Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.37 no.2 Texcoco Mai. 2019 Epub 30-Set-2020
https://doi.org/10.18781/r.mex.fit.1901-1
Phytopathological notes
Inductors of plant resistance in the control of Candidatus Liberibacter asiaticus in Mexican lemon (Citrus aurantifolia) trees
1 Laboratorio de Fitopatología de la línea de Biotecnología Vegetal, Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco A.C., El Bajío del Arenal, C.P. 45019, Zapopan, Jalisco, México;
2 CIIDIR, Unidad Sinaloa, Departamento de Biotecnología Agrícola, Instituto Politécnico Nacional, Bulevar Juan de Dios Bátiz Paredes No. 250, C.P. 81101, San Joachín, Guasave, Sinaloa, México.
The Huanglongbing represents a great challenge for its control, thus, effective alternatives are required to the application of insecticides to the vector insect. The induction of systemic resistance is an alternative to decrease the progress of the disease in infected trees. The objective of this work was to evaluate the effect of inductors of systemic resistance on the concentration of ʻCandidatus Liberibacter asiaticusʼ (CLas) in Mexican lemon trees (Lm) under greenhouse conditions. The resistance inductors used were salicylic acid (T1), Azospirillum brasilense Cd (T2) and chitosan (T3), applied every 20 days for eight months. The quantification of CLas was through qPCR. The CLas concentration was determined at 1, 2, 5 and 8 months after treatment. At 8 months pos-treatment, T1 and T3 did not present significant differences in the reduction of CLas concentration with respect to control without inductor (T4) (6.5×103 bacterial cells/100 ng of DNA), whereas T2 decreased almost three times the titer of CLas with respect to control. Direct soil inoculation of A. brasilense Cd showed a significant effect in the reduction of CLas concentration in Lm trees under greenhouse conditions.
Key words: Salicylic acid; Azospirillum brasilense; HLB; chitosan; growth promoting bacteria
El Huanglongbing representa un gran reto para su control, por lo que se requiere de alternativas efectivas a la aplicación de insecticidas al insecto vector. La inducción de resistencia sistémica es una alternativa para disminuir el progreso de la enfermedad en árboles infectados. El objetivo de este trabajo fue evaluar el efecto de inductores de resistencia sistémica sobre la concentración de ʻCandidatus Liberibacter asiaticusʼ (CLas) en árboles de limón mexicano (Lm) en condiciones de invernadero. Los inductores de resistencia utilizados fueron ácido salicílico (T1), Azospirillum brasilense Cd (T2) y quitosano (T3), aplicados cada 20 días durante ocho meses. La cuantificación de CLas fue mediante qPCR. La concentración de CLas se determinó a los 1, 2, 5 y 8 meses pos-tratamiento. A los 8 meses pos-tratamiento, T1 y T3 no presentaron diferencias postratamiento en la reducción postratamiento de la concentración de CLas con respecto al control sin inductor (T4) (6.5×103 células bacterianas/100 ng de ADN), mientras que T2 disminuyó casi tres veces la concentración de CLas con respecto al control. La inoculación directa al suelo de A. brasilense Cd mostró un efecto significativo en la reducción de la concentración de CLas en los árboles de Lm en condiciones de invernadero.
Palabras clave: Ácido salicílico; Azospirillum brasilense; HLB; quitosano; bacterias promotoras de crecimiento
Mexico ranks second in lemon production and exportation worldwide. In 2017, lemon exports were valued at US $500 million (SIAP, 2018). However, diseases caused by phytopathogens associated with citrus affect lemon yield and consequently its production value. Huanglongbing (HLB), associated with α-Proteobacteria Candidatus Liberibacter spp., is a devastating citrus disease whose control has posed a challenge since its introduction into the Americas (Wang and Trivedi, 2013). In Mexico, Robles-González et al. (2013) reported fruit yield losses (kg tree-1) of up to 40% in Mexican lemon trees showing Huanglongbing symptoms in more than 75% of their treetops compared to asymptomatic trees. The traditional methods for controlling HLB include chemical or biological control of the vector insect, removal of infected trees to reduce inoculum and production of HLB-free patterns and grafts, but these measures have not been completely effective (Hall and Gottwald, 2011). On the other hand, alternative strategies to slow the disease progress and maintain citrus yields have been developed which include improved nutrition programs (Gottwald et al., 2012), antibiotic application (Zhang et al., 2011) and resistance inductors (Hu et al., 2018). Induction of systemic acquired resistance (SAR) or induced systemic resistance (ISR) protects lemon trees against a wide range of phytopathogens or reduces the disease severity (Walters et al., 2013). The use of chemical inductors for controlling HLB in field evaluations has been reported to have significant effects on lowering the concentration of ʻCandidatus Liberibacter asiaticusʼ (CLas) and slowing the disease progress, both when leaves are sprayed (Li et al., 2016) and when the chemical inductors are directly injected into the trunk of the trees (Hu et al., 2018). In field tests, Hu et al. (2018) demonstrated that when salicylic acid (0.8 g/tree) was directly injected into trunks of ʻHamlinʼ sweet orange trees (Citrus sinensis), the CLas concentration was significantly reduced by 65.8% compared to that of the control injected with water. Chitosan is well known to be a response elicitor of the plants defense mechanisms, such as changes in PR proteins accumulation, enzymes and secondary metabolites associated with defense (Xing et al., 2015). Algam et al. (2010) demonstrated that when tomato seeds cv Hezou were pre-treated with chitosan (10 mg mL-1) two weeks before inoculating Ralstonia solanacearum Rs-f.91, there was a significant decrease of wilt incidence (31%) compared to that of the control, as well as an increase of PR proteins activity, such as chitinase and β-1,3-glucanase. On the other hand, there are reports on the use of Plant Growth-Promoting Bacteria (PGPB) as systemic resistance inductors for controlling HLB. Tang et al. (2018) demonstrated that the rate of trees infected with CLas was reduced by 50% when C. sinensis trees roots were irrigated with Bacillus amyloliquefaciens GJ1 under greenhouse conditions. The use of salicylic acid, chitosan and PGPB could be a practical alternative for resistance induction in Mexican lemon trees (Lm) in order to control HLB. Based on the above, the objective of this study was to evaluate the use of systemic resistance inductors on the concentration of ‘Candidatus Liberibacter asiaticusʼ in Mexican lemon trees under greenhouse conditions.
For the study, Mexican lemon trees (Citrus aurantifolia) budded on a 9-month old volkameriana lemon rootstock (Citrus volkameriana) were used. The trees were placed in 40 L pots containing 25 L of a mixture of sphagnum peat substrate-silt soil-perlite (3:3:4/v:v). The trees were kept in the greenhouse under natural light at 23-28 °C and 31-45% RH. Fertilizer was applied to the lemon trees every 20 days with 3 L of a nutrient solution containing 0.25 g L-1 of magnesium sulfate, calcium nitrate and potassium nitrate, 0.15 mL L-1 of phosphoric acid and 0.1 g L-1 of micronutrients (Microfol® Combi P.S.); 40 g of monoammonium phosphate per plant were also applied every six months. When necessary, pest control using chemical (AK-20® 2 mL L-1, Muralla Max® 0.5 mL L-1, Oberon® 2 mL L-1 and Talstar® 2 mL L-1) and organic means (GreenCorp: eBioluzion® PlusvO 5 mL L-1, Akabrown® 5 mL L-1 and Specktron Plus 5 mL L-1) before the experiment was used against insects such as red spiders, leaf miners and aphids. The Mexican lemon trees were inoculated with 2-3 cm long bud graftings (Figures 1A and 1B) obtained from PCR tree twigs positive to CLas showing characteristic HLB symptoms (Bové, 2006), which is considered as an effective pathogen transmission method (Coletta-Filho et al., 2010). The inoculum source was collected in a Mexican lemon tree orchard in Tecomán, Colima, in February 2013.
Before establishing the experiment, the CLas concentration in infected Mexican lemon trees was determined four months after inoculation with bud graftings using quantitative real-time polymerase chain reaction (qPCR) in order to start the experiment with trees that had a similar concentration and thus homogenize this variable among the different treatments. The fifth mature leaf descending from the apex of seven twigs from each tree was collected. The leaves were frozen with liquid nitrogen, lyophilized for 72 h and ground in a TissueLyser II mill (QIAGEN, Hilden, Germany) for 1 min at 30 Hz. 20 mg of the lyophilized and ground tissue were used for genomic DNA extraction and the CTAB method was followed as previously described by Zhang et al. (1998) with slight modifications (Arratia-Castro et al., 2014). The DNA concentration and purity were evaluated using a NanoDrop ND-2000 UV-Vis Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The DNA samples were adjusted to 20 ng µL-1 and stored at -20 °C until they were used. To quantify CLas, all the qPCR assays were performed using the combination of nested PCR and TaqMan® PCR in a single tube (Lin et al., 2010). All the qPCR assays were performed in a 7500 Fast Real-Time PCR System thermal cycler (Applied Biosystems, Foster City, CA). Two technical replications out of four biological replications were used for the qPCR assays. Each qPCR reaction was carried out in a 25 µL volume containing 12.5 µL of TaqMan® master mixture (ABI, Foster City, CA), 0.5 µL of the initial primers Las O-F and Las O-R (0.5 pmol), 0.2 µL of the internal primers Las I-F and Las I-R (20 pmol), 0.5 µL of the Las-P TaqMan® probe (10 pmol), 5 µL of DNA (100 ng) and 5.6 µL of ultrapure water. The nested PCR was carried out as follows: 50 °C for 2 min and 95 °C for 2 min, followed by 20 cycles of 95 °C for 30 s, 67 °C for 45 s and 72 °C for 45 s, and then 35 cycles of 95 °C for 30 s, 57 °C for 45 s and 72 °C for 45 s. The fluorescence signal was recorded at the end of each 57 °C cycle during the second PCR round (Lin et al., 2010). The Ct values were converted to CLas cell concentration using a standard curve previously described by Lin et al. (2010) with the Software 7500 System SDS version 2.0.5. Once the concentration of initial CLas per tree was determined, trees with 610-1700 bacterial cells/100 ng of DNA were selected and randomly distributed among the different treatments (four trees per treatment). Figure 1C shows the experiment conducted in the greenhouse from June 2013 to February 2014.
The resistance inductors tested under greenhouse conditions were chitosan (0.01%), salicylic acid (5 mM) and Azospirillum brasilense Cd (2×107 cfu g-1 of substrate). Chitosan (chitosan≥75% deacetylation, Sigma-Aldrich®) was prepared as 1% stock: 5 g of chitosan were diluted in 100 mL of 1% acetic acid and then the volume was adjusted to 500 mL with 1% acetic acid. The chitosan stock solution was adjusted to 5.75 pH (NaOH 1M) and sterilized at 121 °C for 20 min. The salicylic acid was prepared as a stock solution at 75 mM. Chitosan and salicylic acid were individually applied on leaves with a 5.5 L manual sprinkler (Swissmex®), 15-20 mL per plant (Figure 1D). Azospirillum brasilense Cd was grown in NFb broth (nitrogen-free broth) (Döbereiner et al., 1976) at 30 °C and 200 rpm for 16 h. The solution containing rhizobacteria was adjusted to a concentration of 2x107 cfu g-1 substrate and then directly applied to the substrate in the plant’s rhizosphere. The inductors were applied every 20 days for eight months. A completely randomized design with four replications per treatment was used for the experiment. The treatments were as follows: T1 salicylic acid; T2 Azospirillum brasilense Cd; T3 chitosan, and T4 no inductor. The effect of the resistance inductors to reduce the pathogen level was evaluated by determining the CLas concentration using qPCR, as previously described, at 1, 2, 5 and 8 months after treatment. The CLas concentration variable was analyzed through an analysis of variance (ANOVA), and a means comparison with the least significant difference test (LSD) using the StatGraphics Centurion XV statistical software (StatPoint Inc., 2005).
To evaluate the effect on the decrease of CLas concentration in Mexican lemon trees, two inductors were applied on leaves (salicylic acid and chitosan) and A. brasilense Cd to the soil every 20 days during 8 months under greenhouse conditions. No significant statistical differences were found in the CLas concentration (data not shown) before starting the experiment, so all the treatments started with a similar concentration. However, at the end of the first month after treatment, we observed differences among treatments regarding the rate at which the CLas concentration had increased (Figure 2). Two months after treatment, T3 chitosan showed the lowest CLas concentration (643 bacterial cells/100 ng of DNA) compared to T4 without inductor. Five months after treatment, the three resistance inductors that were used showed a significant decrease (LSD, p≤0.05) in CLas concentration of a little more than twice compared to T4 with no inductor (Figure 2). Finally, at eight months after treatment, T3 Azospirillum brasilense Cd showed a significant effect on the decrease of CLas concentration of almost three times less than T4 with no inductor (Figure 2). T3 chitosan showed a decrease effect on CLas concentration at 2 and 5 months after treatment, but at 8 months after treatment, the CLas concentration in this treatment was statistically similar to that of T4 with no inductor. Regarding the treatments with no inductor (T4), the progress of CLas concentration increased over time but no decrease was observed. At the end of the experiment, the average value of CLas concentration was of 6499 bacterial cells/100 ng of DNA for T4 with no inductor, while that for the three treatments with inductors was of 2253-6463 bacterial cells/100 ng of DNA (Figure 2). The application of chemical or biological inductors is known to boost the systemic or local resistance when a plant is confronted by a phytopathogen and this decreases the disease severity (Walters et al., 2013). In an experiment under field conditions, Hu et al. (2018) demonstrated that when salicylic acid (0.8 g/tree) was directly injected into sweet orange trees (C. sinensis), there was a significant reduction in CLas concentration compared with that of the control treatment injected with water. In this study, applying salicylic acid on leaves reduced the CLas concentration 5 months after treatment; however, 8 months after treatment, even when the concentration continued to decrease, the treatment did not exceed the treatment with Azospirillum in the lemon trees rhizosphere, where significant statistical differences were noted compared to those of the control with no inductor (T4). To this regard, Li et al. (2017) demonstrated that in CLas, the sahA gene encodes the synthesis of the salicylate hydroxylase enzyme that is able to degrade the salicylic acid and its analogous (Li et al., 2017). Li et al. (2017) also demonstrated that when salicylic acid (1 mM) was applied to sweet orange trees, both healthy and infected with CLas, there was an increase in PR gene expression and salicylic acid accumulation. However, the PR expression and the salicylic acid accumulation was significantly lower in trees infected with CLas, while the application of dichloro-isonicotinic and benzothiadiazol (SAR inductors) showed a similar pattern of PR expression among trees healthy and infected with CLas; these molecules are not degraded by the salicylate hydroxylase enzyme (Li et al., 2017). Unlike data reported by Hu et al. (2018), the direct injection of salicylic acid into the tree trunk can overcome degradation caused by the salicylate hydroxylase enzyme. This was demonstrated by an increase of the PR-1 and PR-2 genes expression in orange trees treated with salicylic acid compared to that of the control injected with water. To this regard, Hu et al. (2018) mentioned that the application of salicylic acid has some disadvantages such as degradation and low absorption of salicylic acid by the plant, which could be what occurred in this study.

Figure 1 Inoculation of ʻCandidatus Liberibacter asiaticusʼ (CLas) bacterium in Mexican lemon plants (Citrus aurantifolia) using bud graftings (A and B). Resistance inductors experiments in CLas concentration under greenhouse condi tions (C) and leaves showing the application of inductors solutions (D).
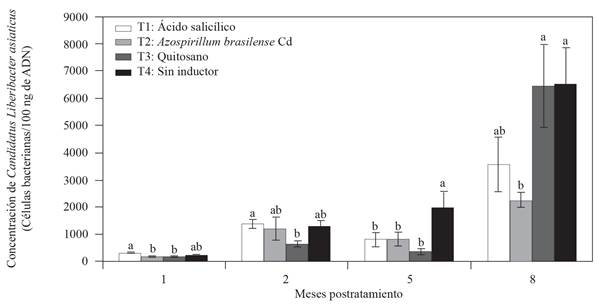
Figure 2 Effect of resistance inductors and Azospirillum brasilense in the ʻCandidatus Liberibacter asiaticusʼ concentration in Mexican lemon tress under greenhouse conditions. The bars in each rectangle indicate the standard error (n= 4 replications); values with the same letter in each rectangle per month are statistically equal (LSD, p≤0.05).
On the other hand, although chitosan has demonstrated to be able to induce plant defense mechanisms, its action mechanism to induce resistance has not been completely elucidated. It also should be noted that the response may vary depending on the pathosystem and other factors (Orzali et al., 2017) such as the type of chitosan and the way the solution is prepared and stored. In this study, the application A. brasilense Cd showed a reduction in the CLas concentration. The induced systemic resistance (ISR) by plant growth-promoting bacteria (PGPB) could be an alternative for controlling HLB. Tang et al. (2018) used healthy navel orange leaves (C. Sinensis)) cv. ʻNewhallʼ to isolate a potential biological control agent for HLB identified as B. amyloliquefaciens GJ1. For tests conducted in the greenhouse navel orange plants infected with HLB were irrigated with 1.5 L of B. amyloliquefaciens GJ1 (OD600 ≈1) solution every week; after seven irrigations, the number of plants infected by the pathogen was reduced by 50% (Tang et al., 2018). It has been demonstrated that the ability to induce plant resistance or tolerance depends on the PGPB (Jain et al., 2014). To this regard, Riera et al. (2018) tested the efficiency of seven bacterial isolates inoculated in ʻDuncanʼ grapefruit trees root to control citrus canker, and found that the Burkholderia territorri A63, B. metallica A53 and Pseudomonas geniculata 95 isolates significantly reduced the disease severity compared to that of the control plants. On the other hand, Zhang et al. (2017) found that when sweet orange cv. ‘Valencia’ trees were inoculated with B. metallica A53 and B. territori A63 bacteria, the relative expression of the PR1, PR2 and SAM genes increased, depending on the bacterium and the time after inoculation. In this context, to continue this study it would be important to evaluate if the reduction in the CLas concentration produced by A. brasilense Cd in Mexican lemon trees is linked to the expression of genes related to the plant defense mechanisms.
In our experiment, some of the pest control products that were applied, when necessary, to all the lemon trees during the first four months before starting the experiment could have contributed to the induction of resistance. Previous studies have demonstrated that some of the components of those products are able to induce resistance, for example, imidacloprid (Francis et al., 2009), some essential oils (Banani et al., 2018) and plant extracts (Fought and Kuć, 1996; Khoa et al., 2017). Despite the above, in our experiment the treatment with no inductor (T4) did not have a significant effect on the reduction of CLas after eight months during which these products were not applied.
In conclusion, the salicylic acid and chitosan evaluated as resistance inductors that were applied to leaves were less effective in reducing the increase rate of the ʻCandidatus Liberibacter asiaticusʼ concentration in Mexican lemon trees. The inoculation of A. brasilense Cd in Mexican lemon trees had a significant effect on the decrease of the CLas concentration under greenhouse conditions. The induction of systemic resistance through PGPB as A. brasilense in citrus could be an alternative for mitigating the effects of HLB and maintain lemon tress productivity for a longer time by slowing the disease progress. We suggest that A. brasilense Cd be inoculated in citrus, under field conditions, in order to evaluate resistance induction through the expression of genes involved in plant defense in infected trees and thus show the reduction in CLas concentration.
Acknowledgments
To the Fondo Mixto of the state of Michoacan and to CONACYT for funding the project “Efecto de distintos bioprotectores en el desarrollo de la enfermedad del HLB en limón mexicano del estado de Michoacán” (MICH-2012-03-193066) (Effect of different bioprotectors on the HLB disease (HuanLongBing) development in Mexican lemon in the state of Michoacán).
REFERENCES
Algam SAE, Xie G, Li B, Yu S, Su T and Larsen J. 2010. Effects of Paenibacillus strains and chitosan on plant growth promotion and control of Ralstonia wilt in tomato. Journal of Plant Pathology 92:593-600. Disponible en línea: http://www.sipav.org/main/jpp/index.php/jpp/article/view/303 [ Links ]
Arratia-Castro AA, Santos-Cervantes ME, Fernández-Herrera E, Chávez-Medina JA, Flores-Zamora GL, Camacho-Beltrán E, Méndez-Lozano J and Leyva-López NE. 2014. Occurrence of ʻCandidatus Phytoplasma asterisʼ in citrus showing Huanglongbing symptoms in Mexico. Crop Protection 62:144-151. http://dx.doi.org/10.1016/j.cropro.2014.04.020 [ Links ]
Banani H, Olivieri L, Santoro K, Garibaldi A, Gullino ML and Spadaro D. 2018. Thyme and savory essential oil efficacy and induction of resistance against Botrytis cinerea through priming of defense responses in apple. Foods 7:11. http://dx.doi.org/10.3390/foods7020011 [ Links ]
Bové JM. 2006. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. Journal of Plant Pathology 88:7-37. Disponible en línea: http://www.sipav.org/main/jpp/index.php/jpp/article/view/828 [ Links ]
Coletta-Filho HD, Carlos EF, Alves KCS, Pereira MAR, Boscariol-Camargo RL, de Souza AA and Machado MA. 2010. In planta multiplication and graft transmission of ‘Candidatus Liberibacter asiaticus’ revealed by Real-Time PCR. European Journal of Plant Pathology 126:53-60. http://dx.doi.org/10.1007/s10658-009-9523-2 [ Links ]
Döbereiner J, Marriel IE and Nery M. 1976. Ecological distribution of Spirillum lipoferum Beijerinck. Canadian Journal of Microbiology 22:1464-1473. http://dx.doi.org/10.1139/m76-217 [ Links ]
Fought L and Kuć JA. 1996. Lack of specificity in plant extracts and chemicals as inducers of systemic resistance in cucumber plants to anthracnose. Journal of Phytopathology 144:1-6. http://dx.doi.org/10.1111/j.1439-0434.1996.tb01479.x [ Links ]
Francis MI, Redondo A, Burns JK and Graham JH. 2009. Soil application of imidacloprid and related SAR-inducing compounds produces effective and persistent control of citrus canker. European Journal of Plant Pathology 124:283-292. http://dx.doi.org/10.1007/s10658-008-9415-x [ Links ]
Gottwald TR, Graham JH, Irey MS, McCollum TG and Wood BW. 2012. Inconsequential effect of nutritional treatments on Huanglongbing control, fruit quality, bacterial titer and disease progress. Crop Protection 36:73-82. http://dx.doi.org/10.1016/j.cropro.2012.01.004 [ Links ]
Hall DG and Gottwald TR. 2011. Pest management practices aimed at curtailing citrus Huanglongbing disease. Outlooks on Pest Management 22:189-192. http://dx.doi.org/10.1564/22aug11 [ Links ]
Hu J, Jiang J and Wang N. 2018. Control of citrus Huanglongbing via trunk injection of plant defense activators and antibiotics. Phytopathology 108:186-195. http://dx.doi.org/10.1094/PHYTO-05-17-0175-R [ Links ]
Jain S, Vaishnav A, Kasotia A, Kumari S and Choudhary DK. 2014. Plant growth-promoting bacteria elicited induced systemic resistance and tolerance in plants. Pp:109-132. In: Ahmad P and Rasool S (eds.). Emerging technologies and management of crop stress tolerance. Academic Press. San Diego, USA. 514p. http://dx.doi.org/10.1016/B978-0-12-800875-1.00005-3 [ Links ]
Khoa NĐ, Xạ TV and Hào LT. 2017. Disease-reducing effects of aqueous leaf extract of Kalanchoe pinnata on rice bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae involve induced resistance. Physiological and Molecular Plant Pathology 100:57-66. http://dx.doi.org/10.1016/j.pmpp.2017.06.005 [ Links ]
Li J, Pang Z, Trivedi P, Zhou X, Ying X, Jia H and Wang N. 2017. ʻCandidatus Liberibacter asiaticusʼ encodes a functional Salicylic Acid (SA) Hydroxylase that degrades SA to suppress plant defenses. Molecular Plant-Microbe Interactions 30:620-630. http://dx.doi.org/10.1094/MPMI-12-16-0257-R [ Links ]
Li J, Trivedi P and Wang N. 2016. Field evaluation of plant defense inducers for the control of citrus Huanglongbing. Phytopathology 106:37-46. http://dx.doi.org/10.1094/PHYTO-08-15-0196-R [ Links ]
Lin H, Chen C, Doddapaneni H, Duan Y, Civerolo EL, Bai X and Zhao X. 2010. A new diagnostic system for ultra-sensitive and specific detection and quantification of Candidatus Liberibacter asiaticus, the bacterium associated with citrus Huanglongbing. Journal of Microbiological Methods 81:17-25. http://dx.doi.org/10.1016/j.mimet.2010.01.014 [ Links ]
Orzali L, Corsi B, Forni C and Riccioni L. 2017. Chitosan in agriculture: A new challenge for managing plant disease. Pp:17-36. In: Shalaby E (ed.). Biological activities and application of marine polysaccharides. IntechOpen. Rijeka, Croatia. 318p. http://dx.doi.org/10.5772/66840 [ Links ]
Riera N, Wang H, Li Y, Li J, Pelz-Stelinski K and Wang N. 2018. Induced systemic resistance against citrus canker disease by rhizobacteria. Phytopathology 108:1038-1045. http://dx.doi.org/10.1094/PHYTO-07-17-0244-R [ Links ]
Robles-González MM, Velázquez-Monreal JJ, Manzanilla-Ramirez MA, Orozco-Santos M, Medina-Urrutia VM, López-Arroyo JI and Flores-Virgen R. 2013. Huanglongbing (HLB) disease in mexican lime trees [Citrus aurantifolia (Christm) Swingle] and its dispersion in Colima state, México. Revista Chapingo Serie Horticultura 19:15-31. http://dx.doi.org/10.5154/r.rchsh.2012.01.005 [ Links ]
SIAP (Servicio de Información Agroalimentaria y Pesquera). 2018. Atlas agroalimentario 2012-2018, 1ª Edición. México, Distrito Federal. https://nube.siap.gob.mx/gobmx_publicaciones_siap/pag/2018/Atlas-Agroalimentario-2018 (consulta, marzo 2019). [ Links ]
StatPoint Inc. 2005. StatGraphics Centurion XV version 15.02.06. Warrenton, Virginia, USA. http://www.statgraphics.com. [ Links ]
Tang J, Ding Y, Nan J, Yang X, Sun L, Zhao X and Jiang L. 2018. Transcriptome sequencing and ITRAQ reveal the detoxification mechanism of Bacillus GJ1, a potential biocontrol agent for Huanglongbing. PLoS ONE 13:e0200427. http://dx.doi.org/10.1371/journal.pone.0200427 [ Links ]
Walters DR, Ratsep J and Havis ND. 2013. Controlling crop diseases using induced resistance: challenges for the future. Journal of Experimental Botany 64:1263-1280. http://dx.doi.org/10.1093/jxb/ert026 [ Links ]
Wang N and Trivedi P. 2013. Citrus Huanglongbing: A newly relevant disease presents unprecedented challenges. Phytopathology 103:652-665. http://dx.doi.org/10.1094/PHYTO-12-12-0331-RVW [ Links ]
Xing K, Zhu X, Peng X and Qin S. 2015. Chitosan antimicrobial and eliciting properties for pest control in agriculture: a review. Agronomy for Sustainable Development 35:569-588. http://dx.doi.org/10.1007/s13593-014-0252-3 [ Links ]
Zhang MQ, Powell CA, Zhou LJ, He ZL, Stover E and Duan YP. 2011. Chemical compounds effective against the citrus Huanglongbing bacterium ʻCandidatus Liberibacter asiaticusʼ in planta. Phytopathology 101:1097-1103. http://dx.doi.org/10.1094/PHYTO-09-10-0262 [ Links ]
Zhang YP, Uyemoto JK and Kirkpatrick BC. 1998. A small-scale procedure for extracting nucleic acids from woody plants infected with various phytopathogens for PCR assay. Journal of Virological Methods 71:45-50. http://dx.doi.org/10.1016/S0166-0934 (97)00190-0 [ Links ]
Zhang Y, Xu J, Riera N, Jin T, Li J and Wang N. 2017. Huanglongbing impairs the rhizosphere-to-rhizoplane enrichment process of the citrus root-associated microbiome. Microbiome 5:97. http://dx.doi.org/10.1186/s40168-017-0304-4 [ Links ]
Received: January 11, 2019; Accepted: March 27, 2019











 texto em
texto em 


