Introduction
Duplex stainless steels contain approximately equal amounts of ferrite and austenite in the annealed condition (Arikan and Doruk, 2007), result of the ferrite to austenite transformation after solidification resulting in a balanced microstructure at room temperature. Since they offer high mechanical properties and good corrosion resistance (Gunn, 1997), they are used in many applications where welding is required exposing the material to thermal cycles that affect the stability of ferrite and austenite either in the heat affected zone or the welding metal, specially if a multipass welding is performed. As a consequence, the formation of secondary phases such as sigma phase (σ), alpha prime (α´) and chromium nitrides within the ferrite phase in the temperature range 400ºC-950ºC (Cortie and Jackson, 1997) is possible, diminishing the mechanical properties and corrosion resistance of the material. In order to avoid the formation of undesirable secondary phases in the welding metal and keeping the balanced microstructure, the filler metal is usually alloyed with 2-4% more Ni than in the base material (Muthupandi et al., 2003), promoting the formation of austenite from ferrite during cooling. This is due to the fact that all duplex alloys solidify as ferrite and the austenite transforms during cooling, first nucleating at the grain boundaries of ferrite. Therefore, if low cooling rates during cooling are experienced, the austenite not only will nucleate at the grain boundaries but also inside the ferrite. Since welding involves high cooling rates, it is expected that the welding metal in the root pass will exhibit a high content of ferrite, increasing the susceptibility to form a high content of austenite and secondary phases when exposed to the thermal cycles of a multipass welding. Eventhough the heat affected zone does not reach the melting temperature, it is affected by the thermal cycles and is also expected to have a higher content of ferrite and more over, the presence of secondary phases due to the deposition of subsequent welding beads which reheats the heat affected zone. However, the drastic microstructural change is more expected in the welding metal than in the heat affected zone since the material experiences a melting process and it has more alloy elements involved. Nowadays, most filler metals for duplex alloys are found to have a high content of nitrogen along with the nickel to keep the austenite balance (Muthupandi et al., 2003) and promoting its formation at high temperatures. On the other hand, they have a high content of chromium and molybdenum in order to avoid the loose of those elements during fusion and to assure the corrosion resistance of the welding metal. Despite the presence of nitrogen and nickel with the aid to increase the volume fraction of austenite, the addition of chromium and molybdenum also increased the volume fraction of ferrite. Therefore, the susceptibility of formation of secondary phases is increased if the welding metal is exposed to high temperatures such as in a multipass welding.
Materials and methods
UNS S32750 superduplex stainless steels plate with dimension of 36 cm by 8 cm and 6 mm of thickness was welded using GMAW process and two filler metals ER 2209 (duplex type) and ER 25.10.4L (superduplex type). Chemical composition of the superduplex stainless steel and the filler metal is shown in Table 1 and welding parameters in Table 2. Two weldings were manufactured: W1 (UNS S32750-ER 2209) and W2 (UNS S32750-ER 25.10.4L), requiring the deposition of three passes to fill completely: root pass, filler pass and cover pass.
Table 1 Chemical composition of materials (wt%)
| Material | C | Si | Mn | Ni | Cr | Mo | N |
| UNS S32750 | 0.03 | 0.40 | 0.88 | 5.70 | 23.40 | 3.20 | 0.27 |
| ER 25.10.4L | 0.04 | 0.49 | 0.50 | 7.30 | 21.70 | 3.00 | 0.25 |
| ER 2209 | 0.03 | 0.45 | 1.20 | 7.70 | 19.90 | 3.10 | .15 |
Table 2 Welding parameters for welding samples
| Sample | Protection gas | Current (A) | Voltage (V) | Welding speed (mm/s) |
| W1 | Ar | 300 | 30 | 3 |
| W2 | Ar | 300 | 30 | 3 |
The microstructural characterization was carried out by standard techniques including grinding with SiC paper and polishing with 1, 3 and 9 microns diamond paste. To reveal the microstructure, the samples were etched with NaOH which attacks ferrite, austenite and sigma phase. In order to analyze the effect of the thermal cycles on the base material, the heat affected zone and the welding metal corresponding to the root pass were analyzed through optical microscopy. The percentage of phases was calculated using an image analyzer. The microanalysis was performed by EDS. Thermal profile for specific points in the heat affected zone corresponding to the three welding passes was calculated according to the heat-flow equation for a thin plate model (Easterling 1992) and the heat input corresponding to the GMAW process. The microhardness test was performed on the cross-section of the specimens with a 150 g indentation load.
Results and discussion
The typical microstructure of the superduplex stainless steel in Figure 1 consists in a ferrite matrix with elongated islands of austenite free of secondary phases. The ferrite content of the base material is ≈54% and ≈46% austenite. The EDX microanalysis of ferrite and austenite in Table 3 shows the composition of both phases in the as-received material, where the ferrite has more chromium and molybdenum compared to the austenite as expected. The hardness of the material in the as-received condition is ≈245 HV.

Figure 1 Microstructure in the as-received condition, depicting the ferrite matrix with islands of austenite
Table 3 Microanalysis of ferrite and austenite in the as-received condition
| Element | (wt%) | |||||
| Phase | Cr | Mo | Mn | Si | Ni | Fe |
| γ | 22.3 | 3.4 | 1.4 | 0.2 | 8.2 | 63 |
| α | 23 | 7.4 | 1.1 | 0.3 | 7.5 | 50 |
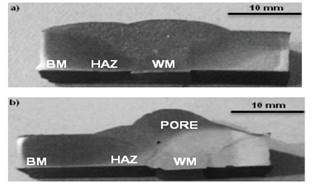
Figure 2 shows the transverse section of both weldings performed free of cracks. However, specimen W2 presents a significant pore probably due to gas bubbles or a dirty weld surface. The welding metal and heat affected zone from W2 showed the biggest hardness with ≈186 HV and ≈163 HV respectively, compared to W1 with ≈128 HV in the welding metal and ≈151 HV in the heat affected zone. It is evident that the welding metal corresponding to W2 presents a drastic variation in the hardness, which can be attributed to the formation of secondary phases such as sigma phase as a result of the deposition of subsequent welding passes. It is well known that sigma phase has a direct implication in the increment of hardness (Filho et al., 2014).
Figure 3 presents the caculated thermal profiles of the heat affected zone for both experimental weldings, with different distances from the welding centerline taking into account the three welding passes. The heat affected zone near the fusion line in point A (root pass) experiences very high temperatures resulting in a drastic microstructural change due to the temperature exposure caused by the deposition of the filler pass and cover pass. The peak temperature in Point A at 8 mm from the welding centerline is ≈1232ºC for the root pass. Then, after the filler pass, the peak temperature in the same point is ≈1160ºC. Finally, the peak temperature after the deposition of the cover pass is ≈1051ºC. On the other hand, the heat affected zone in points B, C and D are exposed to high temperatures that will promote a significant microstructrual change despite they are relatively away from the weld centerline, which is attributed to the high heat input of the GMAW process.
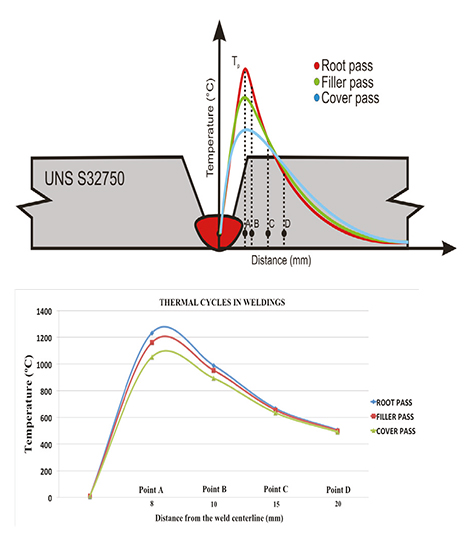
Figure 3 Calculated thermal profiles corresponding to both experimental welds, showing the peak temperatures in specific points in the heat affected zone
Eventhough the exposure to high temperatures, the microstructural evidence in Figure 4 shows that the heat-affected zone near the fusion line in W1 and W2 consists of ferrite, austenite and secondary austenite.
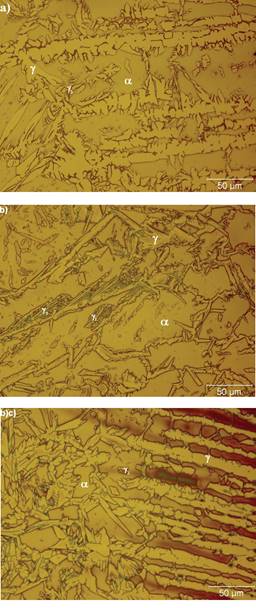
Figure 4 Heat affected zones near the fusion line showing the presence of austenite, ferrite and secondary austenite in a) W1 and b) W2. Sensitization of ferrite is evident in c) W2 along with the ferrite and austenite
However, the sensitization of ferrite is evident in W2 probably due to the presence of chromium nitrides within the ferrite grains (Figure 5a) and in the ferrite-austenite boundaries (Figure 5b) since the filler metal has a high content of nitrogen and chromium compared to the base metal. On the other hand, the partition of ferrite in Figure 5b is visible due to the transformation of ferrite to secondary austenite. This austenite nucleates preferentially at prior grain boundaries of the ferrite matrix or at interdendritic locations within the ferrite by a diffusion controlled process (Leone and Kerr, 1982). It can be assumed that the exposure at high temperatures in the heat affected zone, promoted the diffusion of nitrogen and nickel into the ferrite resulting in the ferrite to austenite transformation. This suggests that the chromium is rejected by the newly formed austenite and goes directly to the nitrogen, resulting in a microstructure where the chromium nitrides and secondary austenite are predominantly.
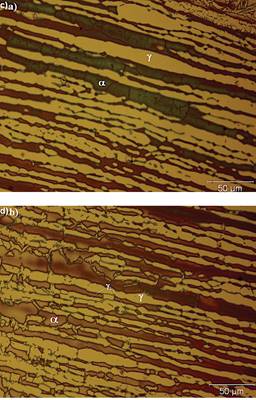
Figure 5 Heat affected zone of W2, showing the partition of ferrite and the sensitization of ferrite probably due to the presence of chromium nitrides and secondary austenite
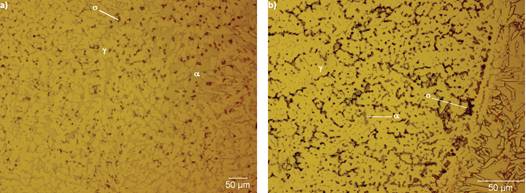
Figure 6 shows the root pass of the experimental welds with a high content of sigma phase (≈33% for W1 and ≈43% for W2) in the ferrite-austenite interface and the ferrite-ferrite interface in a predominatly austenite matrix. It is assumed that during the deposition of the root pass, the welding metal is rapidly cooled from temperatures near the ferrite solvus, resulting in a high content of ferrite at the end of solidifcation (Lippold and Kotecki, 2005). Therefore, the exposure to high temperatures due to the deposition of the subsequent welding passes caused the ferrite to sigma phase transformation, precipitating in the high chromium-concentrated region in ferrite (Hsieh and Wu, 2012), beginning the formation of particles of sigma phase with rounded morphology, nucleating and growing inside the ferrite phase. It can be observed in Figure 6a (W1) that almost all the ferrite was consumed by the formation of austenite and sigma phase through the eutectoid reaction ferrite→sigma+austenite (Nilsson 1992), meaning that the ferrite transforms into sigma phase and secondary austenite. On the other hand, Figure 6b (W2) shows the biggest content of sigma phase, which consumed basically all the ferrite leaving an austenite matrix. The thermal cycles due to the deposition of the filler pass and cover pass, promoted a relatively low cooling rate resulting in the formation of sigma phase and austenite phase in a cooperative way. The presence of sigma phase in the welding metal of the root pass consumes chromium and molybdenum from the ferrite and austenite (Waanders et al., 1999) meaning that the corrosion resistance may be diminished.
Conclusions
Sigma phase nucleates at the ferrite/austenite interface and grows into the ferrite, consuming all the ferrite through the eutectoid reaction of ferrite to sigma phase + austenite.
Thermal cycles caused by the deposition of the filler pass and cover pass led to the formation of sigma phase and secondary austenite in the welding metal.
The presence of nickel and nitrogen in the filler metal promoted the occurrence of an austenite matrix in the welding metal.











 nova página do texto(beta)
nova página do texto(beta)