Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias pecuarias
versão On-line ISSN 2448-6698versão impressa ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.10 no.2 Mérida Abr./Jun. 2019
https://doi.org/10.22319/rmcp.v10i2.4638
Technical notes
Analysis of rotavirus in rabbits in the State of Mexico
a Universidad Autónoma del Estado de México. Centro Universitario UAEM Amecameca, Km 2.5, Carretera Amecameca Ayapango, 59000. México.
b Universidad Autónoma del Estado de México. Centro de Investigación y Estudios Avanzados en Salud Animal, Facultad de Medicina Veterinaria y Zootecnia. Toluca, Estado de México, México.
Enteric diseases can severely impact livestock production systems, causing financial losses due to mortality, compromised growth performance and reduced conversion rates. Rotavirus is considered a principal cause of acute viral gastroenteritis in young humans and animals worldwide, and is associated with dehydrating diarrhea in livestock. A study was done to identify rotavirus in 39 small backyard rabbit production systems in thirteen municipalities in the south-eastern region of the State of Mexico. A total of 147 samples were analyzed with RT-PCR, 99 from healthy rabbits and 48 from animals exhibiting enteric symptoms. Lapine rotavirus was identified in nine (18.7 %) of the diseased animals, and none of the healthy individuals. The results support the hypothesis that in rabbits with enteric disease, viruses play an important but not decisive role, and that rotavirus does not determine disease occurrence. Lapine rotavirus is probably not endemic in rabbit production systems in the studied region, and may occur without inducing serious enteric episodes in individuals. However, in association with other pathogens, it may also be an important contributing factor in enteric outbreaks. This is one of the first reports of rotavirus in rabbits with enteric disease in Mexico.
Key words: Mexico; Rotavirus; Rabbits; Diarrhea
Dentro de las producciones pecuarias, las enfermedades entéricas tienen un papel importante, ya que generan severas pérdidas económicas derivadas de la mortalidad, depresión del crecimiento y disminución del índice de conversión. Rotavirus es considerado una causa importante de gastroenteritis viral aguda en humanos y animales jóvenes en todo el mundo, estando asociado a diarreas deshidratantes en granjas de producción animal. El objetivo de este estudio fue identificar rotavirus en granjas de producción cunícola ubicadas en la región sur-oriente del Estado de México a través de RT-PCR. Se recolectaron muestras de 39 pequeñas granjas de traspatio ubicadas dentro de los 13 municipios que conforman la región estudiada. Un total de 147 muestras se analizaron, 99 de conejos sanos y 48 de conejos con signología entérica, de las cuales se identificó Rotavirus en 9 de ellas (18.7 %), mientras que en conejos sanos no hubo identificación. Los resultados concuerdan con la suposición de que en conejos con signología entérica, los virus parecen tener un papel importante, pero no decisivo, y que la participación de rotavirus no es determinante para la presentación de la enfermedad. Se plantea la posibilidad de que Rotavirus no sea endémico en granjas cunícolas de la región sur-oriente del Estado de México, encontrándose ocasionalmente, sin inducir episodios entéricos graves de forma individual; no obstante, podría ser un factor importante en la presentación de brotes entéricos en asociación con otros agentes patógenos. Este es uno de los primeros reportes de la presencia de Rotavirus en conejos con signología entérica en México.
Palabras clave: México; Rotavirus; Conejos; Diarrea
Rabbit (Oryctolagus cuniculus) production involves their systematic breeding, growing and reproduction to generate profit from the sale of products and by-products1. Production of rabbits is growing in Mexico. In 2016, the Ministry of Agriculture, Livestock, Rural Development, Fisheries and Food (Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación - SAGARPA) estimated that total national rabbit meat production exceeded 15,000 t, with production being highest in the states of Puebla, Tlaxcala, Morelos, Michoacán, Queretaro and the State of Mexico2. This latter state accounts for the highest rabbit meat production and consumption nationwide, and the southeast portion of the state is responsible for most of the production, marketing and consumption(2, 3). Despite constant growth in rabbit production in Mexico, it is not given the importance of other common livestock species, and thus remains a largely rural backyard enterprise done by subsistence farmers in low-income regions4. This type of small-scale production represents 95 % of total national rabbit production5.
One of the principal problems in rabbit production is a lack of information on good production practices, especially on vital issues such as health, biosafety and animal welfare. This can favor the occurrence of pathogenic agents responsible for various diseases6,7. Enteric diseases can seriously impact farms focused on animal production because they can generate severe financial losses due to mortality, compromised growth performance and lower conversion rates8-11. Rotavirus (RV) is among the pathogens that can cause signs of enteric diseases in rabbit production systems9,12, and is one of the leading causes of acute viral gastroenteritis in humans and young animals worldwide13,14, including in rabbits15.
Rotavirus is a double-stranded RNA virus (dsRNA) belonging to the Reoviridae family, subfamily Sedoreovirinae, genus Rotavirus16. Lapine rotavirus (LRV) strains can cause enteric cases, mainly in post-weaning rabbits, but it is also implicated in the etiology of severe enteritis outbreaks in association with bacteria, parasites and other viruses. Rotavirus (RV) infection is most common in rabbits from 35 to 50 d of age, is characterized by a high morbidity rate and the presence of non-specific clinical signs, such as diarrhea, dehydration, anorexia and depression. Diseased rabbits may die from dehydration and secondary infections, while those that recover usually exhibit decreased productivity due to reduced nutrient absorption capacity9,12,17.
In Mexico, there is only one report to date of the presence of RV in rabbits with enteric clinical profiles18. The present study objective was to analyze the presence of LRV in healthy rabbits and those with enteric symptoms in the thirteen municipalities conforming the southeast region of the State of Mexico.
Samples were collected from April 2014 to November 2016 at 39 rural backyard production units. A total of 147 samples were collected from rabbits between 25 and 60 d of age, from both healthy individuals and those manifesting enteric clinical symptoms (e.g. abdominal distension, anorexia, depression, diarrhea, dehydration). Production systems were basic, involving few or no biosafety measures and inadequate sanitation management. The sampled farms were located in the southeast region of the State of Mexico, consisting of thirteen municipalities: Valle de Chalco; Chalco; Temamatla; Cocotitlan; Tlalmanalco; Juchitepec; Tenango del Aire; Ayapango; Amecameca; Atlautla; Ozumba; Tepetlixpa; and Ecatzingo (Figure 1).
Samples were collected from live animals, either as 1 ml of liquid excrement or 2 g of soft or solid excrement. These were placed in sterile vials, transported under refrigeration to the Biotechnology, Molecular Biology and Genetics Laboratory of the Autonomous University of the State of Mexico (Universidad Autonoma del Estado de Mexico - UAEM), UAEM Amecameca University Center, where they were stored at -75 °C until analysis. In dead animals, samples were taken directly from the small intestine within a period no greater than 5 h post mortem. Animals manifesting severe enteric clinical symptoms were killed following established guidelines (NOM-033-ZOO-1995), and samples taken of excrement and small intestine tissue (including contents). The study design was approved by the UAEM Amecameca University Center Bioethics Committee (CBE/13/2014).
Samples were analyzed for LRV identification by amplification of three of its structural proteins; VP6, VP4 and VP7 using reverse transcription polymerase chain reaction (RT-PCR) with total RNA extracted from samples. Detection of VP6 was done using primers designed by Iturriza-Gómara et al19 which amplify a 379 base pair (bp) fragment of the VP6 gene (VP6-F [nt 747 - 766] 5' GACGGVGCRACTACATGGT 3' and VP6-R [nt 1126 - 1106] 5' GTCCAATTCATNCCTGGTGG 3'). For VP4, previously reported primers20 were used for amplification of an 876 bp fragment encoding for the VP4 protein (with 3 [nt 11 - 32] F 5' TGGCTTCGCCATTTTATAGACA 3' and 2 [nt 887 - 868] R 5' ATTTCGGACCATTTATAACC 3'). Complete amplification of VP7 was done with the primers Beg 9 (nt 1 - 28) F 5' GGCTTTAAAAGAGAGAATTTCCGTCTGG 3' and End 9 (nt 1062 - 1036) R 5' GGTCACATCATACAATTCTAATCTAAG 3'21. Total RNA was obtained using the GeneJET Viral DNA and RNA purification kit (Thermo Scientific™), according to the manufacturer instructions. After isolation of virus RNA, a single-step RT-PCR was run using the commercial Superscript® III One Step RT-PCR with Platinum® Taq (Invitrogen™) kit. Reaction positive control was the pentavalent Rotateq vaccine (Sanofi Pasteur MSD), and the negative control was rabbit DNA. PCR products were viewed in 2% agarose gels stained with ethidium bromide.
A total of 147 samples were processed, 99 from healthy rabbits and 48 from rabbits exhibiting enteric symptoms. Lapine rotavirus (LRV) was identified in nine of the diseased animals and was not detected in healthy ones (Table 1).
Table 1 Data on samples collected for lapine rotavirus (LRV) detection in thirteen municipalities in the southeast region of the State of Mexico
| Municipality | Farms | Total Rabbits | Healthy | Diseased | LRV+ |
|---|---|---|---|---|---|
| Valle de Chalco | 2 | 8 | 6 | 2 | 0 |
| Amecameca | 5 | 19 | 12 | 7 | 3 |
| Atlautla | 4 | 14 | 11 | 3 | 1 |
| Ozumba | 2 | 9 | 6 | 3 | 0 |
| Tepetlixpa | 2 | 8 | 6 | 2 | 0 |
| Juchitepec | 4 | 12 | 9 | 3 | 1 |
| Ayapango | 4 | 15 | 8 | 7 | 3 |
| Tenango del Aire | 2 | 9 | 6 | 3 | 0 |
| Temamatla | 3 | 8 | 5 | 3 | 0 |
| Cocotitlán | 2 | 10 | 6 | 4 | 0 |
| Chalco | 3 | 8 | 6 | 2 | 0 |
| Tlalmanalco | 4 | 15 | 10 | 5 | 1 |
| Ecatzingo | 2 | 12 | 8 | 4 | 0 |
| Total | 39 | 147 | 99 | 48 | 9 |
Clinical manifestations in diseased rabbits included abdominal distension, anorexia, depression, diarrhea and dehydration. Lapine rotavirus (LRV) identification was done using excrement samples from all animals, as well as small intestine samples from those manifesting enteric symptoms. The RT-PCR technique was used to amplify 379 bp fragments of VP6 (Figure 2), 1062 bp fragments of VP7 (Figure 3), and 879 bp fragments of VP4 (Figure 4).
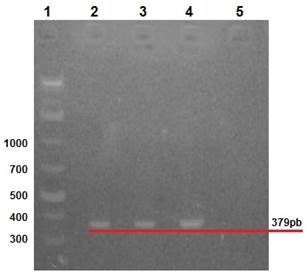
Row 1: 1 Kb molecular weight marker; row 2: positive control (vaccine); rows 3 and 4: rabbit samples; row 5: negative control.
Figure 2 2% agarose gel amplification of 379 bp fragments corresponding to the VP6 gene of LRV
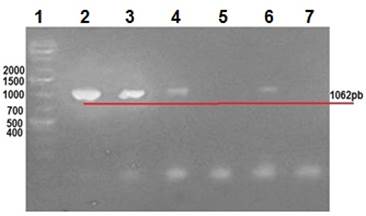
Row 1: 1 Kb molecular weight marker; row 2: positive control (vaccine); rows 3 to 6: rabbit samples; row 5: negative control.
Figure 3 2% agarose gel amplification of 1062 bp fragments corresponding to the VP7 gene of LRV.
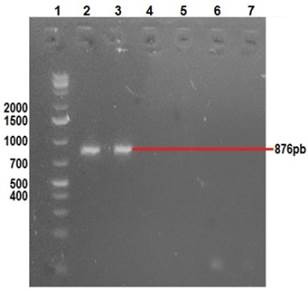
Row 1: 1 Kb molecular weight marker; row 2: positive control (vaccine); row 3: amplification VP4; row 7: negative control.
Figure 4 2% agarose gel amplification of 876 bp fragments corresponding to the VP4 gene of LRV
Enteric diseases can cause substantial financial losses in rabbit production systems9,22. Enteric syndrome is a major disease in rabbits due to its productive and economic impacts. Among the various pathogens that have been identified in rabbits with enteric clinical profiles is a RV strain, which is considered of low virulence23. Alone it should not be able to induce severe episodes, however it can cause enteric disease in post-weaning rabbits and is implicated in the etiology of severe enteritis outbreaks in association with other pathogens24.
Of the 147 samples analyzed here for LRV, 99 were from healthy rabbits and 48 from rabbits manifesting enteric symptoms. Lapine rotavirus was identified in 9 (18.7 %) of the diseased animals. Samples from healthy rabbits were analyzed to identify possible subclinical infections, since once LRV has entered an organism the extent and severity of clinical signs and intestinal lesions depend on the amount of viral particles ingested. Under these circumstances rabbits 4 to 5 wk of age can have a subclinical infection, favoring virus dissemination9,12.
Occurrence of LRV in the diseased rabbits agrees with the assumption that in rabbits with enteric symptoms viruses seem to play an important but not decisive role, and that LRV does not trigger enteric disease6,9. The present results support the hypothesis that enteric syndrome in rabbits is multifactorial in origin with various pathogens acting synergistically to induce gastroenteritis22,25-28. In wild as well as domestic rabbit populations LRV may play an important role in inducing the disease, either by exerting direct pathogenic activity or by facilitating the entry and replication of other pathogenic agents through minimal alteration of the intestinal epithelium17.
The 18.7 % LRV infection rate observed in the present results is higher than the 15.3 %9, 17.6 %25, and 16 %27 reported in studies done in Italy, and the 3 % reported in a study done in Canada28. However, it was lower than the 23 % reported in another study in Italy29. Worldwide, Italy is one of the main producers of rabbit meat30, with highly technical production facilities. Nonetheless, intensive rabbit production is characterized by intense genetic selection, excess productive yield, overpopulation and overcrowding, which contribute to high levels of environmental pollution with facultative pathogens9. Large-scale rabbit production may generate animal health challenges, but the present results suggest that backyard production implies an increased risk of disease occurrence, possibly due to factors such as lack of health and biosafety measures, and inadequate infrastructure. These factors are symptomatic of limited financial resources and a lack of information on good production practices.
The LRV infection rate in the present results is higher than the 10.34 % previously reported in Mexico18. This higher rate may be the result of the constant growth in rabbit meat production2. A larger rabbit population in conjunction with more intra-regional commerce of animals and constant production could explain this increase in LRV, and perhaps in other pathogens. Further studies are needed to determine the epidemiology of LRV and other infectious microorganisms circulating among rabbit farms in this region of intense rabbit meat production, sale and consumption.
In addition to their financial impacts, several animal rotaviruses are potential sources of human infection (zoonoses), with confirmed events31-33. Epidemiological surveillance and molecular characterization of certain strains has been intensified in response, especially in host species in proximity to human populations22.
In the present study samples were collected from backyard rabbit production systems that had similar infrastructure and management practices. This approach was used because 95 % of rabbit meat production in Mexico occurs in small-scale systems5, and several animal species continuously interact in these systems, favoring inter-species disease transmission, as well as zoonotic events. It is vital to know the pathogens present in livestock production systems, especially those posing a potential risk to public health.
The results suggest that LRV is not endemic in rabbit production systems in the southeastern region of the State of Mexico. Apparently it occurs occasionally but without inducing severe enteric episodes in individuals; however, it may be an important factor in the appearance of enteric outbreaks in association with other pathogenic agents.
Continued growth in rabbit meat production in Mexico highlights the need for development and application of good production practices aimed at reducing biological risks to animals and humans, improving production performance and minimizing financial losses. Further research is clearly required to understand the epidemiology of lapine rotavirus and other pathogens that affect rabbit production systems in Mexico. This is among the first reports of the presence of rotavirus in rabbits exhibiting enteric symptomatology in Mexico.
Literatura citada
1. Gamboa RC. Estudio de mercado de la carne de conejo en el municipio de Texcoco [tesis maestría]. Texcoco, Estado de México, México; Colegio de Posgraduados; 2001. [ Links ]
2. SAGARPA. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Todo sobre la producción de carne de conejo. México. 2016. [ Links ]
3. Rodríguez AGI. Competitividad del sistema agroalimentario localizado productor de carne de conejo de la zona sur oriente del estado de México [tesis maestría]. Amecameca, Estado de México, México; Universidad Autónoma del Estado de México; 2012. [ Links ]
4. Comité Nacional Sistema Producto Cunícola. Comité Nacional Sistema Producto Cunícola Comité Nacional Sistema Producto Cunícola http://sistemaproductocunicola.org.mx/cunicola.html Consultado 25 Sep, 2017. [ Links ]
5. SAGARPA. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación . Manual de buenas prácticas de producción de carne de conejo. México. 2015. [ Links ]
6. Percy DH, Muckle CA, Hampson RJ, Brash ML. The enteritis complex in domestic rabbits: a field study. Can Vet J 1993;34(2):95-102. [ Links ]
7. European Food and Safty Authority. The Impact of the current housing and husbandry systems on the health and welfare of farmed domestic rabbits [The EFSA Journal]. 2005;(267):1-31. [ Links ]
8. Peeters JE, Pohl P, Charlier G. Infectious agents associated with diarrhoea in commercial rabbits: a field study. Ann Rech Vet 1984;15(3):335-340. [ Links ]
9. Lavazza A, Cerioli M, Martella V, Tittarelli C, Grilli G, Brivio R, et al. Rotavirus in diarrheic rabbits: prevalence and characterization of strains in Italian Farms. Proc IX World Rabbit Congress. Verona, Italy. 2008:993-998. [ Links ]
10. Dhama K, Chauhan RS, Mahendran M, Malik SV. Rotavirus diarrhea in bovines and other domestic animals. Vet Res Commun 2009;(33):1-23. [ Links ]
11. Papp H, László B, Jakab F, Ganesh B, De-Grazia S, Matthijnssens J, et al. Review of group A rotavirus strains reported in swine and cattle. Vet Microbiol 2013;(165):190-199. [ Links ]
12. Kerr PJ, Donnelly TM. Viral infections of rabbits. Vet Clin North Am Exot Anim Pract 2013;16(2):437-68. [ Links ]
13. Bishop R. Discovery of rotavirus: Implications for child health. J Gastroenterol Hepatol 2009;24(Suppl 3):S81-S85. [ Links ]
14. Estes M, Greenberg H. Rotaviruses. In: Knipe DM, Howley P. et al. editors. Fields virology. 6th ed., Philadelphia, PA; Wolters Kluwer Health/Lippincott Williams & Wilkins 2013:1347-1401. [ Links ]
15. Schoeb TR, Casebolt DB, Walker VE, Potgieter LN, Thouless ME, DiGiacomo RF. Rotavirus-associated diarrhoea in a commercial rabbitry. Lab Anim Sci 1986;(36):149-152. [ Links ]
16. ICTV. International Committee on Taxonomy of Viruses. Virus Taxonomy, EC 48. 2016 2016 https://talk.ictvonline.org/taxonomy/ Consultado 25 Sep, 2017. [ Links ]
17. Ciarlet M, Gilger MA, Barone C, McArthur M, Estes MK, Conner ME. Rotavirus disease, but not infection and development of intestinal histopathological lesions, is age restricted in rabbits. Virology 1998;251(2):343-60. [ Links ]
18. García-Rubio VG, Bautista-Gómez LG, Martínez-Castañeda JS, Romero-Núñez C. Multicausal etiology of the enteric syndrome in rabbits from Mexico. Rev Argent Microbiol 2017;49(2):132-138. [ Links ]
19. Iturriza-Gómara M, Wong C, Blome S, Desselberger U, Gray J. Molecular characterization of VP6 genes of human rotavirus isolates: Correlation of genogroups with subgroups and evidence of independent segregation. J Virol 2002;76(13):6596-6601. [ Links ]
20. Gentsch JR, Glass RI, Woods P, Gouvea V, Gorziglia M, Flores J, et al. Identification of Group A Rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol 1992;(30):1365-1373. [ Links ]
21. Gouvea V, Glass RI, Woods P, Taniguchi K, Clark HF, Forrester B, et al. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from Stool specimens. J Clin Microbiol 1990;(28):276-282. [ Links ]
22. Bányai K, Forgach P, Erdelyi K, Martella V, Bogdan A, Hocsak E, et al. Identification of the novel lapine rotavirus genotype P[22] from an outbreak of enteritis in a Hungarian rabbitry. Virus Res 2005;(113):73-80. [ Links ]
23. Thouless ME, Digiacomo RF, Deeb BJ, Howard H. Pathogenicity of Rotavirus in rabbits. J Clin Microbiol 1988;26(5):943-947. [ Links ]
24. Lavazza A, Capucci L. Viral infection of rabbits. Proc IX World Rabbit Congress. Verona, Italy. 2008:993-998. [ Links ]
25. Martella V, Ciarlet M, Lavazza A, Camarda A, Lorusso E, Terio V. et al. Lapine rotaviruses of the genotype P[22] are widespread in Italian rabbitries. Vet Microbiol 2005;111(1-2):117-124. [ Links ]
26. Licois D. Domestic rabbit enteropathies. Proc. VIII World Rabbit Congress. Puebla, Mexico. 2004:385-403. [ Links ]
27. Nieddu D, Grilli G, Gelmetti D, Gallazzi D, Toccacieli S, Lavazza A. Electron microscopy detection of viral agents in rabbits with enteropathy during the period 1982-1999 in Italy. Proc VII World Rabbit Congress. Valencia, Spain. 2000:4-7. [ Links ]
28. Xie X, Bil J, Shantz E, Hammermueller J, Nagy E, Turner PV. Prevalence of lapine rotavirus, astrovirus, and hepatitis E virus in Canadian domestic rabbit populations. Vet Microbiol 2017;208:146-149. [ Links ]
29. Cerioli M, Cordioli P, Palotta C, Lavazza A. Survey on enteric viruses identified in diarrhoeic rabbits. Proc Cost 848: Workshop pathology and nutrition. Cercedilla, Spain. 2004:26. [ Links ]
30. FAO. Food and Agriculture Organization of the United Nations. FAOSTAT. http://www.fao.org/home/en/ Consultado 28 Sep, 2017. [ Links ]
31. De-Leener K, Rahman M, Matthijnssens J, Van-Hoovels L, Goegebuer T, van-der-Donck I, et al. Human infection with a P[14], G3 lapine rotavirus. Virology 2004;325(1):11-17. [ Links ]
32. Matthijnssens J, Rahman M, Martella V, Xuelei Y, De-Vos S, De-Leener K, et al. Full genomic analysis of human rotavirus strain B4106 and lapine rotavirus strain 30/96 provides evidence for interspecies transmission. J Virol 2006;80(8):3801-10. [ Links ]
33. Bonica MB, Zeller M, Van-Ranst M, Matthijnssens J, Heylen E. Complete genome analysis of a rabbit rotavirus causing gastroenteritis in a human infant. Viruses 2015;7(2):844-856. [ Links ]
Received: September 26, 2017; Accepted: February 09, 2018











 texto em
texto em