Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.49 no.3 Texcoco Abr./Mai. 2015
Recursos naturales renovables
Germination and seedling growth of Enterolobium contortisiliquum as a function of seed weight and temperature and light conditions
Germinación y crecimiento de plántulas de Enterolobium contortisiliquum en función del peso de la semilla y las condiciones de temperatura y luz
B. França da Trindade-Lessa*, J. Paulo Nobre-de Almeida, Charles Lobo-Pinheiro, Fernanda Melo-Gomes, Sebastião Medeiros-Filho
1 Universidade Federal do Ceará. Av. Mister Hull, Campus do Pici-Universidade Federal do Ceará, Bloco 815; CEP 60021-970; Fortaleza, Ceará, Brasil. * Author for correspondence. (brunoftl@yahoo.com.br).
Received: July, 2014.
Approved: February, 2015.
Abstract
Studies of germination behavior and seedling establishment contribute to the development of appropriate methods of forest species management. The aim of this study was to investigate germination and seedling growth of tamboril (Enterolobium contorsiliquum (Vell.) Morong), taking into account environmental factors and seed weight. Seeds were separated into three weight classes (light, medium and heavy), to determine the moisture content percentage and thousand-seed weight per class. Seeds from each class were then tested for germination. The experimental design was completely randomized with a 2x3x4 factorial arrangement of treatments: two light conditions (presence and absence), three weight classes (Light < 0.7 g; Medium 0.7 to 0.8 g; Heavy > 0.8 g) and four temperatures (20, 30, 40 and 20-30 °C), with four replications each. Percentage of germination and germination speed index for seed vigor were determined. For the evaluation of seedling growth, the length and dry mass of both the shoot and root were determined. When the data proved normal, ANOVA and Tukey tests (p≤0.05) were conducted to compare treatment means. Non-normal data were analyzed using nonparametric Mann-Whitney test (p≤0.05). Germination speed index, shoot dry mass and root dry mass data presented a normal distribution, allowing for ANOVA testing. Germination occurred regardless of light conditions, with a higher speed at 40 °C, although this temperature was detrimental to seedling growth. The temperature of 30 °C promoted better germination (>80 %) and seedling growth. Heavy seeds displayed improved germination and produced more vigorous seedlings.
Keywords: Environmental factors, tree species, tamboril, seedling vigor.
Resumen
Los estudios del comportamiento de la germinación y el establecimiento de las plántulas contribuyen al desarrollo de métodos adecuados del manejo de especies forestales. El objetivo de este estudio fue investigar la germinación y crecimiento de plántulas de tamboril (Enterolobium contorsiliquum (Vell.) Morong), tomando en cuenta factores ambientales y el peso de las semillas. Las semillas se separaron en tres categorías de peso (ligeras, intermedias y pesadas) para determinar el porcentaje de contenido de humedad y peso de mil semillas por clase. La germinación de cada clase de semillas se evaluó. El diseño experimental fue completamente al azar con un arreglo factorial de tratamientos 2x3x4: dos condiciones de luz (presencia y ausencia), tres clases de peso (ligeras < 0.7 g, medianas 0.7 a 0.8 g y pesadas > 0.8 g) y cuatro temperaturas (20, 30, 40 y 20 a 30 °C), con cuatro repeticiones cada uno. El porcentaje de germinación e índice de velocidad de germinación fueron evaluados. Para crecimiento de las plántulas se midió la longitud y masa seca del vástago y de la raíz. Con los datos probados normales, se realizó ANDEVA y pruebas de Tukey (p≤0.05) para comparar las medias de los tratamientos. Los datos no normales se analizaron con la prueba no paramétrica Mann-Whitney (p≤0.05). El índice de velocidad de germinación, la masa seca del vástago y de la raíz presentó una distribución normal, lo que permitió realizar el ANDEVA. La germinación se produjo independientemente de la condición de luz, con velocidad mayor a 40 °C, aunque esta temperatura fue perjudicial para el crecimiento de las plántulas. La temperatura de 30 °C promovieron la germinación (>80%) y el crecimiento de las plántulas. Las semillas con peso mayor mostraron mayor germinación y produjeron plántulas más vigorosas.
Palabras clave: Factores ambientales, especies arbóreas, tamboril, vigor de plántula.
Introduction
Many of the Caatinga arboreal species in the Brazilian biome are plants with great importance to various sectors of the economy (Brasil, 2008) and Enterolobium contortsiliquum (Vell.) Morong, known as tamboril, is amongst them. This species, found in different forest formations in Brazil, but especially in the Caatinga biome, is important for apiculture, timber, medicine and landscaping, as well as for recovery projects of degraded lands (Lorenzi, 2002). Therefore, studies of seed germination and seedling establishment of forest species become fundamental for the improvement of techniques that enable appropriate management, whether performed in a natural or modified environment.
Substances from reserves found in the endosperm or in the cotyledons are decomposed, and the soluble products of this process are translocated to the embryonic axis, allowing for growth resumption. Therefore, any accumulation of substances within the storage tissue, as well as physical damages that may result in loss of this tissue, will impair seed quality, resulting in negative consequences for the germination (Ferreira and Borghetti, 2004; Marcos Filho, 2005; Taiz and Zeiger, 2009). Thus, the amount of nutritional reserves in seeds significantly affects germination.
Seed weight at the time of maturity is an important feature that reflects seed quality and it is directly related to the deposition of reserves (Carvalho and Nakagawa, 2012). In cultivated species there is direct, positive correlation between weight and germination or seedling growth (Cicero and de Lima-Orsi, 1977; Frazão et al., 1984; Neves-Martins et al., 2005). For forestry such information is scarce, but the same positive correlation was found for Handroanthus impetiginosus (Mart. ex DC.) Stand. and Moringa oleifera Lam. (Bezerra et al., 2004; Damsceno-Ribeiro et al., 2012).
Environmental factors, such as light and temperature, are the primary regulators affecting the germination process. Temperature directly affects the speed of biochemical reactions, since each group of reactions has its own thermal requirements; this affects both the germination rate and capacity, promoting the onset of primary or secondary dormancy when temperature is unfavorable (Bewley and Black, 1994). Environmental light condition is also critical for the regulation of seed germination, especially for forest species (Marcos Filho, 2005), and light can either promote or inhibit seed germination, depending on its intensity and quality. Such variation is also determined by the features of phytochromes in the embryonic axis of the seed, since they are responsible for perception of light (Kendrick and Frankland, 1981; Takaki, 2001).
Therefore, the purpose of this research was to study germination and growth of E. contortisiliquum seedlings, as a function of seed weight and temperature and light conditions.
Materials and Methods
Mature fruits of 10 E. contortisiliquum trees were collected randomly in July 2012, in a fragment of Caatinga forest, the Daniel de Queiroz district, in the city of Quixadá-CE (4° 49' 0" S and 38° 58' 9" W) and sent to the Laboratory of Seed Analysis at the Universidade Federal do Ceará, Fortaleza-CE, where the seeds were extracted and processed for the experiment.
A sample of 300 seeds was separated from the lot, and each seed was weighed on an analytical balance (0.0001 g) in order to divide the sample into three weight classes, with a similar amount of seeds in each class: Light (<0.7 g), Medium (0.7 to 0.8 g) and Heavy (>0.8 g). For each class, the seeds of the lot were individually weighed and separated into their respective classes. In Figure 1 there is a box plot and the frequency histogram of the sample for a better understanding of the weight distribution of seeds.
After the weighing process, seeds in each class were analyzed to determine the thousand-seed weight and moisture content. Since seeds were large and hard, they were cut in half to facilitate dehydration; afterwards, seeds were left for 24 h in an oven, as described by Brasil (2009). Before the experiment, integumentary dormancy of all seeds was broken by submersion in H2SO4 98 % for 15 min and then washed with water for complete removal of the acid (Eira et al., 1993; Contro-Malavasi and de Matos-Malavasi, 2004; Anderson Gomez de Aquino et al., 2009).
After overcoming dormancy, the experiment was conducted with seeds sown on Germitest® paper moistened with distilled water (2.5 times the weight of the paper). Once the rolls were formed, they were wrapped in transparent plastic bags to reduce water loss and sent for storage in BOD (Biochemical Oxygen Demand)-type germination chambers regulated at constant temperatures of 20, 30 or 40 °C, or at alternating temperatures between 20-30 °C, all with a photoperiod of 12 h. This same procedure was used to conduct experiments in the dark, with the rolls of seeds wrapped in aluminum foil and black polyethylene bags.
A daily germination count was performed, with re-moistening of the substrate (paper rolls) when needed, considering as germinated those seeds which presented radicle size greater than or equal to 2 mm. Thus, germination percentages and germination speed index (GSI) were determined (first and final counts, respectively, on days 7 and 21). During evaluation in the dark, count was conducted under green safelight.
Shoot and root lengths of each seedling were determined using a graduated scale (mm), and then the mean of each plot was calculated. All shoots and roots from each plot were placed separately in bags of Kraft paper and dried at 80 °C for 24 h, with further weighing on an analytical balance (0.0001 g) to determine the dry mass of the parts. Afterwards, the result was divided by the number of seedlings that were initially in the bag to determine the mass per seedling. The parts (shoot + root) were then summed to compose the total length and total dry mass per seedling.
The experimental design was completely randomized with a 2x3x4 factorial arrangement: two light conditions (presence and absence), three weight classes (Light (<0.7 g), Medium (0.7 to 0.8 g) and Heavy (>0.8 g)) and four temperatures (20, 30, 40 and 20-30 °C), with four replications each, with the portion represented by the paper roll containing 25 seeds. Data were submitted to Komolgorov-Smirnov and Watson tests to analyze the normality of distribution. When the data proved normal in one or both tests, ANOVA and Tukey tests (p≤0.05) were conducted to compare treatment means. Non-normal data were submitted to nonparametric Mann-Whitney testing (p≤0.05) in order to compare the treatments, both in pairs and independently. GSI, shoot dry mass and root dry mass data presented a normal distribution, allowing for ANOVA testing. For statistical analyses, Action 2.4 software (Estatcamp, 2013) was used for normality and Mann-Whitney tests. To perform ANOVA and Tukey testing, the Assistat 7.6 beta program was used (Silva and Azevedo, 2009).
Results and Discussion
Enterolobium contortisiliquum seeds presented water content of approximately 11 %, regardless of weight (Table 1), which did not affect the hygroscopic equilibrium between seeds and environment. Seed weight showed a variation greater than 300 g in a sample of 1000 seeds (Table 1). Thus, the great variability in seed weight makes it a decisive factor for its establishment in the ecosystems it inhabits.

Data analysis showed a significant influence of all the tested factors (light, temperature and seed weight) on both germinative and seedling growth variables (Table 2). Significance was also observed for two of the four possible interactions: light influenced the perception of each temperature or vice versa; and seeds with different weights behaved differently under the temperature regimes.
The presence or absence of light did not affect the germinative process of E. contortisiliquum seeds (Figure 2), which exhibited an optimal germination percentage regardless of light conditions. This result suggests that the predominant role of phytochrome A (a photoreceptor pigment in the cells of the embryonic axis of the seeds) is to confer neutral photoblastism properties (Takaki, 2001). This behavior was expected, given the large size of E. contortisiliquum seeds. According to Carvalho and Peres (2013), large seeds should contain sufficient reserves to germinate in the dark and grow unhindered until the emergence of light, thus becoming able to carry out photosynthesis.

Insensitivity to light upon germination is also observed in other tree species native to Brazil: Anadenanthera colubrina (Vell.) Brenan var. colubrina, Guazuma ulmifolia Lam. and Dimorphandra mollis Benth. (Figliolia et al., 2009) Tabebuia serratifolia (Vahl) Nicholson, Tabebuia chrysotricha (Mart. ex DC.) Standl, and Tabebuia roseo-alba (Ridl) Sand) (Santos et al., 2005).
Higher growth values are observed for most variables of seedlings grown in the absence of light (Table 3). This effect suggests the existence of remnants of phytochrome B in control of seedling etiolation (Carvalho and Peres, 2013). Therefore, the dark phase may promote rapid mobilization of seed reserves for the embryonic axis, ensuring a slightly accentuated growth as compared to seedlings grown in the presence of light.
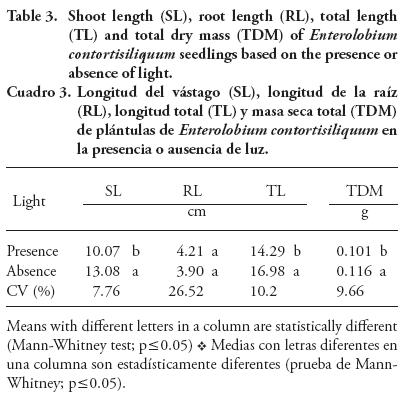
Such light perception behavior explains the ecological succession ranking seen in the literature for the E. contortisiliquum species. Some authors refer to this species as a pioneer, whereas others refer to it as an early secondary succession (Melo et al., 2008; Anderson Gomez de Aquino et al., 2009). The present findings show that both growth patterns are possible for E. contortisiliquum, which explains the large settlement of the tree throughout the Brazilian territory (Lorenzi, 2002). The results also indicate that germination and seedling establishment of this species can occur at any time, day or night. It is important to note that even in the absence of light the seedlings remained alive until the last day of the germination evaluation (three weeks) by using cotyledonary reserves. This reflects the capacity of the seedlings to endure in highly shaded environments until a clearing occurs, allowing the start of photosynthesis for plant growth.
Different behaviors were observed for germination and seedling growth according to the temperature regimes imposed on seeds. Figure 3 shows that the germination percentage was greater at 40 °C and lower at 20 °C one week after sowing (first count). High temperatures accelerate seed metabolism, thus increasing enzymatic reaction speed and a rapid germination process (Ferreira and Borguetti, 2004; Marcos Filho, 2005). After final evaluation (day 21) germination percentages were all similar (p>0.05). These results agree with those reported by Madeiros de Rodrigues-Lima et al. (1997), who found no differences in germination percentages for E. contortisiliquum for temperatures between 18.2 and 38.8 °C. The results also explain the existence of E. contortisiliquum species in forests, other than those of semi-arid regions.

The alternating temperature regime was detrimental to the germination process, providing the second worst performance for the first count, which differ from studies about germination of other tree species native to Brazil, where alternating temperatures were beneficial for the process: Syngonanthus sp. (Goncalves-Oliveira and Souza-Garcia, 2005), Sebastiania commersoniana (Baillon) Smith & Downs) (Garcia dos Santos and Bergemann-Aguiar, 2005) and Caesalpinia pyramidalis Tul. (Madeiros de Rodrigues-Lima et al., 2011). According to Borges and Rena (1993), alternating temperatures would benefit development in some species due to simulation of natural environmental conditions, but our study shows that the E. contortisiliquum tree does not follow that pattern, as well as for Gallesia integrifolia (Spreng.) Harms), Caesalpinia leiostachya (Benth) Duche), Chorizia glaziovii O. Kuntze and Amburana cearensis (Allemão) A. C. Smith (Barros et al., 2005; Biruel et al., 2007; Guedes et al., 2010; Guedes and Alves, 2011).
High germinative capacity was observed for E. contortisiliquum seedlings at 40 °C, but this temperature decreased by approximately 60-67 % seedling length variables and total dry mass, as compared to results found at 30 °C. The latter condition promoted the greatest increments for these variables, with the exception of root size, which had the highest length at 20 °C (Table 4).

The combined effect of the two environmental factors analyzed was significant (p≤0.01) for GSI, shoot dry mass and root dry mass variables (Tables 5 and 6). Thus, there was a significant difference between the presence and absence of light only at alternating temperatures (20-30 °C), and faster germination took place in the dark. Sousa-Albuquerque and Menes-Guimarães (2006) found an opposite result for Bowdichia virgilioides Kunth - a species also found in several forest of Brazil - with non-diverging germination rates under different light conditions in alternating temperatures (20-30 °C), and faster germination in the dark at constant temperatures (20, 30 and 40 °C).
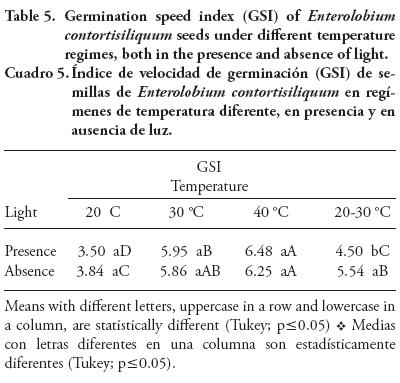

Regardless of light condition, the constant temperatures of 30 and 40 °C accelerated the germination process. Oliviera-Diniz et al. (2008) studied Licania rigida Benth., an important tree species of the Brazilian semiarid region, and show the influence of the interaction of light and temperature on seed germination, with higher speeds at 30 °C.
Table 6 lists the biomass accumulation in each part of the seedling (shoot and root). The absence of light yielded higher values of shoot dry mass, except at 40 °C, which significantly hindered seedling growth and caused death of several seeds (Figure 4). In both light conditions, optimal results were observed at 30 °C. Similar behavior was found for the seedlings of Tabebuia aurea (Silva Manso) Benth. & Hook. f. ex. S. Moore), and a temperature of 30 °C favored biomass accumulation in the shoot of this species Pacheco et al. (2008).

Biomass accumulation in the root was more efficient when tamboril seedlings grew under milder temperatures and regardless of light conditions, a constant temperature of 20 °C was most favorable. This result differs from that found by Pacheco et al. (2008) in Tabebuia aurea seedlings, which showed higher root biomass accumulation at 30 and 35 °C.
Our study shows that root growth and biomass accumulation was increased at 20 °C, whereas the shoot displayed a better performance at 30 °C. Such results are expected since high temperatures maximize seedling metabolism, enhancing competition between the parts for reserves. This is an advantage for the shoots, because roots are not good competitors (Terual and Smiderl, 1999).
Significant differences were observed in germination percentages (first count and final evaluation) of seeds in three weight classes, reflecting the influence of seed weight at the time of physiological maturity, an intrinsic factor. Figure 5 shows that germination percentage increased as seed weight class increased. These results are supported by those of Marcos Filho (2005) and Martins-da Silva et al. (2007), who observed that a higher weight corresponds to a greater seed quality, at the expense of a larger accumulation of reserves and better embryonic axis formation during the seed development in the mother plant.

Seed weight did not change seedling length but it affected biomass accumulation (Table 7). The total dry mass of the seedlings was higher for heavy seeds, followed by medium and light, with 32 % more total biomass for heavy seeds as compared to light seeds. These results corroborate recommendations aimed to separate heavier E. contortisiliquum seeds in order to produce more vigorous and homogeneous seedlings. Literature regarding weight-based classification of forest tree seeds is scarce. For Tabebuia heptaphylla (Vell.), this classification is recommended to ensure more homogeneous seedling production in the nursery, as heavier seeds have higher germination percentages and greater fresh mass accumulation (Damsceno-Ribeiro et al., 2012).

Significant interactions involving weight classes reinforce the previous conclusions. A higher germination speed index is observed for heavy seeds, regardless of temperature (Table 8), which justifies the rationale that these seeds must have greater vigor and, therefore, higher quality.

A significant interaction for biomass accumulation in seedling parts was also observed. Heavy seeds display the greatest results at all temperatures, except at 30 °C, which led to a similar biomass accumulation in both heavy and medium weight seeds (Table 9). A temperature of 40 °C damaged seedling development, causing similar effects on seeds of all weights classes. Data of our study reinforces the importance of continued research on the effects of both environmental and intrinsic factors on seed germination and seedling development, especially for those factors related to seed weight.

Conclusions
Results of this research showed that E. contortisiliquum seed germination is ideal at 30 °C, regardless of light conditions. This temperature also allows for a better development of seedling shoots, whereas roots displayed optimal results at 20 °C. Separation of seeds by weight class was equally important, since heavier seeds produced more vigorous seedlings and better germination quality.
Literature Cited
Anderson Gomez de-Aquino, A. F. M., M. C. Cardoso-Ribeiro, Y. C. Madeiros-Paula, and C. Pereira-Benedito. 2009. Superação de dormência em sementes de orelha-de-negro (Enterolobium contortisiliquum (Vell.) Morang.). Revista Verde 4: 69-75. [ Links ]
Barros, S. S. U., A. da Silva, and I. B. Aguiar. 2005. Germinação de sementes de Gallesia integrifolia (Spreng.) Harms (pau-d'alho) sob diferentes condições de temperatura, luz e umidade do substrato. Braz. J. Bot. 28: 727-733. [ Links ]
Bewley, J. D., and M. Black. 1994. Seeds: Physiology of Development and Germination. New York and London, Plenum Press. 445 p. [ Links ]
Bezerra, A. M. E., V. G. Momenté, and S. Medeiros-Filho. 2004. Germinação de sementes e desenvolvimento de plântulas de moringa (Moringa oleifera Lam.) em função do peso da semente e tipo de substrato. Hortic. Bras. 22: 295-299. [ Links ]
Biruel, R. P., I. B. Aguiar, and R. C. Paula. 2007. Germinação de sementes de pau-ferro submetidas a diferentes condições de armazenamento, escarificação química, temperatura e luz. Rev. Bras. Sementes 29: 151-159. [ Links ]
Borges, E. E. L., and A. B. Rena. 1993. Germinação de sementes. In: Aguiar, I. B., F. C. M. Piña-Rodrigues, and M. B. Figliolia (Org). Sementes Florestais Tropicais. Brasília: ABRATES pp: 83-136. [ Links ]
Brasil. 2008. Manejo sustentável dos recursos florestais da Caatinga / MMA. Ministério do Meio Ambiente. SBF / DF / PNF. Natal. 25 p. [ Links ]
Brasil. 2009. Regras para análises de sementes. Ministério da Agricultura e Reforma Agrária. SNDA / DNDV / CLAV. Brasília. 399 p. [ Links ]
Carvalho, N. M., and J. Nakagawa. 2012. Sementes: Ciência, Tecnologia e Produção. 5 ed. Jaboticabal: FUNEP. 590 p. [ Links ]
Carvalho, R. F., and L. E. P. Peres. 2013. Fotomorfogênese. http://www.miniweb.com.br/ciencia/artigos/fotomorfogenese.pdf. (Access: January 2014). [ Links ]
Cicero, S. M., and E. W. de Lima-Orsi. 1977. Influência do peso da semente de arroz (Oriza sativa L.) sobre a germinação. Anais ESALQ 34: 339-346. [ Links ]
Contro-Malavasi, U., and M. de Matos-Malavasi. 2004. Dormancy breaking and germination of Enterolobium contortisiliquum (Vell.) Morong seed. Braz. Arch. Biol. Technol. 47: 851-854. [ Links ]
Damsceno-Ribeiro, C. A., M. do Prado-Costa, D. Salgado de Senna, and J. Pizzol-Caliman. 2012. Fatores que afetam a germinação das sementes e a biomassa de plântulas de Tabebuia heptaphylla. Floresta 42: 161-168. [ Links ]
Eira, M. T. S., R. W. A. Freitas, and C. M. C. Melo. 1993. Superação de dormência de sementes de Enterolobium contorsiliquum (Vell.) Morong. – Leguminosae. Rev. Bras. Sementes 15: 177-181. [ Links ]
ESTATCAMP. 2013. Software Action. http://www.estatcamp/empresa/software-action. (Access: January 2014). [ Links ]
Ferreira, A. G., and F. Borghetti. 2004. Germinação: Do Básico ao Aplicado. São Paulo: Artmed. 323 p. [ Links ]
Figliolia, M. B., I. B. Aguiar, and A. Silva. 2009. Germinação de sementes de três arbóreas brasileiras. RIF 21: 107-115. [ Links ]
Frazão, D. A. C., J. D. Costa, F. J. Coral, J. A. Azevedo, and F. J. C. Figueiredo. 1984. Influência do peso da semente no desenvolvimento e vigor de mudas de cacau. Rev. Bras. Sementes 6: 31-40. [ Links ]
Galçalves-Oliveira, P. G., and Q. Souza-Garcia. 2005. Efeitos da luz e da temperatura na germinação de sementes de Syngonanthus elegantulus Ruhland, S. elegans (Bong.) Ruhland e S. venustus Silveira (Eriocaulaceae). Acta Bot. Bras. 19: 639-645. [ Links ]
Guedes, R. S., and E. U. Alves. 2011. Substratos e temperatura para o teste de germinação de sementes de Chorizia glaziovii (O. Kuntze). Cerne 17: 525-531. [ Links ]
Guedes, R. S., E. U. Alves, E. P. Gonçalves, J. M. BragaJúnior, J. Silva-Viana, and P. N. Q. Colares. 2010. Substratos e temperaturas para testes de germinação e vigor de sementes de Amburana cearensis (Allemão) A.C. Smith. Rev. Árvore 34: 57-64. [ Links ]
Kendrick, R. E., and B. Frankland. 1981. Fitocromo e Crescimento Vegetal. São Paulo: EPU: Ed. da Universidade de São Paulo. 76 p. [ Links ]
Lorenzi, H. 2002. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas do Brasil. Nova Odessa: Plantarum. 368 p. [ Links ]
Madeiros de Rodigues-Lima, C., F. Borguetti, and M. Valle de Souza. 1997. Temperature and germination of the Leguminosae Enterolobium contortisiliquum. Rev. Bras. Fisiol. Veg. 9: 97-102. [ Links ]
Madeiros de Rodigues-Lima, C., M. Vasconcelos-Pacheco, R. L. Alcantara-Bruno, C. dos Santos-Ferrari, J. MartinsBraga Júnior, and A. K. Dias--Bezerra. 2011. Temperaturas e substratos na germinação de sementes de Caesalpinia pyramidalis Tull. Rev. Bras. Sementes 33: 216 - 222. [ Links ]
Marcos Filho, J. 2005. Fisiologia de sementes de plantas cultivadas. Piracicaba: FEALQ. 495 p. [ Links ]
Martins-da Silva, G., M. de Souza-Maia, and C. O. Costa-Moraes. 2007. Influência do peso da semente sobre a germinação e o vigor de cevadilha vacariana (Bromus auleticus Trinius). CAST 13: 123-126. [ Links ]
Melo, R. R., M. C. L. Cunha, F. Rodolfo Júnior, and D. M. Stangerlin. 2008. Crescimento inicial de mudas de Enterolobium contorsiliquum (Vell.) Morong sob diferentes níveis de luminosidade. Agrária 3: 138-144. [ Links ]
Neves-Martins, G., R. Ferreira-da Silva, E. Fontes-Araújo, M. Gonzaga-Pereira, H. Duarte-Vieira, and A. P. Viana. 2005. Influência do tipo de fruto, peso específico das sementes e período de armazenamento na qualidade fisiológica de sementes de mamão do grupo formosa. Rev. Bras. Sementes 27: 12-17. [ Links ]
Oliviera-Diniz, F., F. J. Carvalho-Moreira, F. D. Barbosa da Silva, and S. Medeiros-Filho. 2008. Influência da luz e temperatura na germinação de sementes de oiticica (Licania rigida Benth.). Rev. Ciênc. Agron. 39: 476-80. [ Links ]
Pacheco, M. V., V. P. Matos, A. L. P. Feliciano, and R. L. C. Ferreira. 2008. Germinação de sementes e crescimento inicial de plântulas de Tabebuia áurea (Silva Manso) Benth. & Hook f. ex S. Moore. Ci. Fl. 18: 143-150. [ Links ]
Santos, D. L., V. Y. Sugahara, and M. Takaki. 2005. Efeitos da luz e da temperatura na germinação de sementes de Tabebuia serratifolia (Vahl) Nich, Tabebuia chrysotricha (Mart. ex DC.) Standl. e Tabebuia roseo-alba (Ridl) Sand – Bignoniaceae. Ci. Fl. 15: 87-92. [ Links ]
Garcia dos Santos, S. R., and I. BergemannAguiar. 2005. Efeito da temperatura na germinação de sementes de Sebastiania commersoniana (Baillon) Smith & Downs separadas pela coloração do tegumento. Sci. For. 1: 77-83. [ Links ]
Silva, F. A. S., and C. A. V. Azevedo. 2009. Principal components analysis in the software Assistat-Statistical Attendance. In: World Congress on Computers in Agriculture, 7, Reno-NV-USA: American Society of Agricultural and Biological Engineers. pp: 1-5. [ Links ]
Sousa-Albuquerque, K., and R. Menes-Guimarães. 2006. Comportamento fisiológico de sementes de Bowdichia virgilioides Kunth. sob diferentes temperaturas e condições de luz. Cerne 13: 64-70. [ Links ]
Taiz, L., and E. Zeiger. 2009. Fisiologia Vegetal. 4 ed. Porto Alegre: Artmed. 819 p. [ Links ]
Takaki, M. 2001. New proposal of classification of seeds based on forms of phitochome instead of photoblastism. Rev. Bras. Fisiol. Veg. 13: 104-108. [ Links ]
Terual, D. A., and O. J. Smiderl. 1999. Trigo: Desenvolvimento das raízes. In: Castro P., R. C. and R. A. Kugle (eds). Ecofisiologia de Cultivos Anuais: Trigo, Milho, Soja, Arroz e Mandioca. São Paulo: Nobel. pp: 18-19. [ Links ]














