Introduction
Hydrocarbon contamination due to accidents or spillage during its exploitation, conduction, and storage, has caused grave environmental damage (White et al., 2006; Gerhardt et al., 2009) and human health problems (Mohsenzade et al., 2009; Shen et al., 2013). Oil hydrocarbons are constituted by a mixture of organic compounds, principally saturated hydrocarbon, resins, and asphaltenes (Altgelt et al., 1993; Farrell-Jones, 2003). Among the most persistent oil components are polycyclic aromatic hydrocarbon (here and after PAHs). PAHs are two or more benzenic ring polynuclear compounds, which are distributed thoroughly across the environment, that has recalcitrant properties (Gao and Zhu, 2004; Fan et al., 2008; Rengarajan et al., 2015) and has cumulative potential (Haritash and Kaushik, 2009). The Environmental Protection Agency (EPA) has classified 16 PAHs as a priority for mitigation due to its ubiquity in the environment and their mutagenic, carcinogenic and teratogenic properties (Zhang et al., 2006; EPA, 2008; Morillo et al., 2008; Olsson et al., 2010). Nowadays, the physicochemical technologies employed for contaminated soils treatments with PAHs, include the extraction by solvent, chemical oxidation and thermal treatments (Gan et al., 2009). However, those treatments tend to have a high cost and a negative effect on the soil properties (Pilon-Smits, 2005; Delgadillo et al., 2011). One of the environmentally friendly mitigation strategies, at a low cost and with acceptable efficiency degrading pollutants, it is the plant-microorganism association, in which is known as phytoremediation (Frick et al., 1999; Pilon-Smits, 2005; Dixit et al., 2011; Ouvrard et al., 2014). Phytoremediation reduces the concentration of various compounds throughout different biochemical processes carried out by plants (Wild et al., 2005; Wang and Zhao, 2007) and microorganisms associated to them i.e. plant-microbiome (Kuiper et al., 2004; Liu et al., 2011; Khan et al., 2013). During phytoremediation, plants are able to carry out one or more of the following processes: accumulation (phytoaccumulation), degradation (phytodegradation), volatilization (phytovolatilization), stabilization (phytostabilization), or degradation in the rhizospheric zone (rhizodegradation) of pollutants. The phytoremediation process depends on the plant species, pollutant characteristics and concentration (Pilon-Smits, 2005; ITRC, 2009). Nevertheless, this technology is not bias-free. It depends mostly of the extension of the radicular system of the plant (Curl and Truelove, 1986; Khan et al., 2013). The phytoremediation process is limited to low contaminants concentration, tends to be a long-term process compared to physicochemical technology, also it depends on the soil’s environmental and physicochemical conditions (ITRC, 2009; Segura et al., 2009). However, phytoremediation causes minor alterations to the soil and has gained public acceptance (Weyens et al., 2010; Nesterenko-Malkovskaya et al., 2012).
It is considered that there are many plant species that have the capacity to establish and degrade oil hydrocarbon present in the soil, that can be used in phytoremediation studies (Cunningham and Ow, 1996; Frick et al., 1999; ITRC, 2009). In recent years it has been carried out experimentation with arboreal vegetal species, grass, and native microorganisms to probe their capacity of degradation and oil hydrocarbon tolerance (Hernández and Mager, 2003; Rivera-Cruz et al., 2005; Sangabriel et al., 2006; Miranda-Martínez et al., 2007; Pérez-Hernández et al., 2013). One of the species thoroughly distributed in hydrocarbon contaminated zones is Mimosa pigra considering that it presents diverse hydrocarbon concentrations (Ochoa-Gaona et al., 2011). M. pigra is a Centroamerican and South American native species that is predominantly found in humid zones (Barneby, 1991). It is catalogued as an invader specie in different parts of the world, which has caused several complications, owing to their excessive growth in countries as Australia, New Zeland and part of the Asian continent (Marambe et al., 2004; Walden et al., 2004; Asyraf y Crawley, 2011). This sort of species possesses great potential for their use in mitigation of contaminated soils due to its quick growth, tolerance to pollutants and easy propagation. For mitigation studies invader species as M. pigra in oil-contaminated soil (Rivera-Cruz and Trujillo-Narcia, 2004) and nickel contaminated soil (Netty et al., 2013), Cyperus rotundus in soils with hydrocarbons (Basumatary et al., 2013) and Leucaena leucocephala in chromium (Sakthivel and Vivekanandan, 2009) and hydrocarbon (López-Ortiz et al., 2012; Rivera-Cruz et al., 2012) contaminated soils, has been employed. Some members of the Mimosa genus present promising characteristics for soil contaminants mitigation, as they present morphological, physiological and geographic regions of distribution variations. M. púdica (Basumatary et al., 2013), M. pilulifera (Inckot et al., 2011) and M. monancristra species (Álvarez-Bernal et al., 2007) have shown tolerance to certain hydrocarbon, presenting various results in morphometry, tolerance and pollutant dissipation. Mimosa pigra, for its hydrocarbon tolerance capacity in this experiment (Rivera-Cruz and Trujillo-Narcia, 2004), as well as for its easy growth and propagation, was selected. Therefore, in this study, the potential of M. pigra for removing and bioaccumulating anthracene and phenanthrene was evaluated, along with the effect of contaminants on soil physicochemical properties throughout a dynamic of 70 days.
Materials and Methods
Experimentation area and soil collection
The experimentation site was located in the installation of the Instituto Tecnológico from Tuxtla (Tuxtla Gutiérrez, Chiapas, México). This experiment has been managed in a transparent roof and 50% shadow net-sides greenhouse conditions. The city has an average altitude of 522 masl, sub-humid warm climate, average annual temperature of 27.1 ºC and an annual rainfall of 1050 mm (INEGI, 2016).
The soil used in the study was collected in “Rancho La Escondida” (16° 1’ 55” N; 92° 50’ 54” W), located in La Concordia, Chiapas. Soil sampling was done using the 5-gold technique (SAGARPA, 2010). The soil organic layer (branches and rocks), i.e. 5 cm top soil, was removed before sampling. Afterward, the soil was sampled to a maximum depth of 20 cm. The collected soil samples were air-dried in the shadow for 7 days, grinded afterward and sifted at 5 mm-mesh. It presented a silt-loam texture (USDA, 2014), pH of 6.76, electrolytic conductivity (EC) of 0.9 dS m-1, water holding capacity (WHC) of 673 g kg-1, total nitrogen content (TN) of 2.66 g N kg-1 and total organic carbon (TOC) of 21.8 g C kg-1.
Seed collection and germination
Mimosa pigra’s fruits were collected in the town of Tucta, Nacajuca, Tabasco 18° 10’ 09” N; 93° 01’ 11” W). Fruits were manually opened to obtain seeds and these were placed in water for 24 h, afterward, they were disinfected in 70% ethanol and 6% NaClO. Disinfected seeds were then washed with distillate water three times. The germination process was done in Petri dishes, where 10 seeds were placed over damped and sterilized No. 1 Whatman® filter paper. A total of twenty Petri dishes were used to obtain enough vegetal material. The germination process was accomplished in a laboratory for 7 days in complete darkness.
Establishment of experiment and Mimosa pigra cultivation
The soil contamination was performed in two phases: (i) 1 kg of soil sample was picked by spraying with 1 g of phenanthrene and 0.5 g of anthracene. Both compounds were dissolved in dimethyl ketone (CH3COCH3) (J.T. Baker, Co.). After contaminant addition, soil was homogenized, and ketone solvent was evaporated overnight in a fume cupboard; (ii) the 1 kg of contaminated soil sample was mixed and homogenized with 4 kg of unpolluted soil. The concentration of both contaminants in the final mixture (5 kg of soil) was 200 mg of phenanthrene and 100 mg of anthracene per kg of soil. The experimental unit consisted of a 40 × 20 cm (height and diameter, respectively) plastic container, where 5 kg of soil (dry base) were deposited. Treatments involved: contaminated soil with M. pigra (C1), contaminated soil without plants (C2) and not contaminated soil with M. pigra (C0). Plantlets with a longitude of 5±1 cm of the stem and 5±1 cm of roots were selected after germination of the seeds. The selected plantlets were used throughout the experiment in C0 and C1. Experimental units were placed in a greenhouse for 70 days and the soil humidity was adjusted at a 40% of soil WHC. The kinetic of removal and bioaccumulation of phenanthrene-anthracene was accomplished, along with the physicochemical properties of the soil and the morphometric development of plants measurement. The kinetics was made for 70 days, in which 6 different samplings were conducted at distinct times: at 7, 14, 25, 35, 50 and 70 days after the beginning of the experiment. In every sampling, three experimental units per treatment (independent replicates) were destroyed to obtain vegetal material and soil for the analysis described below.
Physicochemical analysis of soil and morphometric analysis of the plant
Soil physicochemical variables evaluated in each period during the dynamic were: pH (Thomas, 1996), electrolytic conductivity (EC) (Rhoades, 1996), total organic carbon (TOC) using “Total Organic Carbon” (TOC-Vcn, Shimadzu® Kioto, Japón) semiautomatic analyzer, total nitrogen (TN) by the Kjeldahl method (Bremner, 1996), water holding capacity (WHC) by the gravimetric method, and soil texture by the Bouyoucos method (Gee and Bauder, 1986). The plant morphometric variables evaluated were: root and stem longitudes (cm), aerial and radicular biomass (g) in the treatments C1 and C0.
Determination of anthracene and phenanthrene in soil and plant
Determination of anthracene and phenanthrene concentration in the soil were done as described by Yufang et al. (1995). Briefly, 1.5 g of soil sample from each treatment was added to a Falcon® tube with 12 mL of dimethyl ketone, homogenized and placed in an ultrasonic bath (Cole-Parmer® Mod. 08855-00) for 40 min at 35 ºC. Afterward, the ketone solvent was separated by centrifugation. The same process was repeated twice. The total solvent obtained was mixed and dried by evaporation in fume cupboard overnight. After solvent elimination, the pellet was resuspended in HPLC grade dimethyl ketone for further analysis. The contaminants quantification was done using an Agilent® 7890A chromatograph equipped with a flame ionizing detector (FID). The Elite-5 de Perkin-Elmer® column (30 meters of length, 0.53 mm interior diameter, 1.5 μm film thickness) was used. N2 was employed as driver steam at a flow rate of 7 mL min-1. The temperature of the oven at 140 ºC was increased to 160 ºC at a rate of 2 ºC min-1, then increased to 165 ºC at a rate of 1 ºC min-1 and maintained 2 min. A final increased to 300 ºC at a rate of 35 ºC min-1 was performed. The temperature of injector was set to 250 ºC and the temperature of detector was set to 300 ºC.
In order to extract the accumulated pollutant in the plant’s tissues the ultrasonication extraction method was performed as described by Gao and Zhu (2004). Briefly, plants were oven dried during 3 days at 70 ºC. Aerial and radicular parts were separated and milled using liquid N2 to a 5 mm particle size. Samples were mixed with an acetone:hexane (1:1) solution. Mixtures were placed in an ultrasonic bath (Cole-Parmer® Mod. 08855-00) for one hour and then centrifuged to obtain the extract. These operations were repeated twice and both solvent extracts were mixed. The solvent from the extraction process was overnight evaporate in a fume cupboard. After drying, the pellet was resuspended in HPLC grade dimethyl ketone and stored in vials for their further GC-FID analysis, using the method described above.
Statistical analysis
A one-way analysis of variance (ANOVA one way) with LSD mean comparison test (Least significant difference) was performed on each sampling period using the morphometric data i.e. stem and root longitude data, aerial and radicular biomass, soil physicochemical properties and PAHs concentration levels. Statistical differences were found when P < 0.05 among treatments, using the Statistical Software Centurion XV Statgraphics® software (Statgraphics Technologies, Inc., Virginia, USA).
Results and Discussion
Morphometric variables in plant
After 70 days of kinetics, any significant differences were presented (P > 0.05) in root and stem growth in all treatments. At the 7th day (first period), for C0 a 4.97 cm stem height was observed. At the 25th (third period) it was of 7.33 cm and at 70th day (sixth period) it was of 74.23 cm. C1 presented a height of 4.63 cm, 11.17 cm and 76.50 cm for each period respectively. In root, the values for the first, third and sixth period were of 3.70 cm, 11.93 cm and 44 cm for C0, and 3.83, 13.13 cm and 46.50 cm for C1 (Figure 1a).
In radicular and aerial biomass significant differences were found (P < 0.05) at the 50th. In this day, C0 treatment presented the biggest aerial (4.66 g) and radicular (0.95 g) biomass, in C1 the root and aerial biomass were of 1.41 g and 0.226 g respectively (Figure 1b). In the 7th, 14th, 25th, 35th, and 70th there were no differences presented (P > 0.05).
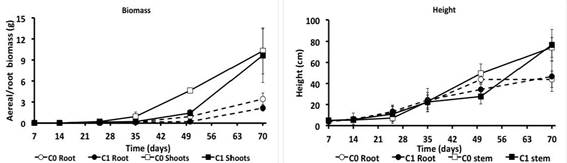
Figure 1: a) Aerial and root biomass; b) Stem and root longitude of Mimosa pigra cultivated in contaminated soil with anthracene and phenanthrene during 70 days, with sampling at the 7th, 14th, 25th, 35th, 50th and 70th days after the beginning of the experiment. C0 = no contaminated soil with the plant; C1 = contaminated soil with the plant. The error bar represents the standard error of the mean (n = 3).
Phenanthrene and anthracene concentration in plants and soil
The removal kinetics of contaminants in the soil showed that after seven days, the biggest PAHs removal was obtained: for phenanthrene a statistical difference between C1 and C2 (P < 0.05), was found, observing a removal percentage of 92% for C1 and 77% for C2 (Figure 2a). For the anthracene, any significant statistical difference was found (P > 0.05) between C1 and C2 treatments, the removal was of an 80% in C1 and 60% in C2 (Figure 2b). In the days after the kinetic, fluctuations without statistical differences were observed. At the end of the experiment, it was not observed a complete removal of anthracene and phenanthrene from the soil.
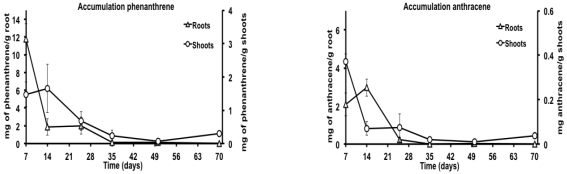
Figure 2: The concentration of phenanthrene (a) and anthracene (b) present in the contaminated soil with anthracene and phenanthrene during 70 days, with sampling at the 7th, 14th, 25th, 35th, 50th and 70th days after initiated the experiment. C1 = contaminated soil with the plant; C2 = contaminated soil without the plant. The error bar represents the standard error of the mean (n = 3).
On the other hand, it was determined that M. pigra accumulated part of the pollutants in the root as well as the aerial part (stem and leaves) (Figure 3); it was observed that most of the accumulation in root was reached at the 7th day with 11.8 mg phenanthrene g-1 root, while in the aerial part the higher content was found at the 14th day with 1.8 g phenanthrene g-1 stem (Figure 3a). For the anthracene, the higher content was at the 7th day with 4.3 mg g-1 root and at the 14th in the stem with 0.22 mg g-1 (Figure 3b). In both cases it was observed that the accumulation was bigger in the root than in the aerial part, also, it was noticed that the concentration of accumulated contaminants decreases after 7 and 14 days after the initiation of the kinetics (Figure 3).
Figure 3: Accumulation of phenanthrene (a) and anthracene (b) in the root zone and the aerial part of Mimosa pigra cultivated in the contaminated soil with anthracene and phenanthrene during 70 days, with samplings at the 7th, 14th, 25th, 50th and 70th days after initiated the experiment. C0 = no contaminated soil with the plant; C1 = contaminated soil without the plant. The error bar represents the standard error of the mean (n = 3).
Soil physicochemical properties
The analysis of the physicochemical properties showed that the pH, EC, and N has not presented significant statistical difference (P > 0.05) between treatments in each period of the kinetics. The pH passed of an initial value of 6.7 to values under 6 at the 7th day, showing level variations during the lapse of these periods (Figure 4a). The electrolytic conductivity (EC) presented a low variation in the experiment (Figure 4b), at the 70th value of 0.42 for C0, 0.35 for C1 and C2 dS m-1 was presented. The total Nitrogen maintained an approximate value of 2 g N kg-1 of soil during the kinetics of all of the treatments (Figure 4c).
The particles of the soil compose the texture, the clay (Figure 4d) did not show statistical difference (P > 0.05) between treatments in the 7th day, the mud (Figure 4e) and the sand (Figure 4f) in soils with contaminants (C1 and C2) did not show differences (P > 0.05) between them but with C0 (P < 0.05), for the 25th day in clay all treatments shown differences (P < 0.05), in mud C1 and C2, did not show differences (P < 0.05) among them but with C0, and the same behavior was found amongst C2 and C0 in relation to C1. For the 70th day, clay, silt, and sand did not show differences among treatments, presenting all of three treatments silt-loam texture.
In the WHC (Figure 4g) at 7th and 35th days any statistical difference was found (P > 0.05). On the 7th day, the WHC was of 490 g H2O kg-1 for C0, 462 g H2O kg-1 for C1 and 463 g H2O kg-1 for C2. On the 50th and 70th day of the kinetics, plant treatments (C1 and C0) did not show differences among themselves but with treatment C2. The WHC on the 70th day was of 611 g H2O kg-1 for C0, 604 g H2O kg-1 for C1 and 530 g H2O kg-1 for C2.
Lastly, the determination of the total organic carbon (TOC) has not presented significant statistical difference (P > 0.05) in the 7th, 14th and 50th days. At the end of the kinetics, treatment C0 presented a significant difference in relation to C1 and C2, these have not presented differences among themselves. Values for this period were of 14.42 g of C kg-1 in C0, 17.51 g of C kg-1 for C1 and 21.08 g of C kg-1 in C2 (Figure 4h).
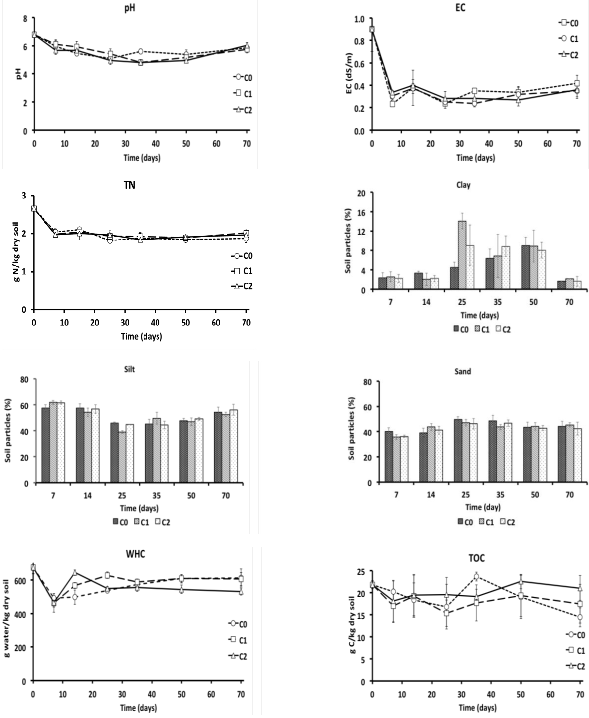
Figure 4: Physicochemical properties of contaminated soils with anthracene and phenanthrene during the 70 days of experiment. a) pH; b) electrolytic conductivity (EC); c) total nitrogen (TN); d) clay; e) slit; f) sand; g) water holding capacity (WHC); h) total organic carbon (TOC). Sampling was performed at the 7th, 14th, 25th, 35th, 50th and 70th days after the onset of the experiment. The error bar represents the standard error of the mean (n = 3).
Discussion
Biomass and height
According to the results, adverse effects in M. pigra biomass and total height due to the presence of contaminants in the soil were not observed. These results are in agreement with previous studies as the one made by Betancur-Galvis et al. (2012) where no variation in biomass and longitude of Tamarix aphylla L. cultivated in saline-alkaline soil contaminated with a mixture of anthracene, phenanthrene and benzo(a) pyrene (120:100:45 mg kg-1 soil) were observed in a 240 day dynamic. In other study Su and Yang (2009), the effect of a PAHs mixture in various concentrations of naphthalene (200 mg kg-1 of soil, maximum concentrations employed), phenanthrene (70 mg kg-1 soil) and pyrene (26 mg kg-1 of soil) in rice plants (Oryza sativa), after 8 weeks any significant effect on the root biomass and shoot was observed. Similarly, Xu et al. (2006), significant effects were not observed upon aerial and root biomass of Ryegrass (Lolium perenne L.), corn (Zea mays L.) and white clover (Trifolium repens) cultivated in diverse phenanthrene and pyrene concentrations. The previous results contrast with the reported results by Dupuy et al. (2015) in a study using Zea mays exposed to different concentrations of phenanthrene through 28 days, were negative effects (P < 0.05) on the plant’s total biomass in relation to control (75% less biomass), due to the presence of the contaminant. Likewise, Álvarez-Bernal et al. (2007) reported negative effects on aerial and radicular biomass (P < 0.05) of Mimosa monancistra cultivated in contaminated soils with a mixture of anthracene, phenanthrene and benzo (a) pyrene (100:200:50 mg kg-1 soil). Similar studies demonstrate such effect on plants as Arabidopsis thaliana (Alkio et al., 2005), Oryza sativa (Li et al., 2008) and Lycopersicon esculentum (Ahammed et al., 2012). These results infer that the effect of the pollutant on the plants depends on the species employed for soil remediation studies, besides the type of contaminant and its concentration (Binet et al., 2000; Gao and Shu, 2004; Merkl et al., 2005). The capacity displayed by the plant to adapt to stress conditions through different mechanisms such as the abscisic acid and ethylene production (Vánôvá et al., 2009; Weisman et al., 2010) and the production of enzymes as superoxide, dismutase, catalase, ascorbate peroxidase, glutathione reductase which help to withstand the oxidative stress provoked by the contaminant (Alkio et al., 2005; Gill and Tuteja, 2010), will be the factor that allow a successful restoration process.
Anthracene and phenanthrene removal kinetics
In the first days of the experiment, the biggest removal of both PAHs present in the soil was observed. This effect could be derived from the capacity of some autochthonous microorganisms with the capacity of removing PAHs from the soil (Kuiper et al., 2004, Khan et al., 2013). Numerous microorganisms with the capacity of metabolizing these compounds as an energy source have been reported (Moody et al., 2001; Mallick et al., 2007; Seo et al., 2007). The phenanthrene removal from the soil was faster than the anthracene’s, which has been reported in other studies (Atagana et al., 2003; Álvarez-Bernal et al., 2007; Betancur-Galvis et al., 2012). This is due to the solubility of each compound 0.045 mg L-1 for the anthracene and 1.1 mg L-1 for the phenanthrene (D’Souza et al., 2015) which facilitates the availability of the compound to be attacked by microorganisms present in the soil (Sun et al., 2010).
The presence of Mimosa pigra could have stimulated the development of microorganisms in the rhizospheric zone, causing a bigger removal of PAHs in the treatment with the plant, as much as the treatment without plant presented a lower removal grade. The effect of the roots in the dissipation of hydrocarbon is due to the upgrading of the conditions of the soil and the release of radical exudate which increase the removal of the pollutants; these exudates promote the development of the microbial population in the rhizospheric zone (Abhilash et al., 2009; ITRC, 2009; Khan et al., 2013). Several studies have demonstrated that the presence of plants can contribute to the increment of hydrocarbon degrader microorganisms in the rhizosphere zone (Merkl et al., 2005; Zhuang et al., 2007; Gaskin et al., 2008). In this zone, the released organic exudates could improve the bioavailability of the PAHs (Ouvrard et al., 2006; Sun et al., 2012) and serve as substrate for the proliferation of an intense microbial activity, favoring the development of bacteria that could remove the phenanthrene and the anthracene through its direct metabolism or by cometabolism.
Besides the biotic degradation of the PAHs, it has been proved that the removal of this could be due to abiotic factors, such as photooxidation (Guieysse et al., 2004; Vione et al., 2006) even though it has been justified that the biggest contribution to the removal is via microorganisms (Gao and Zhu, 2004; Xu et al., 2006; Sun et al., 2012).
In similar studies such as Álvarez-Bernal et al. (2007) employing the legume Mimosa monancistra cultivated in polluted soils with the phenanthrene, anthracene and benzo (a) pyrene mixture at a concentration of 200, 100 and 50 mg kg-1 soil respectively, reported that the phenanthrene was a 90% removed by the 14th day, no existing a difference with or without the presence of the plant. In the case of the anthracene, the presence of the plant has an effect on the removal with 49% and 39% in the treatment without the plant. Xu et al. (2006) applying a mixture of phenanthrene and pyrene in the soil for 60 days has found that the treatments planted with Zea mays and Lolium perenne removed a 98.22% of phenanthrene and 95.81 of pyrene. Similarly, using the same contaminants in a concentration of 133 mg phenanthrene kg-1 soil and 172 mg pyrene kg-1 soil, a dissipation of 55% of phenanthrene and 66% of pyrene in all the used plants at the day 45th was found by Gao and Zhu (2004), reporting that in the removal treatment without the plant, the removal was lower. Similar results of the higher effect of the removal of PAHs in soils with vegetation than in absence of it were reported by Xu et al. (2006), Lee et al. (2008), Gao et al. (2010) and Wang et al. (2015). Therefore, it is deduced that the presence of plants promotes the PAHs dissipation, the removal varies depending on the type of used plant, given that one of the major factors that have an effect upon the xenobiotic is the radicular system and this varies amongst species.
Phenanthrene and anthracene accumulation in plant
Mimosa pigra have the capacity of absorbing the pollutants in the soil and collect them in its tissues (Figure 3). It has been proved that some plants have the capacity of absorbing pollutants through their roots, following by the translocation to superior tissues, where the process of accumulation or degradation can be done (Pilon-Smits and Freeman, 2006; ITRC, 2009). The results indicated that there was a higher accumulation of pollutants in the roots than in the stem. This match with the reported by Gao and Zhu (2004), Tao et al. (2006) and Wang et al. (2015). This behavior is mainly due to the Octanol-water partition coefficient (log Kow) of this PAHs (4.54 for the anthracene and 4.57 for the phenanthrene) that predicts that for highly hydrophobic compounds with log Kow values above 3.5 the absorption of the plant is slower, and they can be not available for the translocation to the higher parts of the plant. (Komives and Gullner, 2006). When the pollutant is assimilated by the roots this are retained in areas where cause as little damage as possible at the principal metabolic process of the cell, when they can be accumulated in the vacuole or the cell wall (Burken, 2004; Pilon-Smits, 2005) and on a tissue level these pollutants can be collected at the epidermis or the trichomes (Jeffers and Liddy, 2003).
Decrease of the pollutant in the plant after 25‑70 days can be due to the capacity of the said plant to metabolize these pollutants. Some plants can have the ability of organic pollutant photo-degradation by a series of enzymes such as cytochrome P450, that allows the improvement of the detoxification capacity. (Khatisashvili et al., 1997; Varazashvili et al., 2001). A lot of vegetal species possess the property of hydrocarbons processing using the cytochrome P450 such as Cyperus laxus Lam (López-Martínez et al., 2008), others are genetically induced to express CYP450 like Nicotiana tabaccum (Dixit et al., 2008), Solanum tuberosum, Oryza sativa and Arabidopsis thaliana (Doty, 2008). The presence of the pollutant in the vegetal tissue trigger a series of adaptive reactions in order to support the stress conditions such as Phyto‑hormones production (ABA, ethylene) (Vánôvá et al., 2009; Weisman et al., 2010), stress oxidative trigger enzymes (superoxide dismutase, catalase, ascorbate peroxidase, glutathione reductase) (Alkio et al., 2005; Gill and Tuteja, 2010) and the degrading enzymes of the pollutants. Phyto-accumulation doesn’t become one of the principal mechanisms of PAHs biodegration of the soil (Gao and Zhu, 2004) consequently it does not have much importance. Su and Zhu (2008) reported that the contribution of PAHs absorption to the soil is just 0.24% comparing with the approved by rhizo-degradation (14%). Phyto-accumulation can come along with other processes such as rhizo-degradation, phyto-degradation or phytovolatilization, that together promote an improvement in the pollutant removal process.
Soil physicochemical variation
Hydrocarbons presence in the soil can cause physical-chemical changes in this by preventing the gaseous exchange with the atmosphere, altering the water penetration and evaporation, as well as the nutrients exchange in the soil. These changes range with the type and the concentration of the hydrocarbon; also affect the texture, moisture and soil temperature (Adams et al., 2008; Adams and Morales, 2008; Martin et al., 2014). In this study, significant changes on pH (being lightly acid), CE (having negligible effects on the salinity), total N (rich in N) during all the kinetics were not observed. In the texture variation of the slit, clay and sand were observed during the kinetic, reaching stability at day 70, showing a silt loam soil.
During the first periods of the kinetics light variations of these parameters created by the stabilization process between microorganisms and the plant were shown. Roots can alter pH conditions through a series of process such as assimilation and production of charged chemical elements, as much as the freedom of organic acids and CO2 (Jones et al., 2004). Pollution of soils causes a direct effect on the quantity of N in the soil (Newman and Reynolds, 2004), this element was not observed during the experiments, but was quantify an effect of this in the content of silt, sand, and clay.
WHC and TOC show a variation at the ending of the experiment. In the WHC the absence of the plant in C2 (retention <530 g H2O kg-1 s. s.) turn out to be a compared factor to C0 and C1 (values >600 g H2O kg-1 s. s.), because the plant root can provoke a higher water infiltration and create a higher quantity of organic materials, which promote water retention (USDA, 1998). Soil texture plays a major role in water retention but in this case, wasn’t a determinant factor in the same type of soil. Besides the properties of the soil, the capacity of the plant for profound rooting will provide a higher quantity of water for its disposition: in more depth, water proportion will be higher (Shaxson and Barber, 2003). Quantity of present water allows an adequate value of humidity in the soil, which facilitates hydrocarbons removal (Infante et al., 2012).
For the TOC at day 70, there wasn’t a statistical difference between C1 and C2 treatments, but it was one between C2 and C0 (P < 0.05); this variation can occur due to the presence of pollutants, which can be quantified as organic carbon by the equipment (TOC) used for this analysis. Zavala et al. (2005) reported a similar result using Brachiaria humidicola cultivated in soil with weathered petroleum. In the C0 treatment, the plant can cause an intense biological activity on the soil, provoking constant recycling of carbon (Martínez et al., 2008), besides the absence of pollutants, this factors could influence the TOC. It has been proved that the presence of plants in polluted soils improves the structure of the soil by the increasing of aeration, humidity and the possibility of proliferation of a diversity of microorganism that influences the soil properties. (Frick et al., 1999; Gerhardt et al., 2009).
Conclusion
The concentration levels of both hydrocarbon compounds use in present study did not affect the soil physicochemical characteristics, e.g. soil pH, electrolytic conductivity (EC) and nitrogen content. However, a reduction in soil carbon content was noticed due the plant presence. Mimosa pigra morphometric measures was unaltered due to soil contaminant at the given concentrations despite the fact that both anthracene and phenanthrene was root and stem accumulated by the plant. A noticeable reduction of soil contaminants by 15 and 20% of anthracene and phenanthrene (respectively) levels was seen due to the presence of M. pigra compared to the control treatment (soil without plant), so we suggest that M. pigra has an outstanding potential for soil bio-remediation use. However, further plant processing must be resolved due to the accumulation of contaminants in the plant biomass and their later environmental impact.
Data Availability
Data sets generated or analyzed during the current study are not publicly available because they are plotted in detail and redundantly appended as supplementary materials, but are available from the author on a reasonable request.
Funding
Valentín Pérez-Hernández and Mario Hernández-Guzmán received grant-aided support from ‘National Council of Science and Technology (CONACyT, Mexico)’, ‘304568’ and ‘306905’, respectively. Given for the master’s degree in Biochemical Engineering into the postgraduate program of the TNM: Tuxtla Gutierrez Institute of Technology.
Authors Contributions
Writing, revision and edition: V. Pérez-Hernandez, M. Hernández-Guzman, I. Pérez-Hernández, S. Enciso-Saenz, F Gutierrez-Miceli, L. M. C. Ventura-Canseco. Supervision, courses: S. Enciso-Saenz. Research, visualization, conceptualization: V. Pérez-Hernández. Writing, preparation of the original draft: V. Pérez-Hernández, M. Hernández-Guzmán











 nova página do texto(beta)
nova página do texto(beta)



