Introduction
Benthic communities have an important role in the structure and function of aquatic ecosystems. Benthos is regulated by geographic location and climate (Giere, 2009), bathymetry (Goldman & Horne, 1983), water column and sediment characteristics (Horne & Goldman, 1994; Wetzel, 1981), as well as direct and indirect trophic interactions (Horne & Goldman, 1994); therefore all these factors define the benthos species composition, adaptations, distribution, growth, productivity, and reproductive potential (Wetzel, 1981).
Benthic communities also have an important role in trophic food webs by linking benthic and pelagic food webs (i.e., bottom-up and top-down controls) by feeding on detritus, algae, bacteria, protozoa, and other small invertebrates, and having a relevant role in nutrient recycling and organic matter decomposition (Fierro et al., 2015). On the other hand, they constitute the energy source of larger organisms of higher trophic levels that prey on them (Fierro et al., 2015; Vander & Vadeboncoeur, 2002).
Most research on the role of benthos in aquatic ecosystems comes from temperate lakes (e.g., Babler et al., 2008; Brodersen et al., 2004; Fierro et al., 2015; Hamburguer et al., 2000; Jørgensen & Revsbech, 1985; Jyväsjärvi et al., 2009; Nagell & Landahl, 1978; Peeters et al., 2004; Vander & Vadeboncoeur, 2002), while contributions from tropical lakes are rather scarce (e.g., Cleto-Filho & Arcifa, 2006; Hernández et al., 2014).
Species diversity of terrestrial ecosystems increases in the tropics, which results in a high biodiversity in tropical rain forests (Lewis, 1987). Contrarily, Lewis (1996) suggested that biological diversity at deep zones of tropical lakes would diminish by the effect of their prolonged anoxia, a consequence of their higher temperatures (i.e., low solubility of oxygen and increased microbial metabolism), regardless of their trophic state. In contrast, the bottom layer of temperate oligotrophic lakes remains well oxygenated.
Mexico is considered a megadiverse country. It is one of the few countries that possesses, all together, 70% of the vertebrate and vascular plants diversity worldwide (Llorente-Bousquets & Ocegueda, 2008); however, little is known about the diversity of aquatic invertebrates of Mexican freshwaters, and it is unknown whether these ecosystems present the same trend of high biodiversity that terrestrial communities show.
The aim of the present study was to provide accurate data on the biodiversity of benthic communities of 18 tropical lakes in the Protected Natural Area and RAMSAR site “Lagunas de Montebello”, Chiapas, southern Mexico. It should be noted that there are only 2 previous published studies (Guadarrama-Hernández et al., 2015; Palacios-Vargas et al., 2018) concerning benthic macroinvertebrates of this area.
Materials and methods
Lagunas de Montebello National Park (LMNP), with 64.2 km2, is at the southwestern portion of Chiapas State, Mexico, at the border with Guatemala; it covers part of 2 municipalities: La Trinitaria and La Independencia (Fig. 1). Extreme coordinates are 16°04’40” - 16°10’2” N, 91°37’40” - 91°47’40” W (Conanp, 2007), at an elevation of 1,200 to 1,800 m asl (Durán-Calderón et al., 2014).
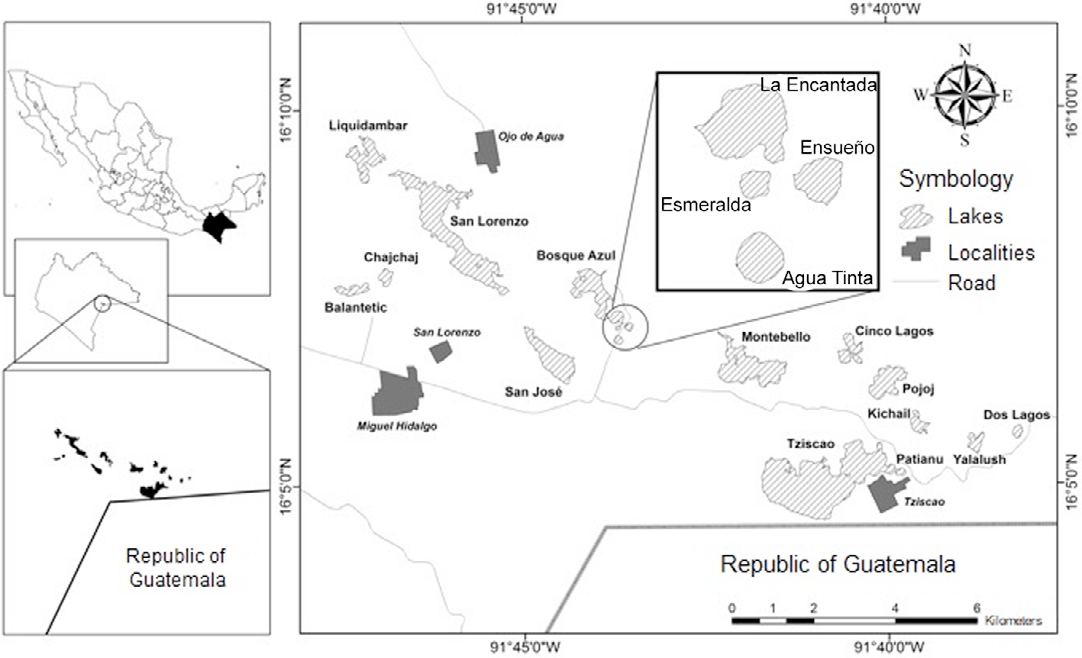
Figure 1 Location of the lakes in the "Lagunas de Montebello" National Park (taken from Alcocer et al. [2016]).
Two physiographic provinces are found in the LMNP: the High Mountains of Chiapas and the Coastal Plain of the Gulf of Mexico. The LMNP is in an endorheic basin from the sub-basin of the Comitán River, fed by the Grande River, which belongs to the hydrologic region No. 30 (RH30) Grijalva-Usumacinta (Conanp, 2007). The region has a temperate, wet climate with long summers, mean annual temperature of 17.3 °C and precipitation of 1,800 mm, and a warm-rainy season lasting from May to December (García, 2004).
The area is covered by Cretaceous limestones associated with the formation of a lake complex of karstic origins. The lakes are NW-SE aligned, following the orientation of the main tectonic units (Durán-Calderón, 2013). There are more than 50 lakes in the zone displaying a wide range of morphometric and bathymetric characteristics (Alcocer et al., 2016).
The water of the bottom of the lakes is warm, with temperatures varying from 17.1 to 23.2 °C, dissolved oxygen concentration range from anoxia (under detection limit, < 0.01 mg L-1) up to 18 mg L-1, electric conductivity fluctuate from 172 to 1,659 μS cm-1, and pH from neutral to slightly basic, within a range of 6.9-8.8. The sediments are silty, organic (15-75%), and with highly variable (2-98%) carbonate content (Alcocer et al., 2018). Deep lakes (14 out of 18) are warm-monomictic; the thermal stratification period takes place throughout summer, the warm-rainy/warm season, during which the hypolimnion becomes anoxic. In the winter (cold-dry/cold season), circulation takes place in the lakes and the bottom water re-oxygenates. The remaining 4 lakes are shallow, warm polymictic, with water circulating throughout the year.
Eighteen lakes were selected (Fig. 1, Table 1) illustrating different morphometric features (shallow and deep, small and large) and different trophic states (oligotrophic and eutrophic). Five sampling campaigns were carried out between 2013 and 2016 at different seasons along the annual cycle, so the sampling frequency varied between one and 5 times per lake (Table 2).
Table 1 General characteristics of the Montebello Lakes, Chiapas, Mexico. Abb: Abbreviation, Zmax: maximum depth, Zmean: mean depth, L: maximum length, B: maximum width, A: surface area, S: shallow, D: deep, O: oligotrophic, E: eutrophic. Lakes are arranged in a NW-SE orientation. Taken from Alcocer et al. (2016).
| Lake | Abb | Zmax | Zmean | L | B | A | S/D | O/E |
| (m) | (m) | (km) | (km) | (ha) | ||||
| Liquidámbar | LI | 24 | 11.2 | 0.95 | 0.70 | 40.5 | D | E |
| San Lorenzo | SL | 67 | 11.8 | 3.09 | 1.29 | 181.3 | D | E |
| Chajchaj | CH | 12 | 5.3 | 0.45 | 0.31 | 9.2 | S | E |
| Balantetic | BL | 3 | 1.7 | 0.81 | 0.23 | 13.6 | S | E |
| Bosque Azul | BA | 58 | 20 | 1.32 | 0.82 | 52.5 | D | E |
| La Encantada | EC | 89 | 29.4 | 0.39 | 0.31 | 8.2 | D | E |
| Esmeralda | ES | 7 | 3.6 | 0.14 | 0.11 | 1.1 | S | O |
| Ensueño | EN | 35 | 21.6 | 0.22 | 0.19 | 2.7 | D | O |
| Agua Tinta | AT | 24 | 14.7 | 0.21 | 0.20 | 3 | D | O |
| San José | SJ | 30 | 10.3 | 1.76 | 0.66 | 60.6 | D | O |
| Montebello | MO | 45 | 12.3 | 1.69 | 1.14 | 96.2 | D | O |
| Cinco Lagos | CL | 162 | 42.5 | 0.82 | 0.60 | 23.7 | D | O |
| Pojoj | PO | 198 | 35.2 | 1.06 | 0.74 | 43.7 | D | O |
| Kichail | KI | 22 | 9.5 | 0.58 | 0.44 | 12.5 | D | O |
| Tziscao | TZ | 86 | 28.9 | 3.20 | 1.48 | 306.6 | D | O |
| Patianú | PA | 26 | 10.8 | 0.26 | 0.18 | 3.4 | D | O |
| Yalalush | YA | 23 | 9.9 | 0.54 | 0.33 | 11.5 | S | O |
| Dos Lagos | DL | 42 | 25.2 | 0.34 | 0.23 | 5.2 | D | O |
Table 2 Date, season, and sampling frequency at each lake in Montebello, Chiapas, México, throughout sampling campaigns (2013- 2016).
| Lake | Sampling date | Season | Sampling frequency |
| Liquidámbar | March 2013 | Cold-dry | 2 |
| June 2014 | Warm-rainy | ||
| San Lorenzo | February 2015 | Cold-dry | 1 |
| Chajchaj | March 2013 | Cold-dry | 2 |
| June 2014 | Warm-rainy | ||
| Balantetic | February 2015 | Cold-dry | 1 |
| Bosque Azul | March 2013 | Cold-dry | 4 |
| June 2014 | Warm-rainy | ||
| October 2015 | Warm-rainy | ||
| March 2016 | Cold-dry | ||
| La Encantada | February 2015 | Cold-dry | 1 |
| Esmeralda | March 2013 | Cold-dry | 3 |
| June 2014 | Warm-rainy | ||
| February 2015 | Cold-dry | ||
| Ensueño | March 2013 | Cold-dry | 5 |
| June 2014 | Warm-rainy | ||
| February 2015 | Cold-dry | ||
| October 2015 | Warm-rainy | ||
| March 2016 | Cold-dry | ||
| Agua Tinta | March 2013 | Cold-dry | 3 |
| June 2014 | Warm-rainy | ||
| February 2015 | Cold-dry | ||
| San José | March 2013 | Cold-dry | 2 |
| June 2014 | Warm-rainy | ||
| Montebello | March 2013 | Cold-dry | 2 |
| June 2014 | Warm-rainy | ||
| Cinco Lagos | March 2013 | Cold-dry | 3 |
| June 2014 | Warm-rainy | ||
| February 2015 | Cold-dry | ||
| Pojoj | March 2013 | Cold-dry | 2 |
| June 2014 | Warm-rainy | ||
| Kichail | February 2015 | Cold-dry | 1 |
| Tziscao | March 2013 | Cold-dry | 4 |
| June 2014 | Warm-rainy | ||
| October 2015 | Warm-rainy | ||
| March 2016 | Cold-dry | ||
| Patianú | March 2013 | Cold-dry | 2 |
| February 2015 | Cold-dry | ||
| Yalalush | March 2013 | Cold-dry | 2 |
| June 2014 | Warm-rainy | ||
| Dos Lagos | March 2013 | Cold-dry | 5 |
| June 2014 | Warm-rainy | ||
| February 2015 | Cold-dry | ||
| October 2015 | Warm-rainy | ||
| March 2016 | Cold-dry |
Benthic invertebrate samples were taken from one point at the deepest zone of each lake with an Ekman dredge (15 × 15 × 15 cm) and 3 repetitions were made at each sampling point. By convention, macroinvertebrates are defined as all invertebrate fauna that can be captured by a 500 μm sieve (Hauer & Resh, 2007); however, early stages of some species may be smaller, so the samples were sieved through a 250 μm mesh size (Hauer & Resh, 2007). Organisms were preserved in 96% ethanol + Bengal Rose as a vital dye. Organisms were separated and identified to genus level following general keys (Brinkhurst & Marchese, 1991; Epler, 1992; Merritt et al. 2008; Wiederholm, 1983), and confirmed by experts.
Taxonomic richness differences between lakes were tested using Student´s t-tests and Anovas. The taxonomic composition similarity between lakes was tested using the Jaccard index (presence-absence), excluding those lakes where no organisms were found. Statistical analyses were performed in SigmaPlot 12.0 and PAST (Hammer et al., 2001).
Results
No benthic organisms were found in 5 (27.8%) out of the 18 sampled lakes: Liquidámbar, Chajchaj, San José, Montebello, and Yalalush, while in the remaining 13 lakes (72.2%) there were 49 taxa, belonging to 14 orders and 25 families, of which 40 genera were certainly identified and 9 taxa remain undetermined (Table 3). Except for Montebello, where the deep bottom is hard rock without sediments, we do not know the reason for the absence of benthic organisms in the other 4 lakes, since they include shallow, deep, oligotrophic and eutrophic lakes, and all of them were sampled at least twice.
Table 3 List of benthic invertebrate taxa found in the deep bottom of the Montebello Lakes. (un.: undetermined).
| Order | Family | Genus |
| Dorylaimida | Dorylaimidae | Laimydorus |
| Haplotaxida | Haplotaxidae | Pelodrilus |
| Haplotaxidae genus 1 | ||
| Naididae | Chaetogaster | |
| Homochaeta | ||
| Naididae genus 1 | ||
| Tubificidae | Aulodrilus | |
| Limnodrilus | ||
| Gasteropoda | Ancylidae | Ancylidae genus 1 |
| Podocopida | Candonidae | Cypria |
| Amphipoda | Talitridae | Chelorchestia |
| Amphipoda genus 1 | ||
| Unionicolidae | Koenikea | |
| Entomobryomorpha | Entomobryidae | Americabrya |
| Lepidocyrtus | ||
| Entomobryidae genus 1 | ||
| Isotomidae | Ballistura | |
| Proisotoma | ||
| Paronellidae | Salina | |
| Tomoceridae | Tomocerus | |
| Poduromorpha | Hypogastruridae | Ceratophysella |
| Symphypleona | Sminthurididae | Sminthurides |
| Ephemeroptera | Caenidae | Caenis |
| Odonata | Coenagrionidae | Chromagrion |
| Diptera | Ceratopogonidae | Bezzia |
| Culicoides | ||
| Chaoboridae | Chaoborus | |
| Chironomidae | Cardiocladius | |
| Chironominae genus 1 | ||
| Chironomus | ||
| Cladotanytarsus | ||
| Cryptochironomus | ||
| Dicrotendipes | ||
| Einfeldia | ||
| Harnischia | ||
| Microchironomus | ||
| Micropsectra | ||
| Parachironomus | ||
| Polypedilum | ||
| Procladius | ||
| Rheotanytarsus | ||
| Tanypus | ||
| Tanytarsini genus 1 | ||
| Culicidae | Culex | |
| Syrphidae | Syrphidae genus 1 | |
| Coleoptera | Dytiscidae | Agabus |
| Hydrophilidae | Sphaeridiinae genus 1 | |
| Megaloptera | Sialidae | Ilyobius |
| Trichoptera | Polycentropodidae | Polyplectropus |
Taxonomic richness at each lake varied widely between none and 19 taxa with and average of 5 ± 6 taxa (Fig. 2, Table 4). Chironomidae is the family with the highest taxonomic richness with 17 genera, followed by Entomobryidae and Naididae with 3 taxa each. The highest taxonomic richness was found in Tziscao (19), Ensueño (13), and Patianú (13), all of them deep oligotrophic lakes; although other lakes with similar characteristics had lower taxonomic richness (≤ 10). The taxonomic richness in the eutrophic lakes (2.2 ± 2.1, 0-5) tended to be lower than in the oligotrophic lakes (6.7 ± 6.3, 0-19), although no statistically significant differences were found (T = 44, p = 0.23). Similarly, the taxonomic richness in the shallow lakes (3.8 ± 4.8, 0-10) tended to be lower than in deep lakes (5.6 ± 6.0, 0-19) but once again, no statistically significant differences were found (T = 32, p = 0.59).

Figure 2 Taxonomic richness of benthic invertebrates found in the deep zone of the Montebello Lakes. Lakes are arranged in a NW-SE orientation. Lake abbreviations follow table 1. Black bars indicate eutrophic lakes, white bars indicate oligotrophic lakes, and asterisks indicate shallow lakes.
Table 4 Benthic invertebrates of the Montebello Lakes (X: presence of taxon, S: taxonomic richness, un.: undetermined). Lakes with no organisms were not included. Lake abbreviations follow table 1.
| Taxa / lake | SL | BL | BA | EC | ES | EN | AT | CL | PO | KI | TZ | PA | DL |
| S | 1 | 5 | 3 | 4 | 10 | 13 | 2 | 7 | 3 | 3 | 19 | 13 | 10 |
| Agabus | X | ||||||||||||
| Americabrya | X | ||||||||||||
| Amphipoda un. | X | ||||||||||||
| Ancylidae un. | X | ||||||||||||
| Aulodrilus | X | ||||||||||||
| Ballistura | X | ||||||||||||
| Bezzia | X | ||||||||||||
| Caenis | X | ||||||||||||
| Cardiocladius | X | ||||||||||||
| Ceratophysella | X | ||||||||||||
| Chaetogaster | X | ||||||||||||
| Chaoborus | X | X | X | X | |||||||||
| Chelorchestia | X | ||||||||||||
| Chironominae 1 | X | X | X | X | X | ||||||||
| Chironomus | X | ||||||||||||
| Chromagrion | X | X | |||||||||||
| Cladotanytarsus | X | ||||||||||||
| Cryptochironomus | X | X | X | ||||||||||
| Culex | X | ||||||||||||
| Culicoides | X | ||||||||||||
| Cypria | X | ||||||||||||
| Dicrotendipes | X | X | X | ||||||||||
| Einfeldia | X | ||||||||||||
| Entomobryidae 1 | X | ||||||||||||
| Ephemeroptera 1 | X | ||||||||||||
| Haplotaxida 1 | X | X | X | ||||||||||
| Harnischia | X | X | |||||||||||
| Homochaeta | X | X | X | X | |||||||||
| Ilyobius | X | ||||||||||||
| Koenikea | X | X | X | X | X | ||||||||
| Laimydorus | X | X | X | ||||||||||
| Lepidocyrtus | X | ||||||||||||
| Limnodrilus | X | X | X | ||||||||||
| Microchironomus | X | X | X | ||||||||||
| Micropsectra | X | ||||||||||||
| Naididae 1 | X | X | X | ||||||||||
| Parachironomus | X | ||||||||||||
| Pelodrilus | X | ||||||||||||
| Polycentropodidae 1 | X | X | |||||||||||
| Polypedilum | X | X | X | ||||||||||
| Procladius | X | X | X | ||||||||||
| Proisotoma | X | ||||||||||||
| Rheotanytarsus | X | ||||||||||||
| Salina | X | ||||||||||||
| Sminthurides | X | X | X | X | X | X | |||||||
| Sphaeridiinae 1 | X | ||||||||||||
| Syrphidae 1 | X | ||||||||||||
| Tanypus | X | ||||||||||||
| Tomocerus | X |
In general, the taxonomic richness of shallow eutrophic lakes (2.5 ± 3.5, 0-5) is lower than in shallow, oligotrophic lakes (5.0 ± 7.1, 0-10), and the taxonomic richness of the deep, eutrophic lakes (2.0 ± 1.8, 0-4) is lower than in deep, oligotrophic lakes (7.0 ± 6.5, 0-19), although in both cases the differences were not statistically significant (F = 0.91, p = 0.5).
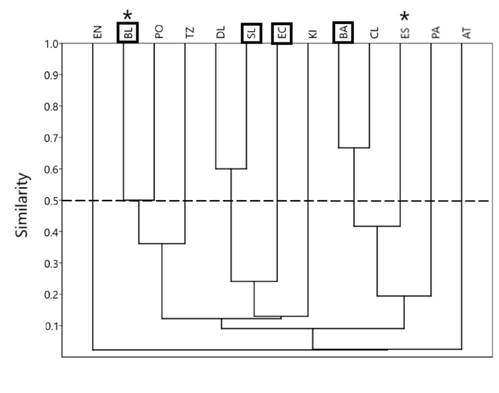
Thirty-two taxa (65.3%) were found in a single lake, 3 taxa (6.1%) in 2 lakes, and 14 taxa (28.6%) in 3 or more lakes. Sminthurides was the most frequent genus, present in 6 lakes (Table 4). To calculate the Jaccard similarity index based on the lakes’ taxonomic composition, those taxa found only in a single lake were not included. The index was low (Fig. 3) indicating little coincidence of taxa among lakes; in other words, the lakes have few common taxa. Only 6 out of the 13 lakes were clustered in 3 groups, 2 lakes each, and all of them with low values of similarity (≥ 0.5): Balantetic and Pojoj (0.5), Dos Lagos and San Lorenzo (0.6), and Bosque Azul and Cinco Lagos (0.65). The other 7 lakes remained ungrouped (Fig. 3). The lakes of the 3 groups are not coincident in size, depth, or trophic state.
Discussion
The regional taxonomic richness is made up by 49 taxa and the average richness per lake is 5 ± 6 (0-19), which is higher or similar to the richness reported from deep benthic zones of other Mexican (Hernández et al., 2014), and tropical lakes around the world (Jiménez & Springer, 1996; Sibaja-Cordero & Umaña-Villalobos, 2008; Tudorancea & Harrison, 1988), where taxonomic richness varied from 2 to 15 taxa.
No relationship or trend between lake morphometry (maximum depth, average depth, length, width, surface area) and taxonomic richness was found. Regardless of being shallow, deep, large or small, lower taxonomic richness in eutrophic compared with oligotrophic lakes was consistently found. Additionally, of the 11 genera found in eutrophic lakes, only 3 (27%) were exclusive to them, while 8 (73%) were also present in oligotrophic lakes (Table 3). In terms of composition, eutrophic lakes make a lower contribution to regional richness than do oligotrophic lakes. These data reveal that eutrophication is diminishing the regional lake biodiversity.
An elevated regional taxonomic richness (49) accompanied by a low average taxonomic richness (5 ± 6) is associated to the fact that very few taxa are found in more than a single lake. Most taxa inhabiting one lake are absent in the others, even though all lakes are in the same lake district and not far from each other. Whittaker (1977) defined β diversity as the magnitude of change in the composition of species along an environmental gradient or between different communities in a landscape. It is a fundamental component of species diversity in a región (Whittaker, 1960). Following this, β diversity of benthic invertebrate communities of the Montebello Lakes is high, since each lake’s taxonomic composition showed a great level of singularity, which points to a large fragility of the ecosystem’s biodiversity. As the lakes become eutrophic, individual and global biodiversity is lost.
Causes of elevated β diversity may be related to 3, not mutually exclusive mechanisms: niche differentiation, spatial configuration, and species dispersal capacity (Soininen et al., 2007). Physical barriers and low dispersal ability from macroinvertebrates may explain the low similarity among lakes. From 14 orders found, 6 have flying adults (Ephemeroptera, Odonata, Diptera, Coleoptera, Megaloptera, and Trichoptera), and the remaining 8 have non-flying aquatic adults, which have lower dispersal capacity.
Two taxonomic groups found in the Montebello Lakes are worth mentioning: the Chironomidae, because of their high taxonomic richness in the lakes with 17 taxa, and the springtails (Collembola), because they are rarely found in the benthos of aquatic ecosystems. Chironomids are recognized by their ability to tolerate low dissolved oxygen concentrations and even anoxia; some members of the family have been used as bioindicator of poor water quality or eutrophy (Merritt et al., 2008). Brodersen et al. (2004) found that warm water assemblages represented by Chironomus, Dicrotendipes, and Procladius, all found in the Montebello Lakes, have a higher oxygen regulatory capacity than the cold water assemblages characterized by Hydrobaenus, Diamesa, Heterotrissocladius, and Micropsectra, from which only Micropsectra was found in Dos Lagos.
Springtails are uncommon in aquatic environments; some of them are neustonic, living in wet caves or the interstices of marine littoral zones (Deharveng et al., 2008). This adaptation may allow them to stay above the water, but prevents them from submerging, which makes it unexpected to find them inhabiting the bottom of the lakes, at great depth, and under anoxic conditions. From all collembolans found, only Sminthurides had been reported from aquatic environments, particularly neustonic (Folsom & Mills, 1938). Palacios-Vargas et al. (2018) provide a more detailed analysis on the presence of collembolans in the Montebello Lakes.
The benthic invertebrate diversity of the deep zone of the Montebello Lakes, with 49 taxa, constitutes a significant contribution to the regional biodiversity of the LMNP and Chiapas. Our findings suggest macroinvertebrate aquatic biodiversity follows the same trend -highest diversity- found for terrestrial arthropods, plants and vertebrates in Mexico (Llorente-Bousquets & Ocegueda, 2008).
It is important to highlight that although the studied water bodies belong to the same lake district, few taxa (34.7%) were found in more than a single lake, which stresses the singularity of the benthic invertebrate fauna from each lake, making evident the fragility of these ecosystems. The disappearance of the fauna from a single lake means a high probability of its disappearance from the entire region. Eutrophic water bodies showed lower taxonomic richness compared to the oligotrophic lakes. This means that if the eutrophication process that has been observed in some of the lakes continues to the rest of the system, important aquatic biodiversity will be lost, so the relevance of their conservation is based on the elevated biodiversity harbored by these lakes.











 nueva página del texto (beta)
nueva página del texto (beta)