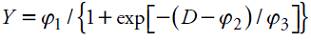Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.50 no.5 Texcoco Jul./Ago. 2016
Crop Science
Sink-source relation on abortion of reproductive structures, photosynthetic rate and yield in Capsicum annuum
1Departamento de Biología, Facultad de Ciencias, Universidad de Los Andes. La Hechicera, Núcleo Universitario Pedro Rincón Gutiérrez, Edificio A, Avenida Alberto Carnevalli, Mérida 5101, Venezuela. (albaniajose@gmail.com).
2Laboratorio de Ecofisiología de Cultivos. Facultad de Ciencias Forestales y Ambientales. Instituto de Investigaciones Agropecuarias. Universidad de Los Andes. Santa Rosa 77, Mérida 5101-A, Venezuela.
There is evidence of a relationship between the development of the initial fruits and the abortion of reproductive structures in upper nodes of Capsicum annuum (paprika, pepper), which makes its harvest difficult in continuous production systems. In this study, the effect of the sink size during fruiting, growth rate of fruits and rate of CO2 assimilation (A) on three flower removal intensities treatments was evaluated. At the same time, photosystem II (PSII) photochemical activity and fruit growth (size and biomass) were evaluated. The experimental design was completely randomized, with three replications and 16 plants per experimental unit. The maximum flower removal (up to the third flower node) increased fruit diameter, length, fresh weight and dry weight (p≤0.05) compared to the other two treatments (flowers removed at the first or second node). A minimum percentage of fruit with less than 100 g weight was obtained under the same condition. According to A and the photochemical activity, leaf age determines photosynthetic efficiency. The relationship of the latter with the development state of the fruit is not straightforward.
Key words: Floral abortion; photochemical activity; Capsicum annuum; photosynthesis; sink-source strength; floral pruning
En Capsicum annuum (pimentón, pimiento) existen evidencias de una relación entre el desarrollo de los frutos iniciales y el aborto de estructuras reproductivas en nudos superiores, lo cual dificulta la cosecha continua en sistemas de producción. En este estudio se evaluó el efecto de la magnitud de la fuerza de la demanda de asimilados en la fructificación, tasa de crecimiento del fruto y tasa de asimilación de CO2 (A) con tres intensidades de eliminación de flores. Al mismo tiempo, se evaluó la actividad fotoquímica del fotosistema II (PSII) y crecimiento de los frutos (dimensiones y biomasa). El diseño experimental fue completamente aleatorizado, con tres repeticiones y unidades experimentales de 16 plantas. La eliminación máxima de flores (hasta la tercera flor) produjo frutos con diámetro, longitud, peso fresco y peso seco mayor (p≤0.05) que los otros dos tratamientos (eliminación de flores en el primer nudo o hasta el segundo nudo). En esa misma condición se obtuvo el porcentaje mínimo de frutos con peso menor a 100 g. Según la A y la actividad fotoquímica, la edad de la hoja determina la eficiencia fotosintética. La relación de esto último con el estado de desarrollo de los frutos no es directa.
Palabras claves: Aborto floral; actividad fotoquímica; Capsicum annuum; fotosíntesis; fuente-fuerza de la demanda; poda floral
Introduction
The falling or abortion of flowers and immature fruits are a major production constraint for Capsicum annuum (Minges and Warholic, 1973; Greenleaf et al., 1978; McGraw and Greig, 1986; Wien et al., 1989). This creates wide fluctuations when weekly harvested, with maximum yields ranging from 5 to 10 fruits per m2 and intermediate periods of less than 2 per m2 (Buwalda et al., 2006). This cyclical growth pattern has periods of slow growth and large fruit load alternating with periods of rapid growth and low load (Kato and Tanaka, 1971; Hall, 1977; Marcelis, 1992; Marcelis and BaanHofman-Eijer, 1995; Heuve-link et al., 2004).
Different abiotic factors promote fruit abscission (Figure 1). The decrease in light intensity affects flower production (Kato and Tanaka, 1971; Quagliotti et al., 1974; Park and Jeong, 1976; Sawahata et al., 1980) and produces the drop of up to 60 % of reproductive structures that fall from the first four nodes (Wien, 1990). Also, increasing the plant density decreases light availability and promotes greater flower abortion (Marcelis et al., 2004). Besides, at high ambient temperatures, increased ethylene production promotes flowers abscission, which intensifies during periods with high night temperatures (Aloni et al., 1991). Additionally, flower abortion increases with water deficit (Fernández et al., 2005).
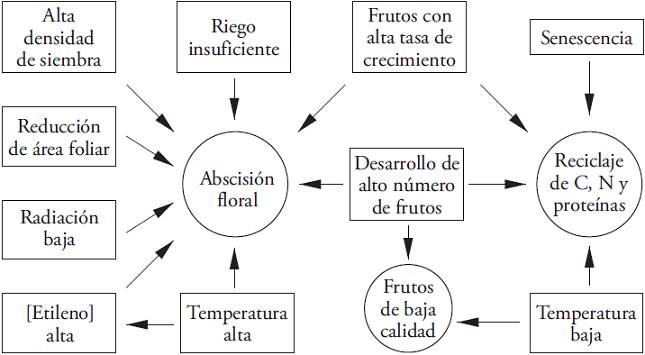
Figure 1: Interactions between biotic and abiotic factors involved in the floral abscission of Capsicum annuum.
The fruits development also increases floral abscission dramatically (Bakker, 1989; Wien et al., 1989; Marcelis and BaanHofman-Eijer, 1997; Heuvelink et al., 2004; Marcelis et al., 2004). The cyclical fluctuations are related to the high sink strength of carbohydrates that each developing fruit represent (Hall, 1977; Bakker, 1989; Marcelis et al., 2004; González-Real et al., 2008) and the competition to assimilate the latter, pollinated flowers and the first fruits (Aloni et al., 1996; Marcelis et al., 2004; Jaimez et al., 2010). The increase of sink strength during the highest growth phase of the fruits coincides with high CO2 assimilation rate (A) and high concentration of N in leaves close to these fruits (González-Real et al., 2009). Besides, A is lower in leaves neighboring ripe fruits or those located in already harvested nodes (Hall, 1977; Gucci and Flore, 1989; González-Real et al., 2009). This allows inferring that increasing carbohydrates available for fruit growth decrease the abortion rate.
For C. annuum there is no available information that links the fruit growth and the photosynthetic efficiency at different heights of the plant. It has not been established whether the handling of the initial number of flowers influences the biomass accumulation in fruits and their competition for photoassimilates. Although there is evidence that the presence of the first fruits influence a decrease of the total yield of the plant (Jaimez et al., 2010). In that study the relation with fruit abortion rate was not evaluated. Up to now, the effect of fruit harvesting on the growth rate of the rest of the fruits is unknown. It is to be expected that the reduction of the sink strength by flowers removal, would increase fruit growth rate. The source-sink relationship in C. annuum must be understood in greater detail before proposing a management system that raises the quality of the fruit and reduce the production fluctuation.
In this study we considered that the source of assimilates available before the reproductive stage gradually increases with the intensity in which flowers are removed (Jaimez et al., 2010); and that the sink strength is reflected in the diameter of the fruits, which is closely related to the growth rate (Bozokalfa and Kilic, 2010). The objectives of the present study were to evaluate how the initial variation of the sink strength affects the growth rate of fruits, fruiting dynamics and abortion; and relate the sink strength at different heights with A and the photochemical activity.
Materials and Methods
This research was conducted in greenhouses at the Santa Rosa experimental station of the Instituto de Investigaciones Agropecuarias of the University of Los Andes in Merida, Venezuela (8° 37' 38.7" N, and 71° 9' 23.6" W) at 1936 mas1. The greenhouses characteristics were those reported by Jaimez et al. (2013). The maximum and minimum average temperatures during the study were of 26.5 °C and 13.2 °C.
Seedlings were obtained from seeds of the P1216 hybrid (Syngenta®). These were sown in trays with a commercial substrate. Then, at 45 d they were transplanted to polyethylene bags (12 kg) containing substrate prepared with sifted sand mixed with rice husks-horse manure (1:1). Each plant received 450 mL of water daily. Plants were watered at 09:00, 12:00 and 15:00 h. The daily fertilization was of 1 g L‒1, with commercial full fertilizers with a NPK 18-18-18 ratio, and then changed to 5-30-15 during the flowering onset. Calcium was added (1 g L‒1) 150 d after transplanting (ddt). All plants were two stems tutored.
Experiment 1: Effect of the flower eliminating intensity upon the growth rate, abortion rate, and size and fruits number
Plants were established at a density of six plants per m2, the experimental design was completely randomized design with three replications and experimental units of 16 plants. The treatments were: 1) flowers removal at first node or central fork of the plant (27 ddt), 2) flowers removal at the first two nodes (46 ddt) and, 3) flower removal of from the first three nodes (53 ddt).
In the three central plants of each plot the length and diameter of the fruits were measured weekly, starting once 15 mm were reached. Harvested fruits were weighed and length and diameter were measured. Weekly harvests started at 119 ddt and ended at 319 ddt.
Additional plants were used to remove fruit during the growth, covering all stages up to maturity. They were measured (diameter and length), weighed, oven dried and weighed again. Two regression curves were developed for fresh weight and biomass, according to the product of the fruits dimensions (diameter×length).
Using the measures of the sampled fruits, regression equations of fresh weight and weekly accumulated biomass during growth were estimated. Data in diameter, length, fresh weight and biomass were treated in the same manner and separately, setting the next (logistics) nonlinear model:
where Y: response variable; D: days of observation; φ 1: asymptotic value or maximum value of Y; φ 2: time in which half the value of the asymptote is reached; φ 3: elapsed time between the time half was reached and 3⁄4 of the asymptote.
As a result of this model, the instantaneous growth rates were estimated using the first derivative based on the daily observation. The aborted and developed reproductive structures up to 15th node were also counted.
Experiment 2: Influence of the sink strength on the CO 2 assimilation rate at different plant nodes
The plants were distributed in nine rows inside a greenhouse. Five plants randomly distributed in the central row were weekly used to measure the diameter of the first five fruits: one from the first node (central), two from the second and two from the third node (without floral pruning).
In the same plants gas exchange parameters (CO2 assimilation (A), stomatal conductance (gs), transpiration (E), radiation (PAR), ambient CO2 concentration (Ca) and intercellular CO2 concentration (Ci) were measured with an open type IRGA gas analyzer (Infra-Red gas Analyser ADC, model LCA4). Those leaves that accompanied the first five fruits and an extended apical leave were taken into account. The gas exchange measurements were performed at 109, 126 and 150 ddt.
Chlorophyll a fluorescence was measured with a portable fluorimeter (Model PAM-2100, Heinz Walz GmbH, Germany) in the same leaves used to measure gas exchange. The fluorescence variable evaluated were: quantum yield of photosystem II
The chlorophyll a fluorescence was measured three times during the crop cycle (119, 167 and 185 ddt). That is to say, at full fruit production, post-harvest and the end phase of the leaves; these measurements were done at 28 °C temperature and 1000 𝛍mol m‒2 s of photon flux density (DFFF).
In all cases, Kruskal Wallis ranking test method was applied with p≤ 0.05.
Results and Discussion
Effect of the intensity of flowers removal on the growth rate, abortion rate, size and fruit number
The yield showed fluctuations in all three treatments. In all cases two periods of high yield (p≤0.05) were recorded. The first of these occurred between 119 and 167 ddt and the second between 230 and 245 ddt (Figure 2). The offset of the start of production between treatments is related to the flower removal intensity. This procedure delayed fruiting. The mismatch disappeared and all plants coincided the following harvest maximum (217 ddt). During periods of high harvest, yield was similar for all treatments (p>0.05).
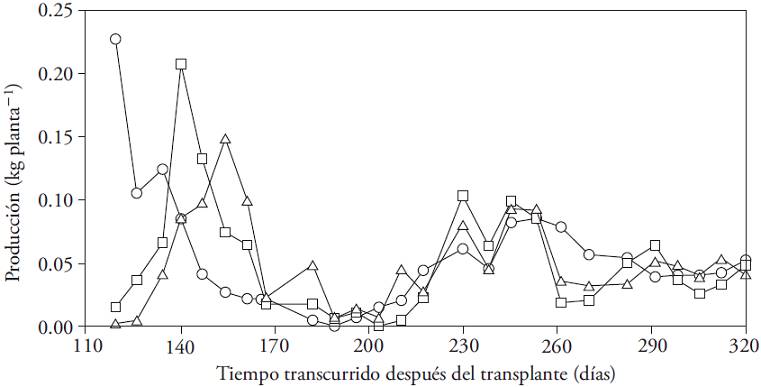
Figure 2: Dynamics of production (kg per plant) of Capsicum annuum (cv. P1216) cultivated in three conditions of flowers elimination: first node (○), up to second node (□), up to the third node (∆).
This cyclic behavior seems characteristic of the Capsicum genus, given what is reported for C. annuum cultivars with different fruits sizes (Wubs et al., 2009a) and C. chinense (Jaimez et al., 2000; Jaimez and Rada, 2006).
In C. annuum cultivation the flowering rate decreases during the fruit development and vegetative growth alternates with the reproductive growth (Fontes et al., 2005). When fruiting rate approaches zero, the growth rate of stem, root and flower buds rapidly increases (Clapham and Marsh, 1987), resulting in different fruiting intensities during the crop cycle. The time between the harvesting maximum varies among cultivars (Wubs et al., 2009a).
In all treatments flowers of the first node remained on the plant and developed mature fruits. In the second node reached 100 % of harvest with the lower flower removal and 99 % was reached in the other two treatments. No other node reached this percentage of high fruiting, regardless of how much the initial sink strength was reduced. Except for the first two nodes, the abortion of reproductive structures was always greater than 40 % in the plants of the two treatments with the lowest flower removal, and greater than 60 % in those with the most intense flower removal (Figure 3). Similar abortion-fruiting patterns were observed in the treatments with flower removal up to the first and second node. After the maximum fruit production in the first two nodes, a total fruiting decrease was observed. This happened in the fifth and sixth node on plants of the first treatment (without flowers on the first node) and the seventh node in the plants of the second treatment (without flowers to the second node). Fruiting percentage increased up to 60 % from the seventh and eighth nodes in the first and second treatment. The highest fruit development was at the 12° and 13° nodes with the removal of flowers on the first node and in the 9° and 10° nodes with the removal of flowers up to the 2nd node.
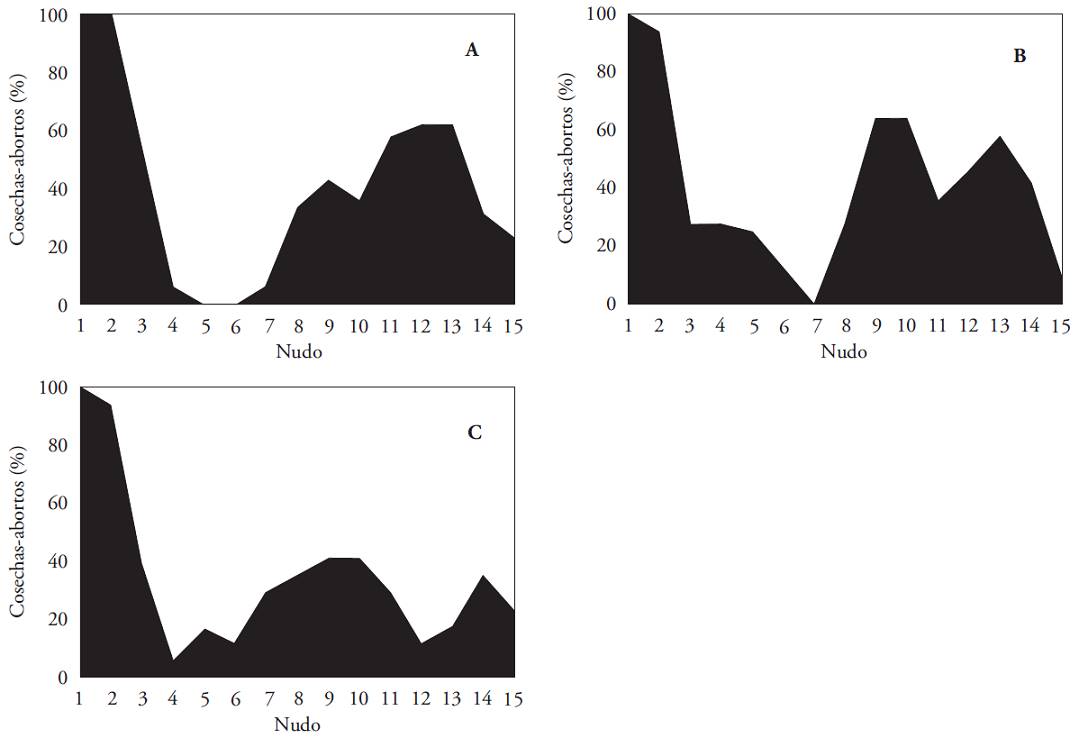
Figure 3: Percentage ratio between fruits set and abortions in the first fifteen nodes of Capsicum annuum plants grown in three conditions of flower removal: A) on the first node, B) up to the second node, C) up to third node.
The most important difference in the behavior of plants with the highest flower removal was the absence of nodes with a 100 % aborted flowers; although a similar pattern was observed in treatments one and two. An area with preponderant flower loss was clearly delimited between the fourth and sixth node along with increased fruit production between the seventh and 11° node; maximum fruiting (40 %) occurred at the 9° and 10° nodes.
Fruit development in lower nodes is related to competition between reproductive structures of the upper nodes and with increased flower abortion. Other studies have shown that with increasing sink strength the source/sink relationship decreases and the overall rate of abortion linearly increases (Hall, 1977; Clapham and Marsh, 1987). All together, the increase in the overall abortion rate of the plant is related to the high growth rates of the fruits (Marcelis et al., 2004).
The three flower removal treatments did not cause a significantly different effect on yield (p>0.05) (Table 1). This result does not match with the paradigm established the beginning of the studies about since this phenomenon, which states that dry matter accumulation in the root and fruiting efficiency increase with the removal of flowers (Hall, 1977; Clapham and Marsh, 1987). In other study higher yield was obtained by removing the flowers on the second node (Jaimez et al., 2005).
Table 1: Average production (kg per plant, kg m‒2 and fruits per plant) of fruits of Capsicum annuum in three treatments of flower removal intensity, cultivated for 320 d in greenhouse.
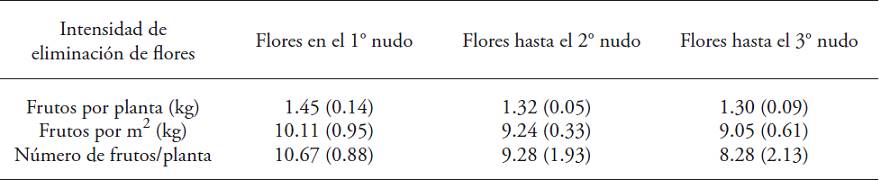
♦ Between parenthesis, the standard error. There were no statistical differences between treatments (Kruskal-Wallis; p>0.05).
The fruits of treatments with maximum flower removal reached the highest average weight (158 g±53); in treatments one and two, average fruit weight was lower (140 g±67 and 136 g±62), respectively (p≤0.05). There was no significant difference in the yield of plants with different flower removal treatments (p>0.05).
The quality of the fruits varies in relation to the removal of flowers (p≤0.05) (Table 2). With less flower removal, the fruits distribution between weight classes was more homogeneous. In all treatments fruit production with weights greater than 300 g was low. The difference in the amount of harvested fruits with less than 100 g weight was evident among treatments, and they were the lowest quality. The plants of both treatments with less flower removal had a higher percentage of fruit with these characteristics (30 % and 28 %). In plants with higher flower elimination, fruits of that class accounted for only 11 % of total production.
Table 2: Percentage distribution by classes regards fresh weight (g) of Capsicum annuum fruits. Plants subjected to three flower removal intensities and cultured for 320 days in a greenhouse.
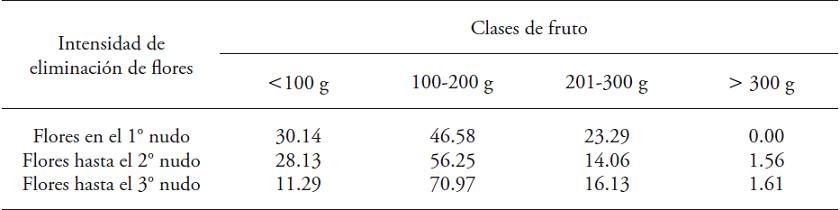
♦ Chi-square test:
The fruits of all treatments showed the same growth rate in the previous lapse to the maximum instantaneous fresh weight accumulation rate period. Afterwards, (about 20 d), the growth rate was higher, whereas flower removal increased (Figure 4).
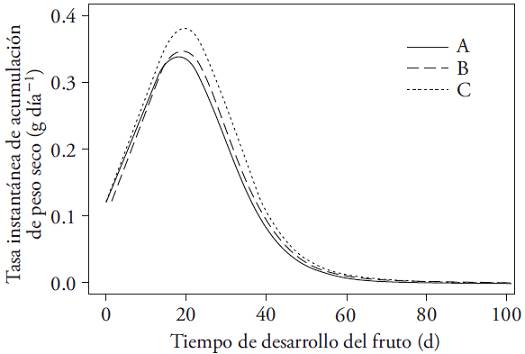
Figure 4: Instantaneous growth rate (g d‒1) in dry weight of Capsicum annuum fruits until harvest. Plants subjected to three flowers removal intensities (A) on the first node, (B) up to the second node, (C) up to the third node.
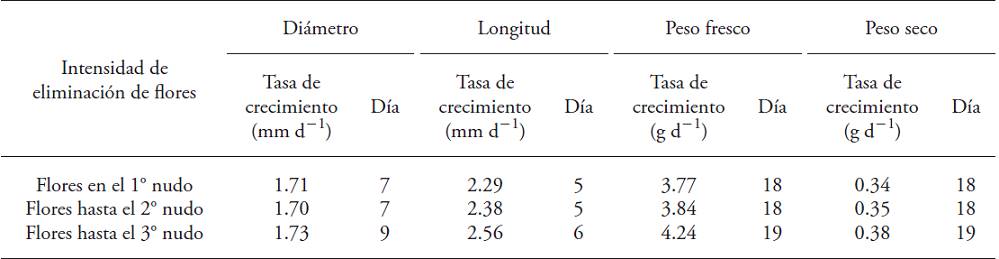
The maximum growth rate of the fruit plants with larger flowers removal took place one or two days after the fruits of the other two treatments (Table 3). This behavior disappears after day 60, when the fruit growth rate is minimal and unchanged in all treatments. This allows linking the fruit high growth rate from the highest flower removal treatments with the overall floral abortion rate in those plants. Wubs et al. (2009b) reported that cultivars with fruit average greater than 140 g reach the highest growth rates by the twentieth day. However, in the literature reviewed there were no researches linking flowers elimination intensity with fruits classification by weight or with the instantaneous growth rate.
Table 3: Maximum instantaneous growth rates (mm d‒1 or g d‒1) and time to reach (d) developing fruits of Capsicum annuum plants submitted to three flower removal intensities.
We showed that by using the fruits classification according to its fresh weight, after 260 d of cultivation, the fruits quality significantly decreased, regardless of the flower removal treatment applied. This decrease in fruits quality is consistent with results reported by Khah and Passam (1992), who found that during the cultivation of C. annuum in greenhouse conditions, the fruit size and number of seeds are gradually reduced. Thus, we propose that in tropical mountain conditions a cultivation period of 260 d in order to optimize the harvests quality and take advantage of the remaining time for new planting. Then, within a year the advantage of a greenhouse structure could be used to develop a full crop and a third from another.
Fruits rapidly increased their diameter and longitude during the first developmental phase After fruits reached 15 mm in diameter, it took 7 to 9 d to increasing greater diameter and 5 to 6 d for the length; whereas the maximum gain in weight occurred between days 18 and 19 (Table 3). The backwardness of the mass gain with respect to the dimensional growth was not associated with treatments of removal flowers.
The initial reduction of the sink strength (by removing flowers) and the concomitant increase in vegetative growth prior to fruiting seems to increase the rate of biomass accumulation during fruit development. However, there is no evidence that this effect is maintained in the plant for indefinite time, because the observations were made up to the 15° node.
Relationship between dimensions and weight of the fruit during its development
The relationship between the fresh fruit weight and the product of the diameter × length is described by the equation: Y=6.473e‒7X2+0.016X‒4.426 (R2=0.95). The relationship between the dry weight of the fruit (biomass) and the product of the diameter × the length is described by the equation: Y=2.112e‒8X2+1.673e‒3X‒1.140 (R2=0.91). Through these equations it was possible to estimate the fresh and dry weight of the fruits still attached to plants. This result concur with those of Bozokalfa and Kilic (2010), who showed that the volume of the fruits of C. annuum depends on 95 % in the relationship between diameter, length and fresh weight. The main difference between their results and the ones here presented is that they used the volumetric estimation of the fruit, which is used in the calculation of yield (commercial); and in the present research we used the fresh and dry weight estimation in order to apply a growth model and calculate the daily rate of weight gain.
Influence of the sink strength on photosynthetic traits of leaf at different heights of the plant
The fruit diameter when harvested was almost invariable in all fruit, regardless of the node where it was formed (Figure 5); but the average fresh weight at harvest did present wide variations. There was a tendency to decrease the weight of the fruit with rise of its position along the plant. The fruits developed on the first node were significantly heavier than those from the second and third (p≤0.05).
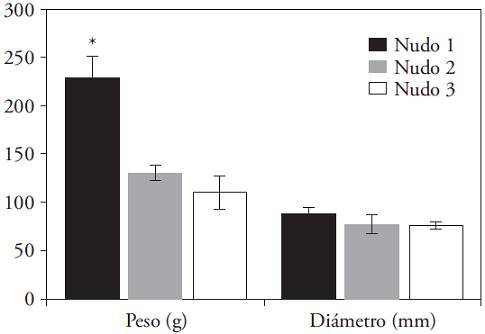
Figure 5: Final average weight (g) and final average diameter (mm) of fruits of C. annuum in the first three nodes. (*) Indicates significant difference between nodes (Kruskal-Wallis; p≤0.05). Bars indicate standard error.
With the increase of the fruit diameter the gs of the accompanying leave decreased and A showed a similar behavior (Figure 6) and three sets of leaves, with significant differences defined. The apical leaves, with larger A (14.55 𝛍mol m‒2 s‒1); leaves on two and three nodes, with intermediate rates (9.81-10.82 𝛍mol m‒2 s‒1) and leaves the first node with A 5.93 𝛍mol m‒2 s‒1.
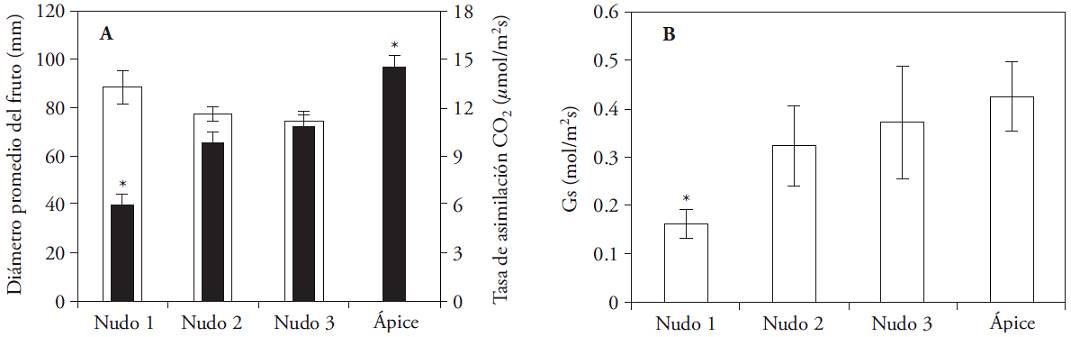
Figure 6: (A) Mena fruit diameter (mm) on C. annum on the first three nodes (white columns) and rate of CO2 assimilation (𝛍mol m‒2 s‒1) (± standard error). (B) Mean stomatal conductance (mol m‒2 s‒1) on accompanying and apical leaves (± standard error). Mean irradiance 900 𝛍mol m‒2 s‒1 and mean temperature 30 °C. (*) Indicates significant differences among nodes (Kruskal-Wallis; p≤0.05).
Leaves on nodes with fruits which already was mature or harvest fruits have low A due to decreased organic foliar N and low demand of the fruit; whereas leaves that are near of developing fruits have the highest A (González-Real et al., 2009). Our results agree with this approach, because A decreased with increasing fruit diameter adjacent, in their last phase of development. However, the A in the apical leaves was higher than in leaves that accompanied the developing fruits; which contradicts previous reports (González-Real et al., 2009). The latter was attributed to discrepancies in the characteristics of the apical leaf used in both investigations.
The simultaneous A measurement and fruit growth showed that the assimilation capacity of the leaves declined as the diameter increased (Figure 7). This shows that due to the demand from developing fruits, mobilization of nitrogen compounds take place from the surrounding leaves, which reduces photosynthesis (González-Real et al., 2009). In this regard, the management of turnover and the number of leaves per node can positively impact the sink/source ratio of carbohydrates, and thereby productivity.
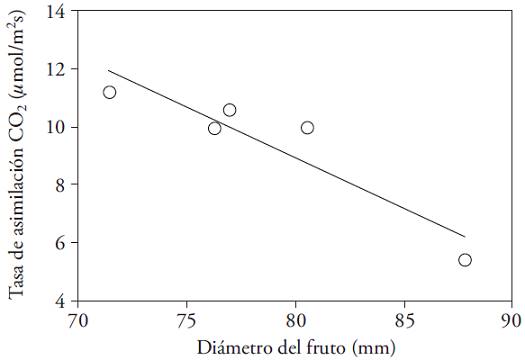
Figure 7: Rate of CO2 assimilation (𝛍mol m‒2 s‒1) as a function of fruit diameter (mm) nearest to leave, for two cultivars of Capsicum annuum. Y=‒0.35X+37.00 (R2=0.86).
The photochemical apparatus of photosystem II efficiency decreases with the aging of the leaf. That is, as leaves became senescent the photochemical yield and photochemical extinction coefficient (q p ) decreased and the no-phootochemical extinction coefficient (q N ) increased (Figure 8). This means that with time, the photoprotection mechanisms increase as the proportion of photon directed to CO2 fixation is reduced. This trend towards decreased photochemical yield (Φ PSII ) of the plants was very markedly shown on the lower leaves, which accompanied the harvested fruit. Besides, in the first experiment no relationship was found between the progressive decrease in the weight of the fruit and the flowers removal treatments applied. This suggests that the decrease is due to the aging of the plants and the concomitant reduction in photosynthetic capacity.
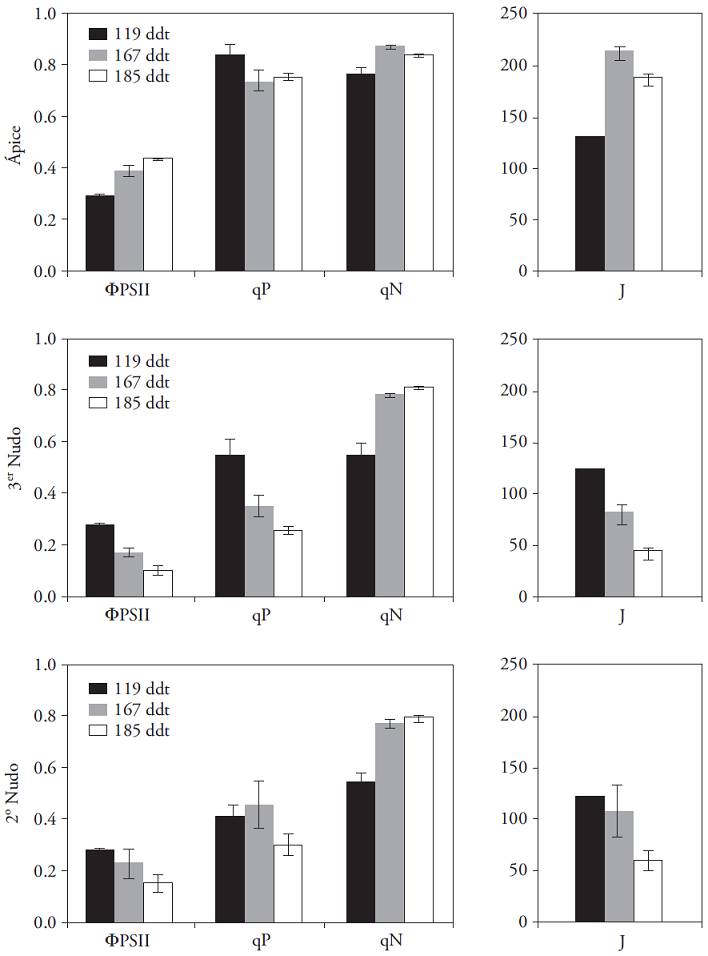
Figura 8: Fluorescence parameters of chlorophylla in apical leaves of the third and second node of Capsicum annuum. Values obtained in three stages of cultivation (± standard error). Average radiation 1000 𝛍mol m‒2 s‒1 and constant temperature (28 °C).
This decrease in the photosynthetic capacity during aging is correlated with a decrease in chlorophyll content, leaf nitrogen, leaf proteins and Rubisco enzyme activity (Field, 1987). Under this proposal, Imai et al. (2008) showed that the Rubisco synthesis with leaf senescence. Olesinski et al. (1989) stated that the decrease in photosynthetic rates decrease, as leaves get older, responds to the redistribution of resources during which it favors the youngest leaves. This would make senescent leaves act as resources for the other that eventually accompany developing fruits demanding carbohydrates.
The turnover of the leaves on the stem depends on the availability of N in the soil and in the plant and the allocation-distribution carbon rate in the plant. This process of foliar turnover maximizes the efficient use of resources and can determine the success and dominance in the competition and demand between neighboring plant organs (Kouki, 2005). In our study, N supply was continuous during the growing season, so, this would not be a limiting factor; however, González-Real et al. (2009) showed that even with continuous fertilization, organic foliar N content decrease depending on the leaf senescence.
Consequently, recycling of resources from basal leaves (senescent), distribution of N or change in the rate of synthesis of enzymes responsible for CO2 fixation might be some of the factors determining the decrease of the photosynthetic capacity of leaves over time.
Conclusions
Crop management of C. annuum should encourage leaves renewal in order to maintain the photosynthetic efficiency and flower removal up to the third node, so that a greater number of leaves transcends from sink organ to source organ before fruiting. With this, availability of photoassimilates increases, competition between organs decreases, floral abortion is reduced and fruit quality is improved.
Literatura Citada
Aloni, B., L. Karni, Z. Zaidman, Y. Riov, and A. Schaffer. 1996. Changes of carbohydrates in pepper (Capsicum annuum L.) flowers in relation to their abscission under different shading regimes. Ann. Bot. 78: 163-168. [ Links ]
Aloni, B., T. Pashkar, and L. Karni. 1991. Partitioning of [14]-C sucrose and acid invertase activity in reproductive organs of pepper plants in relation to their abscission under heat stress. Ann. Bot. 67: 371-377. [ Links ]
Bakker, J. 1989. The effects of temperature on flowering, fruit set and fruit development of glasshouse sweet pepper (Capsicum annuum L). J. Hort. Sci. 64: 313-320. [ Links ]
Bozokalfa, M., and M. Kilic. 2010. Mathematical modeling in the estimation of pepper (Capsicum annuum L.) fruit volume. Chilean J. Agric. Res. 70: 626-632. [ Links ]
Buwalda, F., E. Henten, A. Gelder, J. Bontsema, and J. Hemming. 2006. Toward an optimal control strategy for sweet pepper cultivation: a dynamic crop model. ActaHorticulturae 718: 367-374. [ Links ]
Clapham, W., and H. Marsh. 1987. Relationships of vegetative growth and pepper yield. Can. J. Plant Sci. 67: 521-530. [ Links ]
Fernández, M., M. Gallardo, S. Bonachela, F. Orgaz, R. Thompson, and E. Fereres. 2005. Water use and production of a greenhouse pepper crop under optimum and limited water supply. J. Hort. Sci. Biotechnol. 80: 87-96. [ Links ]
Field, C. B. 1987. Leaf-age effects on stomatal conductance. In: Zeiger E., G. Farquhar, and I. Cowan (eds). Stomatal Function. Stanford University Press. Stanford. pp: 367-384. [ Links ]
Fontes, P. C. R., E. N. Dias, e D. J. Henriques. 2005. Dinâmica do crescimento, distribuição de matéria seca e produção de pimentão em ambiente protegido. Horticulta Bras. 23: 94-99. [ Links ]
Genty, B., J. M. Briantais, and N. R. Baker. 1989. The relationships between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fuorescence. Biochim. Biophys Acta 990: 87-90. [ Links ]
González-Real, M. M., A. Baille, and H. Q. Liub. 2008. Influence of fruit load on dry matter and N-distribution in sweet pepper plants. ScientiaHorticulturae 117: 307-315. [ Links ]
González-Real, M. M., H. Q. Liub, and A. Baille. (2009). Influence of fruit sink strength on the distribution of leaf photosynthetic traits in fruit-bearing shoots of pepper plants (Capsicum annuum L.). Environ. Experimen. Bot. 66: 195-202. [ Links ]
Greenleaf, W., M. Hollingsworth, J. Turner, and W. Hearin. 1978. Pimiento peppers-history, breeding and evaluation. Proc. National Pepper Conference Pickle Packers International 4: 13-16. [ Links ]
Gucci, R., and J. Flore. 1989. The effects of fruiting or fruit removal on leaf photosynthesis and dry mater distribution of tomato. Adv. Hortic. Sci. 3: 120-125. [ Links ]
Hall, A. 1977. Assimilate source-sink relationships in Capsicum annuum L.: The dynamics of growth in fruiting and deflorated plants. Aust. J. Plant Physiol. 4: 623-636. [ Links ]
Heuvelink, E., L. Marcelis, and O. Körne. 2004. How to reduce yield fluctuations in sweet pepper? ActaHorticulturae 633: 349-355. [ Links ]
Imai, K., Y. Suzuki, T. Mae, and A. Makino. 2008. Changes in the synthesis of Rubisco in Rice leaves in relation to senescence and N influx. Ann. Botany 101: 135-144. [ Links ]
Jaimez A., R. E, B. Añez, y W. Espinoza. 2010. Desfloración: su efecto sobre el aborto de estructuras reproductivas y rendimiento en pimentón (Capsicum annuum). Revista de la Facultad de Agronomía de La Universidad del Zulia 27: 418-432. [ Links ]
Jaimez A., R. E., O. Araque, W. Espinoza, y C. Azócar. 2013. Dinámica de producción de flores de cultivares de Gerbera (Gerbera jamesonii H. Bolus): relación con tasas de fotosíntesis. Revista de Facultad de Agronomía. Universidad del Zulia 29: 161-178. [ Links ]
Jaimez A., R. E., R. Da Silva, A. D’Aubeterre, J. Allende, F. Rada, y R. Figueiral. 2005. Variaciones microclimáticas en invernadero: efecto sobre las relaciones hídricas e intercambio de gases en pimentón (Capsicum annuum). Agrociencia 39: 41-50. [ Links ]
Jaimez A., R. E., and F. Rada. 2006. Flowering and fruit production dynamics of sweet pepper (Capsicum chinense Jacq) under different shade conditions in humid tropical region. J. Sustain. Agric. 27:97-108. [ Links ]
Jaimez A., R. E., O. Vielma, F. Rada, and C. García-Núñez. 2000. Effects of water deficit on the dynamics of flowering and fruit production in Capsicum chinense Jacq in a tropical semi-arid region in Venezuela. J. Agron. Crop Sci. 185: 113-119. [ Links ]
Kato, T., and M. Tanaka. 1971. Studies on the fruit setting and development of sweet peppers. J. Jap. Soc. Hort. Sci. 40: 359-366. [ Links ]
Khah, E., and H. Passam. 1992. Flowering, fruit set and development of the fruit and seed of sweet pepper (Capsicum annuum L.) cultivated under conditions of high ambient temperature. J. Hort. Sci. 67: 251-258. [ Links ]
Kouki, H. 2005. Leaf canopy as a dynamic system: ecophysiology and optimality in leaf turnover. Ann. Bot, 95: 521-533. [ Links ]
Marcelis, L. 1992. Non-destructive measurements and growth analysis of the cucumber fruit. J. Hort. Sci. 67: 457-464. [ Links ]
Marcelis, L., and L. BaanHofman-Eijer. 1995. Growth analysis of pepper fruits (Capsicum annuum L). ActaHorticulturae 412: 470-478. [ Links ]
Marcelis, L., and L. BaanHofman-Eijer. 1997. Effects of seed number on competition and dominance among fruits in Capsicum annuum L. Ann. Bot. 79: 687-693. [ Links ]
Marcelis, L., E. Heuvelink, L. Baan Hofman-Eijer, D. Baker, and L. Xue. 2004. Flower and fruit abortion in sweet pepper in relation to source and sink strength. J. Exp. Bot. 55: 2261-2268. [ Links ]
McGraw, B., and J. Greig. 1986. Effect of transplant age and pruning procedure on yield and fruit-set of bell pepper. Hort. Sci. 21: 430-431. [ Links ]
Minges, P., and D. Warholic. 1973. Pepper problems in New York. Proc. National Pepper Conference Pickle Packers Int. 1: 14. [ Links ]
Olesinski, A., S. Wolf, J. Rudich, and A. Marani. 1989. Effect of leaf age and shading on photosynthesis in potatoes (Solanum tuberosum). Ann. Bot. 64: 643-650. [ Links ]
Park, S., and H. Jeong. 1976. The effect of shading on blossomdropping and fruit-dropping of the hot pepper plant (Capsicum annuum L.). Res. Rep. Office Rural Dev. 18: 1-8. [ Links ]
Quagliotti, L., G. Lepori, and P. Bigotti. 1974. Responses to solar radiation by two varieties of pepper (Capsicum annuum L.). Korean J. Breed. Sci. 6: 29-33. [ Links ]
Sawahata, K., K. Onuma, and G. Saruta. 1980. Investigations on the formation of abnormally-shaped fruit and the early drop of fruit of sweet pepper, Capsicum annuum L. in semiforcing culture. Bulletin. Ibaraki-Ken Hort. Exp. Station 8: 1-18. [ Links ]
Wien, H. 1990. Screening pepper cultivars for resistance to flower abscission: a comparison of techniques. Hort. Sci. 25: 1634-1636. [ Links ]
Wien, H., K. Tripp, R. Hernández, and A. Turner. 1989. Abscission of reproductive structures in pepper: causes, mechanisms and control. In: Green, S. (ed). Tomato and Pepper Production in the Tropics. Asian Vegetable Research and Development Center. Shanhua, Taiwan. 619 p. [ Links ]
Wubs, A. M., Y. Ma, L. Hemerik, and E. Heuvelink. 2009a. Fruit set and yield patterns in six Capsicum cultivars. Hort. Sci. 44: 1296-1301. [ Links ]
Wubs, A. M., Y. Ma, E. Heuvelink, and L. F. M. Marcelis. 2009b. Genetic differences in fruit-set patterns are determined by differences in fruit sink strength and source:sink threshold for fruit set. Ann. Bot. 104: 957-964. [ Links ]
Received: April 2015; Accepted: September 2015











 texto em
texto em