, the lower the adsorption equilibrium capacity, and the greater availability of the substrate to adhere to the adsorbent (Koshy & Singh, 2016). Regarding interaction between factors (zeolite dose - initial organic matter concentration), a statistically significant effect (p < 0.05) was found with respect to the removal of ammoniacal nitrogen (positive effect) and Zn2+ (negative effect), which impacts the removal process. An additional contribution to the removal process are second-order interactions between two factors (zeolite dose2 and initial organic matter concentration2) that individually influence the response. This shows that the zeolite dose2 has a negative second-order effect on the removal of organic matter, Fe3+, Zn2+, and water hardness, while the concentration of MWWs2 has a positive second-order effect on Cu2+ and Zn2+ removal.
Some authors have reported similar results in studies where Pb2+ removal was greater than the removal of Cu2+ in a multicomponent water matrix. However, when higher doses of zeolite are used the effectiveness of metal ion removal is close to 100% (Zheng & Zaoui, 2011; Visa, 2016).
There is evidence of a matrix effect on Pb2+ and hardness removal, which shows that an increase in the organic matter concentration in MWWs leads to a decrease in the zeolite's removal ability. The opposite effect occurs regarding removal of Fe3+ and Zn2+, since an increase in initial concentrations of organic matter in MWWS results in an increase in ion removal. Metal cations (Zn2+, Fe3+, Pb2+, Cu2+) behave like Lewis acids, since their acid strength depends on their ionic radius, charge, and electronegativity. In general, these cations have such a high charge/radius ratio that they quickly interact with other ions, atoms and molecules to produce a more thermodynamically stable structure. In this case, stabilization was achieved by the interaction with oxygen and/or organic molecules found in the water, and the interaction with the negatively-charged zeolite, which acts as a Lewis base, providing the cation with additional stability.
Organic molecules found in MWWs can interact with transition-metal ions (Fe3+, Cu2+, and Zn2+) in order to form organometallic complexes. These compounds can affect metal adsorption since the metal complex exhibits different characteristics (solubility, charge, size) (Mekatel et al., 2012). The formation of complexes was not assessed by this work. However, the results obtained for Zn2+ (Figure 5) suggest the formation of complexes with organic matter, which promotes adsorption in MWW containing high organic matter concentrations. Meanwhile, Pb2+ is not able to form metal complexes, and its adsorption is better in MWW systems with low concentrations of organic matter.
Among metal ions, Fe3+ has the lowest removal rate (between 50% to 65%) with a 0.5 g.L-1 zeolite dose. This is explained by the precipitation of Fe3+, since it tends to form ferric hydroxide at pH values higher than 2.2. In addition to being a flocculent precipitate, this element has a strong basic character (Zhao et al., 2017). A level of hardness removal of up to 100% was obtained in this study. Therefore, the lower the initial concentration of MWW the higher the removal percentage.
The removal of organic matter (Figure 6A) is improved by the low initial concentration of organic matter in MWWs, where removal levels of up to 94% have been achieved by using a 0.5 g.L-1 zeolite dose. In MWWs with high initial organic matter concentrations, the percentage of removal does not exceed 85%. Competition for the zeolite exchange sites occurs between colloidal organic matter having a cationic surface charge and other cations present in MMWs. Therefore, an increase in the organic matter and ion concentrations leads to an increase in cations that have a greater affinity towards exchange and adsorption sites, which also causes a decrease in the levels of organic matter removal (Manrique, Bonilla, Chica, Otálora, & Salamanca, 2015).
Figure 6B shows the percentages of ammoniacal nitrogen removal achieved by using different zeolite doses and initial concentrations. A level of removal of this ion of up to 94% was reached in MWW with low initial concentration. As for MWWs with medium initial concentrations, the level of removal was 91%, while in MWWs with high ammoniacal nitrogen concentration the removal percentage was 90% using a 0.5 g.L-1 zeolite dose. The lower the initial concentration of the NH4 + ion, the higher the percentage of removal, which can be attributed to the availability of active sites in the zeolite. Some authors have reported that cation exchange with NH4 + Na+, K+, Ca2+, and Mg2+ ions accounts for much of NH4 + removal (Rožić, Cerjan-Stefanović, Kurajica, Vančina, & Hodžić, 2000). A previous study with naturally-occurring zeolite (clinoptilolite) found that greater ammoniacal nitrogen removal occurs when Na+ is present in the exchange sites. In addition, a competitive effect occurrs between the ammonium ions and other cations such as Ca2+, Mg2+, and K+. Nevertheless, selectivity remains predominant for the ammonium ion (Cooney, Booker, Shallcross, Stevens, & Geoffrey, 1999). The zeolite used in this study exhibited greater Mg2+ and Ca2+removal levels.
Adsorption isotherms
Unlike the result obtained (R 2 ) from the Freundlich adsorption model, the Langmuir model best fits the adsorption results, with coefficients of determination greater than 0.97. It is possible to assume that monolayers form where ion adsorption occurs in specific sites that are energetically equivalent and far enough apart, so that interaction between adsorbed ions in adjacent sites will not occur.
Table 1 shows the parameters corresponding to this type of isotherm in certain ions. The maximum adsorption rate (q m ) is given in descending order as follows: Zn2+> Fe3+ - Cu2+> NH4 +> Pb2+. The zeolite's maximum Pb2+ adsorption capacity is induced by weak attractive forces caused by the size of the hydrated ion, as previously mentioned (Mekatel et al., 2012). The q m capacity of Pb2+ on the synthesized zeolite is lower when compared to other types of adsorbents, such as sawdust (Leyva, Berber, Mendoza, & Aragón, 2004).
Table 1 Langmuir parameters for adsorption isotherms of cations found in synthetic water, where q m accounts for the maximum adsorption capacity and K is the adsorption equilibrium constant.
| Ion | q m (mg.g-1) | K (L.g -1) |
|---|---|---|
| Ammoniacal nitrogen (NH4 +) | 28.49 | 0.060 |
| Lead (Pb2+) | 9.33 | 0.268 |
| Zinc (Zn2+) | 93.46 | 0.332 |
| Copper (Cu2+) | 55.26 | 0.064 |
| Iron (Fe3+) | 68.55 | 0.651 |
The maximum adsorption capacity of ammoniacal nitrogen (28.49 mg g-1) is comparable with values obtained for two synthetic zeolites with an adsorption capacity ranging from 10.2 mg.g-1 to 24.8 mg.g-1. Other adsorbents, such as sugarcane bagasse ash, have a maximum adsorption capacity of 19.9 mg.g-1 (Prieto-García, Quintana-Puchol, Rodríguez-Díaz, Arteaga-Pérez, & Mollineda-Trujillo, 2012). These results show that synthesized faujasite has a higher adsorption capacity for ammonium ion compared to other materials.
A similar behavior occurs for Zn2+ and Cu2+, with q m values ranging from 93.46 mg.g-1 to 55.26 mg.g-1, which are higher than those reported for clinoptilolite-type zeolites (Pavón & Briones, 2009). The same happens with other adsorbents, such as tuff and bentonite (Rueda, Volzone, Lago, & Ortiga, 2007).
The synthesized faujasite has a maximum adsorption capacity q m of 68.55 mg.g-1 for Fe3+, while the maximum adsorption capacity of iron ion reported for a natural zeolite is 24.29 mg g-1, which shows that the zeolite synthesized in this study had better performance (Pavón & Briones, 2009).
All in all, the maximum adsorption values for zeolite in solutions where ions are found independently are either comparable or exceed the results reported for other adsorbents with respect to each ion.
As for the Langmuir adsorption constant (K) for each ion, the results suggest that the values were higher than those reported by other authors, who reported constants of K = 0.251 Lg-1 for Fe3+ adsorption , K = 0.150 Lg-1 for Zn2+ , K = 3.4x10-6 for Cu2+ and K = 2x10-6 for Pb2+ ((Shavandi et al., 2012; Visa, 2016).
Table 2 shows the fraction of sites occupied on the surface, also known as coverage fraction (θ = X/q m ), where X accounts for the number of ions (mg) adsorbed per gram of zeolite, and q m is the maximum adsorption capacity. In all cases, the formation of a monolayer on the surface of the zeolite (θ > 0.9) occurred for Cu2+ and Pb2+ ions, as ion concentration increased.
Table 2 Coverage fraction of each ion, at different concentrations, on the zeolite surface in synthetic water.
| Zeolite dose g.l-1 | θ Pb2+ | θ Zn2+ | θ Cu2+ | θ Fe3+ | ||||
|---|---|---|---|---|---|---|---|---|
| 3 mg.l-1 | 10 mg.l-1 | 10 mg.l-1 | 50 g.l-1 | 8 mg.l-1 | 50 mg.l-1 | 15 mg.l-1 | 50 mg.l-1 | |
| 0.013 | 0.121 | 0.863 | 0.135 | 0.235 | 0.171 | ≈1 | 0.135 | 0.235 |
| 0.025 | 0.212 | 0.886 | 0.135 | 0.401 | 0.300 | ≈1 | 0.135 | 0.401 |
| 0.038 | 0.303 | 0.908 | 0.139 | 0.387 | 0.429 | ≈1 | 0.139 | 0.387 |
| 0.050 | 0.394 | 0.931 | 0.141 | 0.385 | 0.557 | ≈1 | 0.141 | 0.385 |
| 0.063 | 0.280 | 0.946 | 0.135 | 0.391 | 0.397 | ≈1 | 0.135 | 0.391 |
| 0.075 | 0.401 | 0.954 | 0.138 | 0.449 | 0.568 | ≈1 | 0.138 | 0.449 |
| 0.085 | 0.288 | 0.984 | 0.135 | 0.449 | 0.407 | ≈1 | 0.135 | 0.449 |
| 0.100 | 0.318 | 0.954 | 0.136 | 0.455 | 0.450 | ≈1 | 0.136 | 0.455 |
With respect to zeolite’s ion adsorption capacity, when ions are present in MWW (real matrix), a competition for adsorption sites occurs, as well as a competition for sites that are conducive to ion exchange. Ions with a better affinity towards zeolite will be able to occupy larger coverage fractions.
To determine the coverage fraction by using this matrix, it was necessary to independently take into account the fraction corresponding to each ion, and then group them in order to calculate ion behavior. These values were obtained by the Langmuir equation. Table 3 shows the aforementioned results. According to these values, the coverage fractions were higher for cations at low concentrations, since a lower number of ions are competing for the active sites on the zeolite surface. The cations with the highest coverage fraction were Cu2+ at 8 g.L-1, followed by Fe3+ and Pb2+, which had similar coverage fractions at both high and low concentrations.
Table 3 Mole fraction of occupied sites by each ion present in MWWs on the zeolite surface
| Ө Mix | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ϴ NH4 + | ϴ Pb2+ | ϴ Zn2+ | ϴ Cu2+ | ϴ Fe3+ | |||||
| 12 g.l-1 | 25 g.l-1 | 3 g.l-1 | 10 g.l-1 | 10 g.l-1 | 50 g.l-1 | 8 g.l-1 | 50 g.l-1 | 15 g.l-1 | 50 g.l-1 |
| 6.90E-04 | 6.21E-05 | 2.37E-03 | 2.48E-03 | 3.68E-03 | 8.88E-04 | 4.49E-02 | 6.28E-05 | 2.66E-03 | 6.42E-03 |
Conclusions
The method used in this study enabled obtaining a faujasite-type zeolite. The zeolite did not exhibit significant changes in its structure and thermal stability after the adsorption process. This behavior suggests that zeolite could be reused after undergoing regeneration treatment.
The synthesized zeolite exhibited a high ability to remove the cations present in the MWW, and was also more selective regarding the adsorption of cations associated with water hardness (Ca2+ and Mg2+). This behavior could be related to factors such as cation exchangeability and the adsorption of hydrated ions in the zeolite pores, and depends on its size and affinity.
The affinity between zeolite and the ions found in wastewater varied significantly with MWW containing a low organic matter concentration. The level of effectiveness of the removal was Pb2+> Cu2+ > Zn2+ > Fe3+. As for high organic matter concentrations, the order was Zn2+ > Cu2+ > Fe3+ > Pb2+, which demonstrated the effect caused by the matrix. The level of selectivity may be associated with factors such as the size and charge of the cation, the concentration of species in solution, the crystal structure, and the distribution and availability of cation exchange sites on the zeolite surface. The potential formation of complexes between the organic components present in MWW and the ions also plays an important role. High concentrations of organic matter hinder Pb2+ adsorption while promoting the adsorption of Zn2+. For applications using a real matrix (high, medium and low concentrations), the results suggest that higher zeolite doses promote the adsorption of ions with lower affinity or interaction ability.
The best adsorption rate for batch applications can be obtained by using zeolite doses equal to or greater than 0.4 g.L-1. The zeolite adsorption capacity is higher for ions such as Na+ and Ca2+ (water hardness), NH4 + and organic matter present in concentrated or diluted MWWs.
The high effectiveness of the removal capacity (greater than 70%) obtained for the ions evaluated in this study makes this process a viable alternative for adsorption processes associated with heavy metal ions, water hardness, organic matter and ammoniacal nitrogen found in MWW.
Acknowledgments
The authors would like to acknowledge the financial support from the Office of the Vice-Rector for Research at Universidad de la Amazonia and its valuable contribution to the project entitled “Estudio de la capacidad de remoción de contaminantes presentes en aguas residuales afluentes a la Quebrada la Perdiz mediante una zeolita tipo faujasita sintética”. The authors would also like to thank the financial support provided by Universidad de Antioquia through the program “Sostenibilidad de Grupos de Investigación”.
REFERENCES
Ahmaruzzaman, M. (2011). Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Advances in Colloid and Interface Science, 166(1-2), 36-59. Recuperado de https://doi.org/10.1016/j.cis.2011.04.005 [ Links ]
Ansari, M., Aroujalian, A., Raisi, A., Dabir, B., & Fathizadeh, M. (2014). Preparation and characterization of nano-NaX zeolite by microwave assisted hydrothermal method. Advanced Powder Technology, 25(2), 722-727. Recuperado de https://doi.org/10.1016/j.apt.2013.10.021 [ Links ]
APHA-AWWA-WEF, American Public Heal Association-American Water Works Association-Water Environment Federation. (2012). Standard Methods for the Examination of Water and Wastewater (22nd ed.) Rice, E. W., Baird, R. B., Eaton, A. D., & Clesceri, L. S. (eds.). Washington, DC, USA: American Public Health Association, American Water Works Association, Water Environment Federation. [ Links ]
Baur, G. B., Héroguel, F., Spring, J., Luterbacher, J. S., & Kiwi-Minsker, L. (2016). Hydrothermally-treated Na-X as efficient adsorbents for butadiene removal. Chemical Engineering Journal, 288, 19-27. Recuperado de https://doi.org/10.1016/j.cej.2015.11.096 [ Links ]
Bevilacqua, M., Montanari, T., Finocchio, E., & Busca, G. (2006). Are the active sites of protonic zeolites generated by the cavities. Catalysis Today, 116(2), 132-142. Recuperado de https://doi.org/10.1016/j.cattod.2006.01.024 [ Links ]
Burakov, A. E., Galunin, E. V., Burakova, I. V., Kucherova, A. E., Agarwal, S., Tkachev, A. G., & Gupta, V. K. (2018). Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicology and Environmental Safety, 148, 702-712. Recuperado de https://doi.org/10.1016/j.ecoenv.2017.11.034 [ Links ]
Busca, G. (2014). Zeolites and other structurally microporous solids as acid-base materials. Heterogeneous Catalytic Materials, 1, 197-249. Recuperado de https://doi.org/10.1016/B978-0-444-59524-9.00007-9 [ Links ]
Castaldi, P., Santona, L., Enzo, S., & Melis, P. (2008). Sorption processes and XRD analysis of a natural zeolite exchanged with Pb2+, Cd2+ and Zn2+ cations. Journal of Hazardous Materials, 156(1-3), 428-434. Recuperado de https://doi.org/10.1016/j.jhazmat.2007.12.040 [ Links ]
Chowdhury, S., Mazumder, M. A. J., Al-Attas, O., & Husain, T. (2016). Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Science of The Total Environment, 569-570, 476-488. Recuperado de https://doi.org/10.1016/j.scitotenv.2016.06.166 [ Links ]
Chung, B. Y., Song, C. H., Park, B. J., & Cho, J. Y. (2011). Heavy metals in brown rice (Oryza sativa L.) and soil after long-term irrigation of wastewater discharged from domestic sewage treatment plants. Pedosphere, 21(5), 621-627. Recuperado de https://doi.org/10.1016/S1002-0160(11)60164-1 [ Links ]
Cooney, E. L., Booker, N. A., Shallcross, D. C., Stevens, G. W., & Geoffrey, W. (1999). Ammonia removal from wastewaters using natural Australian Zeolite. I. Characterization of the zeolite. Separation Science and Technology, 34(12), 2307-2327. Recuperado de https://doi.org/10.1081/ss-100100774 [ Links ]
Dussan, A., Otálora, J., Chica, R., Bonilla, N., & Otálora, B. D. M. (2012). Síntesis y estudio de las propiedades estructurales de zeolitas crecidas a partir del sistema NaOH + H2O + A* (A* = SiO2, A ) en un medio alcalino (pH > 10). Avances Investigación en Ingeniería, 9(2), 53-59. [ Links ]
El-Nemr, A., Khaled, A., Abdelwahab, O., & El-Sikaily, A. (2008). Treatment of wastewater containing toxic chromium using new activated carbon developed from date palm seed. Journal of Hazardous Materials, 152(1), 263-275. Recuperado de https://doi.org/10.1016/j.jhazmat.2007.06.091 [ Links ]
Fricke, R., Kosslick, H., Lischke, G., & Richter, M. (2000). Incorporation of gallium into zeolites: Syntheses, properties and catalytic application. Chemical Reviews, 100, 2303-2045. [ Links ]
Gómez, J. M. (2001). Síntesis, caracterización y aplicaciones catalíticas de zeolitas básicas (tesis de doctorado). Facultad de Ciencias Químicas, Departamento de Ingeniería Química, Universidad Complutense de Madrid, Madrid, España. [ Links ]
Khaleghian-Moghadam, R., & Seyedeyn-Azad, F. (2009). A study on the thermal behavior of low silica X-type zeolite ion-exchanged with alkaline earth cations. Microporous and Mesoporous Materials, 120(3), 285-293. Recuperado de https://doi.org/10.1016/j.micromeso.2008.11.027 [ Links ]
Koshy, N., & Singh, D. N. (2016). Fly ash zeolites for water treatment applications. Journal of Environmental Chemical Engineering, 4(2), 1460-1472. Recuperado de https://doi.org/10.1016/j.jece.2016.02.002 [ Links ]
Leyva, R. R., Berber, M., Mendoza, B., & Aragón, A. (2004). Intercambio iónico de Pb (II) en solución acuosa sobre clinoptilolita modificada por intercambio catiónico. Revista de la Sociedad Química de México, 48(2), 130-136. [ Links ]
Ltaief, O. O., Siffert, S., Fourmentin, S., & Benzina, M. (2015). Synthesis of Faujasite type zeolite from low grade Tunisian clay for the removal of heavy metals from aqueous waste by batch process: Kinetic and equilibrium study. Comptes Rendus Chimie, 18(10), 1123-1133. Recuperado de https://doi.org/10.1016/j.crci.2015.03.013 [ Links ]
Manrique, L., Bonilla, N., Chica, R., Otálora, J., & Salamanca, M. (2015). Estudio preliminar de la capacidad de remoción de iones inorgánicos de una zeolita sintética tipo faujasita. Revista Facultad de Ciencias Básicas, 11(2), 114-123. [ Links ]
Martínez, C., & Corma, A. (2013). Comprehensive inorganic Chemistry II. Comprehensive Inorganic Chemistry II, 5, 103-131. Recuperado de https://doi.org/10.1016/B978-0-08-097774-4.00506-4 [ Links ]
Mekatel, H., Amokrane, S., Benturki, A., & Nibou, D. (2012). Treatment of polluted aqueous solutions by Ni2+, Pb2+, Zn2+, Cr6+,Cd2+ and Co2+ ions by ion exchange process using faujasite zeolite. Procedia Engineering, 33(2011), 52-57. Recuperado de https://doi.org/10.1016/j.proeng.2012.01.1176 [ Links ]
Moulin, B., Oliviero, L., Maugé, F., Groust, J. F., Krafft, J. M., Costentin, G., & Massiani, P. (2008). Probing the strength, concentration and environment of basic sites in zeolites by IR spectroscopy. Studies in Surface Science and Catalysis, 174(B), 861-864. Recuperado de https://doi.org/10.1016/S0167-2991(08)80024-9 [ Links ]
Orozco, C. A., Triviño, C. C., & Manrique, L. (2014). Arranque de un reactor UASB para el tratamiento de aguas residuales domésticas en condiciones andino-amazónicas. Revista Facultad de Ciencias Básicas, 10(2), 170-185. [ Links ]
Pavón, T., & Briones, R. I. K. (2009). Evaluación del efecto de la temperatura en la remoción de cadmio, cobre, níquel, plomo y zinc del agua utilizando zeolita natural tipo clinoptilolita. México, DF, México: Instituto de Ingeniería, Universidad Nacional Autonoma de México, 1-7. Recuperado de http://www.zeocat.es/spanish/documentos.htm [ Links ]
Prieto-García, J., Quintana-Puchol, R., Rodríguez-Díaz, J., Arteaga-Pérez, L., & Mollineda-Trujillo, Á. (2012). Estudio termodinámico de la adsorción de amoniaco en ceniza de bagazo de caña de azúcar. Revista Cubana de Química, 24(2), 181-184. [ Links ]
Qureshi, A. S., Hussain, M. I., Ismail, S., & Khan, Q. M. (2016). Evaluating heavy metal accumulation and potential health risks in vegetables irrigated with treated wastewater. Chemosphere, 163, 54-61. Recuperado de https://doi.org/10.1016/j.chemosphere.2016.07.073 [ Links ]
Rezania, S., Taib, S. M., Md-Din, M. F., Dahalan, F. A., & Kamyab, H. (2016). Comprehensive review on phytotechnology: Heavy metals removal by diverse aquatic plants species from wastewater. Journal of Hazardous Materials, 318, 587-599. Recuperado de http://dx.doi.org/10.1016/j.jhazmat.2016.07.053 [ Links ]
Ríos, C. A., Williams, C. D., & Castellanos, O. M. (2006). Síntesis y caracterización de zeolitas a partir de la activación alcalina de caolinita y subproductos industriales (cenizas volantes y clinker natural) en soluciones alcalinas. Bistua Revista de la Facultad de Ciencias Básicas, 4(2), 60-71. [ Links ]
Ríos, C. A., Williams, D. C., & Roberts, L. C. (2010). Zeolitas a base de cenizas volantes del Reino Unido como adsorbentes para la remoción de metales pesados y amonio a partir de soluciones contaminadas artificialmente. Ingeniería y Competitividad, 12(1), 57-71. [ Links ]
Rosas, P. G., & Mesa, J. P. (2002). Diagnóstico preliminar de la calidad del agua y condiciones socioambientales presentes en el área de influencia de la Quebrada La Perdiz, en el Municipio de Florencia Caquetá (trabajo de pregrado). Programa de Ingeniería Agroecológica, Universidad de la Amazonia, Florencia, Colombia. [ Links ]
Rožić, M., Cerjan-Stefanović, Š., Kurajica, S., Vančina, V., & Hodžić, E. (2000). Ammoniacal nitrogen removal from water by treatment with clays and zeolites. Water Research, 34(14), 3675-3681. Recuperado de https://doi.org/10.1016/S0043-1354(00)00113-5 [ Links ]
Rueda, M. L., Volzone, C., Lago, D. C., & Ortiga, J. (2007). Aprovechamiento de tobas y bentonitas regionales para la adsorción de metales pesados en solucón acuosa. Memorias XII Encontro Nacional de Tratamento de Minérios e Metalurgia Extrativa y VII Meeting of the Southern Hemisphere on Mineral Technology (pp. 451-456). Volumen II. Ouro-Preto Brazil, Nov 20-24. [ Links ]
Sánchez, A. G. (2005). Isomerización de epóxidos lineales en fase líquida sobre catalizadores heterogéneos (tesis doctoral). Universidad Rey Juan Carlos, Madrid, España. [ Links ]
Shavandi, M. A., Haddadian, Z., Ismail, M. H. S., Abdullah, N., & Abidin, Z. Z. (2012). Removal of Fe (III), Mn (II) and Zn (II) from palm oil mill effluent (POME) by natural zeolite. Journal of the Taiwan Institute of Chemical Engineers, 43(5), 750-759. Recuperado de https://doi.org/10.1016/j.jtice.2012.02.014 [ Links ]
Treacy, M. M. J., & Higgins, J. B. (2001). Collection of simulated XRD powder patterns for zeolites. Elsevier, 21(2), 388-389. Recuperado de https://doi.org/10.1016/S0166-9834(00)81382-2 [ Links ]
Visa, M. (2016). Synthesis and characterization of new zeolite materials obtained from fly ash for heavy metals removal in advanced wastewater treatment. Powder Technology, 294, 338-347. Recuperado de https://doi.org/10.1016/j.powtec.2016.02.019 [ Links ]
Wang, S., & Peng, Y. (2010). Natural zeolites as effective adsorbents in water and wastewater treatment. Chemical Engineering Journal, 156(1), 11-24. Recuperado de https://doi.org/10.1016/j.cej.2009.10.029 [ Links ]
Yuna, Z. (2016). Review of the natural, modified, and synthetic zeolites for heavy metals removal from wastewater. Environmental Engineering Science, 33(7), 443-454. Recuperado de https://doi.org/10.1089/ees.2015.0166 [ Links ]
Zhang, X., Yang, S., Tang, D., & Yang, R. (2015). Synthesis of zeolite NaX at 25 °C and 95 °C: Characterization, cobalt exchange and catalytic performance in epoxidation of styrene. Materials Research Bulletin, 70, 343-347. Recuperado de https://doi.org/10.1016/j.materresbull.2015.04.049 [ Links ]
Zhao, M., Zhou, X., Tang, J., Deng, Z., Xu, X., Chen, Z., Ma, L.-J. (2017). Pyrene excimer-based fluorescent sensor for detection and removal of Fe3+ and Pb2+ from aqueous solutions. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 173, 235-240. Recuperado de https://doi.org/10.1016/j.saa.2016.09.033 [ Links ]
Zheng, Y., & Zaoui, A. (2011). How water and counterions diffuse into the hydrated montmorillonite. Solid State Ionics, 203(1), 80-85. Recuperado de https://doi.org/10.1016/j.ssi.2011.09.020 [ Links ]
Received: February 06, 2017; Accepted: February 14, 2018











 texto em
texto em