Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.35 no.3 Texcoco Set. 2017
https://doi.org/10.18781/r.mex.fit.1609-6
Phytopathological notes
In vitro evaluation of extracts from the Lilium genus to control Fusarium oxysporum
1Instituto de Horticultura, Universidad Autónoma Chapingo, km 38.5 Carretera México-Texcoco, Chapingo, Estado de México, 56230, México.
2Departamento de Fitotecnia, Universidad Autónoma Chapingo, km 38.5 Carretera México-Texcoco, Chapingo, Estado de México, 56230, México.
3Departamento de Biotecnología, Universidad Politécnica de Pachuca, km 20 Carretera Pachuca-Ciudad Sahagún, Ex Hacienda Santa Bárbara, Zempoala, Hidalgo, 43830, México.
4Departamento de Fitotecnia, Universidad Autónoma Chapingo. km 38.5 Carretera México-Texcoco, Chapingo, Estado de México, 56230, México.
The effect of antifungal plant extracts of bulbs of Lilium hybrid L/A “Indian summer” were evaluated on Fusarium oxysporum, which is considered a pathogenic fungus that causes serious damage to agriculture and economic losses to various sectors of industry. The crude extract was fractionated into three sub-extracts according to their polarity (polar, moderately polar and non-polar), and then subject to bioassays of inhibition being potato-dextrose-agar (PDA) the control. The moderately polar extract showed the highest inhibition, decreasing the growth rate by 27 % compared to the mycelial growth in the control. However, when it was compared to plant extracts reported under similar conditions, it was discarded as a growth inhibitor and considered as fungistatic.
Key words: fungicide; fungistatic; phenols
Se evaluó el efecto antifúngico de extractos de bulbos de Lilium hibrido L/A “Indian summer” sobre Fusarium oxysporum considerado como hongo patógeno que provoca daños severos en la agricultura y pérdidas económicas en diversos sectores de la industria. El extracto crudo se fraccionó en tres sub-extractos de acuerdo a la polaridad (polar, medianamente polar y no polar), los cuales se sometieron a bioensayos de inhibición siendo papa-dextrosa-agar (PDA) el tratamiento testigo. Las muestras que se trataron con el extracto medianamente polar presentaron la mayor inhibición, al disminuir la tasa de crecimiento en un 27 %, en comparación al crecimiento micelial del testigo. Sin embargo, al comparar con extractos vegetales que reportaron bajo condiciones similares, se descarta como inhibidor del crecimiento y se considera como fungistático.
Palabras clave: fungicida; fungistático; fenoles
Lilium is an ornamental crop of major importance in the market worldwide and ranked 11th in demand and 2nd among bulbous plants in Mexico (Gómez, 2009). This is mainly attributed to its flower color diversity, which has been possible through hybridization among Asiatic and Eastern species, as well as the availability of flowers almost all the year round through intensive production systems (Álvarez et al., 2008).
These practices lead growers to implement pest and disease control programs that do not succeed in eradicating them, but do help decrease the high rate of pathogen development. However, those programs do not take into consideration the consequences of the indiscriminate use of chemical fertilizers or pesticides that directly affect natural resources as soil, air and water (Ortega et al., 2006). For this reason, it is convenient to establish pathogen control programs in time and seek environment-friendly alternatives which, aside from cutting costs, help prevent pollution caused by the use of chemical agents that induce resistance in microorganisms (Keng et al., 2010; Bhromsiri and Bhromsiri, 2010). That is the case of plant extracts, essential oils, organic and enzymatic extracts that mark the beginning of pathogens control in intensive agriculture because they have proven to be effective at controlling the growth of bacteria, nematodes and phytopathogens isolated from a wide variety of crops (Rodríguez et al., 2012; Alcalá de Marcano et al., 2005; Chávez and Aquino, 2012; Vinueza et al., 2006). In the specific case of Lilium, bulbs are disposed after the crop cycle ends, so a way of reusing them could be extracting active compounds for the control of Fusarium oxysporum fungus, since mycosis causes loss of tissue turgence and fruit rot, stem end or root rot, minor infections, and eventually the plant dies. In summary, these are the problems that growers face every year.
The objective of this study was to determine the antifungal activity of extracts of Lilium bulbs collected after flower harvest as a way of reusing an organ considered a residue at the end of the production cycle and which is not properly managed and could be a source of contamination. The isolate used was Fusarium oxysporum kept in glycerol and nutrient agar that was obtained from soils allocated to experiment crops by the Parasitology Department of Universidad Autónoma Chapingo. Lilium bulbs were taken from a single lot imported from Dijkweg, Honselersddijk, The Netherlands, by the company Van den Bos Flowerbulbs B.V. The lot consisted of 300 size 14/16 bulbs of the Lilium genus L/A hybrids Indian Summer variety. The bulbs were first washed with running water to remove impurities and damaged septa, then with distilled water and disinfected with 1.5 % sodium chloride for 5 min. Finally, they were rinsed in deionized water. Fifty g of bulbs were weighed and macerated with a minimum of deionized water (5 mL) in a disinfected mortar. The extract obtained was filtered with a cotton cloth and then with filter paper (Papel Whatman®); this procedure was repeated three times. The filtered extracts were placed in sterile vials and kept at 4 °C until they were used. For morphological characterization, Fusarium oxysporum was sown in Petri dishes filled with agar-potato-dextrose (PDA), according to BD Bioxon® directions. Each of the developed colonies were isolated and purified using the hypha-point method. Considering 10 sampling units (10 Petri dishes containing axenic culture), a macroscopic-morphological characterization was performed and permanent preparations were made and observed under a microscope. Atypical structures were observed whose length and width were measured in each unit. Their genus was confirmed according to the Barnett and Hunter procedure (1998). The incubation conditions for the extract were as follows: after obtaining the raw plant extract (EVC), it was split according to the polarity affinity of the compounds analyzed by thin-layer chromatography (CCP) using 25-mm-thick silica gel plates GF 254, and run in 100 % eluent hexane, 100 % ethyl acetate and 100 % ethanol. Later, when defining the eluents to split the plant extracts, the non-polar (ENP) extract was obtained using the 100 % ethyl acetate eluent at a 1:1 ratio, the polar extract (EMP) using the 100 % ethyl acetate eluent at a 1:1 ratio, and the polar extract (EVP) using 100 % ethanol at a 1:1 ratio.
The first step in molecular identification was DNA extraction, which was performed with 30 to 50 mg of mycelium. The procedure was repeated using 3 samples of the fungus with the oligos ITS-1 5’-tccgtaggtgaacctgcgg-3’ and ITS-4 5’-tcctccgcttattgatatgc-3’ (White et al., 1990) that amplify an internal transcribed spacer (ITS) and yields a product of variable size that contains approximately 800 to 1200 base pairs (pb). This task was performed with a mixture of reaction in a 25 µL final volume whose final components were: reaction buffer (1X, MgCl2 2mM, dNTP’s), 200 nM of each, 20 pmoles of each oligonucleotide, and 1 unit of Taq DNA polymerase. The thermal program involves keeping 2 min at 94 °C, 30 seg at 55 °C and 1 min at 72 °C (35 cycles). The products resulting from the PCR reaction were separated by electrophoresis in 1.5% agarose gels, the PCR bands were observed in an UV Transilluminator (UVP brand) and pictures were taken. The amplified fragments were directly sequenced and the results compared with the sequences stored in the (GenBank) Centro Nacional de Información Biotecnológica (NCBI).
For pathogenicity tests, 7 bulb flakes of the Lilium hybrid L/A Indian summer variety were used. They were disinfected with 1.5 % sodium hypochlorite for 5 min and then rinsed with deionized water. Each flake was considered as an experiment unit in a complete randomized design. The isolate that was previously isolated was sown in PDA medium for 8 days for inoculum increase, and then a suspension of conidia was prepared in sterile distilled water, adjusted to a concentration of 80x103 propagules mL-1 as stated by Díaz et al. (2014), and the flakes were spread with spore suspension (approximately 2mL in volume). As control we used the flake that was rinsed only with deionized water. The 7 experiment units were placed in 8 previously sterilized Petri dishes and incubated at 5 at 26 °C ± 2 °C. Symptoms were recorded daily for 15 days. From the areas affected by mycelial invasion, tissue was taken, disinfected with 1.5 % sodium hypochlorite for 2 min and rinsed twice with deionized water. Then, the samples were sown in Petri dishes containing PDA medium. The concentration of the plant extract in the medium was 30 %, incubation period: 23 h, incubation temperature: 25 °C +/-1.
The morphological-macroscopic features (color, shape and colony spreading) and microscopic (hypha, sclerotium, conidium, metula, phialid, etc.) were compared with those originally inoculated to prove Koch postulates. For repellency bioassays (antibiograms), 20 mL of plant extract were previously added for analysis along with 100 mL of PDA for sterilization; then it was distributed in Petri dishes 100 X 155 mm in diameter (Araujo et al., 2007). When the culture medium solidified, sowings were made in the middle of each plaque using a 5x5 mm sample of F. oxysporum that was previously reactivated in liquid medium (nutrient broth) for 48 h and then transferred to solid medium (PDA) for the next 48 h. Mycelial growth was measured every 24 h at the four quadrant directions of the Petri dishes and the diameter of the inhibition halo was measured (in mm) until complete development was observed in the Petri dish containing the control (without plant extract). We carried out an analysis of the data using variance analysis (ANAVA). Similarly, a median comparison test with 5 % significance level was performed according to Tukey’s method. All the statistical analyses were performed using the Statistical Analysis System software.
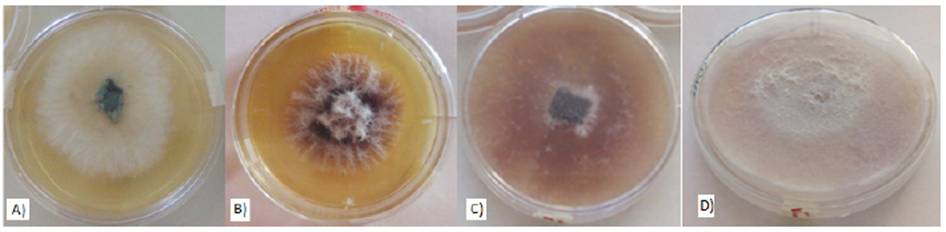
In their macroscopic morphology, the Fusarium oxysporum colonies isolated showed whitish and cottony shapes during the first 72 h (Figure 1a) that started to appear when they were sown and extended as new hyphae developed. After 144 h, we observed a pinkish-purple color that grew in intensity when the colonies started to grow (Figure 1b) and became lighter with lilac-pinkish tones towards the edges. The purple dark pigmentation was uniform after 312 h (Figure 1c and Figure 1d). The mature isolate produced branched hyphae 3-4.5µm in diameter, single and short phialides, abundant cylindrical micronidia and thin macronidia with a slightly curve and apical cell (Figure 1d).
In the molecular characterization, the size of the isolate identified as Fusarium was of 1040 pb, which agrees with the size expected for this species. The obtained sequence was 99 % similar to the ITS region whose alignment was in agreement with F. oxysporum CM000589.1.
The pathogenicity test confirmed the ability of the available isolate to develop typical fusariosis symptoms in septa of Lilium hybrids L/A bulbs. After 96 h, we observed the first signs of infection on the septum’s meristematic tissue as yellowish-brownish spots (Figure 2). After 144 h, the damaged area showed degraded tissue, and from these areas an aliquot was taken to be resown in Petri dishes and confirm the presence of Fusarium oxysporum, according to Koch’s postulates.
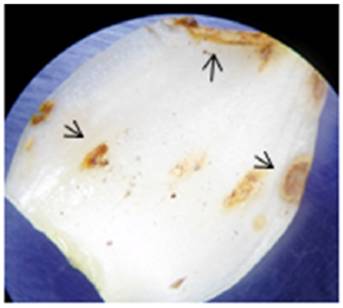
Figure 2 Lilium L/A septo after 96 h under induced infection by a Fusarium oxysporum axenic culture. Arrows show tissue damage.
As for inhibition bioassays, the effect of the plant extracts on Fusarium sp. mycelial growth in vitro is shown in Table 1.
Table 1 Median comparison of Fusarium oxysporum mycelial growth in different mediums to which plant extracts at 30% were added.
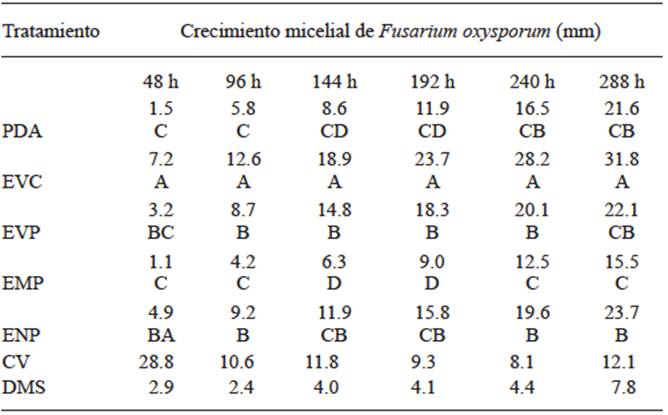
* medians with different letter in each column denote significant difference at 5 % probability.
There is a significant difference among the treatments being the row plant extract (EVC) the statistically higher treatment at 48 h with an increase of 79% compared with the PDA treatment, since it acted as mycelial growth stimulator. In the case of the intermediately polar extract (EMP), there was a statistical difference lower than the difference in the control with a decrease of 27% and a low inhibitory effect. At 96 h, EVC decreased the mycelial growth ratio, but it was kept as the treatment with a higher statistical difference with an increase of 54% compared with the control. On the other hand, EMP presented a lower statistical difference with a decrease of 28% compared with the mycelial growth of the control, which was greater at 48 h and increased its inhibitory effect. At 144 h, EVC was statistically higher with an increase of 67 % compared with the control and recovered its ability to stimulate growth that has shown 48 h earlier. However, EMP was statistically lower with a decrease of 27 % on the control’s mycelial growth and maintained its inhibitory activity. At 192 h, EVC continued to be the highest but different from the control with an increase of 50 %. It should be noted that the growth stimulating activity decreases but the inhibitory effect of the EMP treatment is continuous and presents a statistically lower difference than that of the PDA treatment with a decrease of 26 %. At 240 h, EVC remains as the statistically highest treatment with a growth median of 28.167 mm but shows a difference of 41 % where the lowest kinetics of mycelial growth was obtained. On the other hand, EMP is 25 % statistically lower than the control but maintains its inhibitory activity 10 days after inoculation. At 288 h, EVC resumes its activity to stimulate growth but there is no significant increase than that at 192 h, and shows a statistically higher difference of 51 % in growth compared with the control. In the case of EMP, increased inhibitory activity was observed, being statistically different from the control with an increase of 28 %. Chávez and Aquino (2012) state that plant extracts of pirii (Cyperus spp.) showed a low inhibitory effect in Fusarium sp. using 20 % concentrations. However, the authors mention that garlic extract completely inhibited Fusarium sp. mycelial growth. This result is in agreement with López et al. (2005) statement, who reported that at 5 and 10 % concentrations, garlic inhibits 68.5 and 69.8 % of Fusarium oxysporum f. sp. lycopersici growth, respectively. For this reason, it is assumed that the higher the concentration of plant extracts, the higher the inhibitory effect. Nettle extract (Urtica dioica) showed a low inhibitory effect in Fusarium sp. (Chávez and Aquino, 2012) in contrast with the effects observed on tests using Colletotrichum gloeosporioides, in which 100 % growth inhibition was observed when aqueous and alcoholic extracts of such plant were used. Based on the favorable response of EMP, we characterized and determined the organic structure of the extract using gas chromatography with a universal column and obtained the chromatogram shown in Figure 3.
In the plant extract (EMP), we found hexadecanoic acid methyl ester (Palmitic acid) and octadecadienoic acid 9,16 methyl ester (Table 2) called Omega 6 that are compounds known to have the capacity to capture free radicals and anti-inflammatory, antiallergic, antithrombotic, antimicrobial and antineoplastic activity (Kuskoski et al., 2005).
Table 2 Compounds obtained within the retention periods shown in the intermediate polar extract chromatogram.
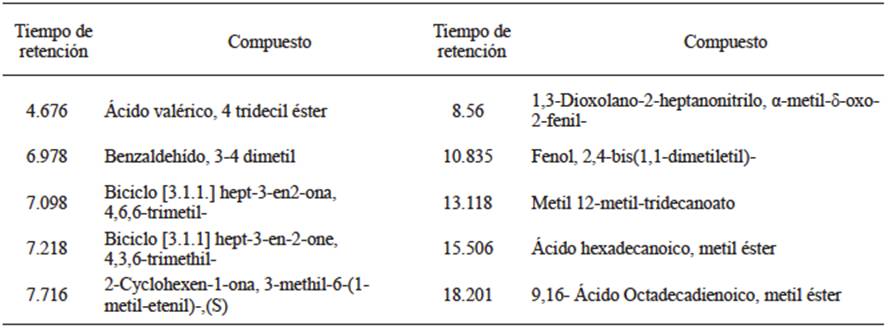
The presence of phenolic compounds such as 2,4-diphenol 1,1-dimethyl, ethyl and 1,3 dioxolane 2 heptanenitrile α-methyl δ-oxo- 2- phenyl (precursor of thymol, and carvacrol and eugenol intermediary) suggest fungal activity of the plant extracts, which is favored by the acid nature of their hydroxyl group that creates a hydrogen bridge with an active enzymatic site (Kalemba and Kunicka, 2003). On the other hand, the biosynthesis of these molecules is taken either constitutively, pathogen-independent (phytoanticipins), or, if induced as part of the defense mechanism of plants against infections caused by bacteria, fungi or nematodes (phytoalexins). To this group belong flavonoids, isoflavones, aurones and phenalenes. (Tanaka et al., 2002. Kalemba and Kunicka (2003) suggest that plant extracts and essential oils with phenol as the main component express the highest spectrum of antimicrobial activity.
The Fusarium oxysporum white fungal species was confirmed with the sequence key CM000589.1 stored in the gene bank in NCBI and based on DNA and PCR extraction protocols. The most abundant compounds (3-4 benzaldehyde dimethyl, 1,3-dioxolane-2-heptanenitrile, α-methyli-δ-oxo-2- phenyl and 9,16- octadecadienoic acid, methyl ester) in the intermediately polar plant extract (EMP) have shown fungal activity in crops of interest and are potential components for further studies. The raw plant extract of Lilium hybrid L/A “Indian summer” bulbs presented stimulating activity in Fusarium oxysporum mycelial growth. The intermediate polar extract (EMP) of the bulbs presented low inhibitory activity and it is considered useful as a Fusarium oxysporum fungistatic.
LITERATURA CITADA
Alcalá de Marcano D, Vargas N, Pire A. 2005. Efecto de extractos vegetales y fungicidas sintéticos sobre el crecimiento micelial in vitro de Sclerotium rolfsii y Thielaviopsis basicola. Revista de la Facultad de Agronomía 22:315-323. http://revfacagronluz.org.ve/PDF/octubre-diciembre2005/d-alcala.pdf [ Links ]
Álvarez SME, Maldonado TR, García MR, Almaguer VG, Rupit AJ, Zavala EF. 2008. Suministro de calcio en el desarrollo y nutrición de Lilium asiático. Agrociencia 42:881-889. http://www.colpos.mx/agrocien/Bimestral/2008/nov-dic/art-3.pdf [ Links ]
Araujo D, Rodríguez D, Sanabria ME. 2007. Respuesta del hongo Fusarium oxysporum f sp. cubense causante del mal de Panamá, en algunos extractos vegetales y funguicidas. Fitopatología Venezolana 21:2-8. http://www.sovefit.com.ve/boletines/21-1/documento1.pdf [ Links ]
Barnett, H.L., Hunter, B.B. (1998). Ilustrated genera imperfect fungi. 4 ed. The American Phythopatological. Society Minnesota, USA. 218 p. [ Links ]
Bhromsiri C, Bhromsiri A. 2010. The effect of plant growth promoting Rhizobacteria and Arbuscular mycorhizal fungi on the growth, development and nutrient uptake on different vetiver ecotypes. Thai Journal of Agricultural Science 43:239-249. http://www.thaiagj.org/images/stories/Journal_online/2010/43-4/06-TJ-AGR-1010-49.pdf [ Links ]
Chávez AR, Aquino AS. 2012. Control de los hongos Rhizoctonia sp., Fusarium sp y Sclerotium sp con extractos vegetales. Investigación Agraria 14:17-23. http://www.agr.una.py/revista/index.php/ria/article/view/242/228 [ Links ]
Díaz JF, Vargas M, Ayvar S, Alvarado OG, Solís JF, Durán JA, Díaz HL, Hernández A. 2014. Morphological and PCR identification of Rhizoctonia solani Kühn isolated from pipiana pumpkin fruits and greenhouse management. Biotecnia 16:17-21. DOI: 10.18633/bt.v16i3.107 [ Links ]
Gómez AA. 2009. La situación de las flores de corte mexicanas dentro de la política comercial internacional de México Tecsis-Tecatl 2:1-30 http://www.eumed.net/rev/tecsistecatl/n9/aagg.htm [ Links ]
Kalemba DF, Kunicka A. 2003. Antibacterial and antifungal properties of essential oils. Current Medicinal Chemistry 10:813-829. DOI: 10.2174/0929867033457719 [ Links ]
Keng HC, Rung YW, Keng CC, Ting FH, Ren SC. 2010. Effects of chemical and organic fertilizer on the growth, flower quality and nutrient uptake of Anthurium andreanum, cultivated for cut flower production. Scientia Horticulturae 125:434441 DOI: 10.1016/j.scienta.2010.04.011 [ Links ]
López BA, López BSR, Vásquez BME, Rodríguez HSA, Mendoza EM, Padron CE. 2005. Inhibición del crecimiento micelial de Fusarium oxysporum schlechtend f. sp. lycopersici (Sacc.) Snyder y Hansen, Rhizoctonia solani Kunh y Verticillium dahliae Kleb. Mediante extractos vegetales acuosos. Revista Mexicana de Fitopatología 23:183-190. http://www.redalyc.org/pdf/612/61223212.pdf [ Links ]
Ortega BR, Correa BM, Olate ME. 2006. Determinación delas curvas de acumulación de nutrientes en tres cultivares de Lilium spp. para flor de corte. Agrociencia 40:77-88. http://www.colpos.mx/agrocien/Bimestral/2006/ene-feb/art-8.pdf [ Links ]
Rodríguez PAT, Ramírez AMA, Bautista BS, Cruz TA, Rivero D. 2012. Antifungal activity of Acacia farnesiana extracts on the in vitro growth of Fusarium oxysporum f. sp. lycopersici. Revista Científica UDO Agrícola 12:91-96. http://udoagricola.udo.edu.ve/V12N1UDOAg/V12N1Rodriguez91.htm [ Links ]
Tanaka H, Sato M, Fujiwara S, Hirata M, Etoh H, Takeuchi H. 2002. Antibacterial activity of isoflavonoids isolated from Erythrina variegate against methicillin-resistant Staphylococcus aureus. Letters in Applie Microbiology 35:494-498. DOI: 10.1016/j.1472-765x.2002.01222.x [ Links ]
Vinueza PSM, Crozzoli R, Perichi G. 2006. Evaluación in vitro de extractos acuosos de plantas para control de nematodo agallador Meloidogyne incognita. Fitopatología Venezolana 19:26-31. [ Links ]
White, T.J., Bruns, T., Lee, S. y Taylor, J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenies. In: M.A., Inns, D.H., Gelfland, J.J., Sninsky, and T.J., White (eds.). PCR protocols. Pp 315-322. Academic Press. San Diego, CA. [ Links ]
Received: September 26, 2016; Accepted: April 11, 2017











 texto em
texto em