Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.52 no.8 Texcoco Nov./Dez. 2018
Crop science
Vegetable growth promoter microorganisms with agricultural plaster on potatoes (Solanum tuberosum L.) under shadow housing
1Instituto Tecnológico de Sonora. 85000. Calle 5 de febrero 818 sur, Colonia Centro, Cajeme Sonora.
2Instituto Nacional de Investigación Forestal Agrícola y Pecuaria. 85000. Calle Dr. Norman E. Borlaug Km. 12, Colonia Valle del Yaqui, Cajeme, Sonora.
As an alternative to the excessive use of synthetic fertilizers, farmers use microorganisms that promote growth in plants, to increase roots growth, strengthen natural mechanisms that react to diseases and insects and increase production. The objective of this study was to evaluate the potential of agricultural plaster combined with Bacillus cereus, B. subtilis, Pseudomonas fluorescens and Trichoderma harzianum as plant growth promoter. Its soil application will allow selecting the most productive combination, with higher quality and crop yield. The design was completely random, with three treatments applied to potatoes cultivation (Solanum tuberosum L.) in shaded house conditions and drip irrigation. The treatments were: control (T1), T. harzianum combined with 40 kg ha-1 calcium (T2), B. cereus, B. subtilis, P. fluorescens and T. harzianum combined with 40 kg ha-1 calcium (T3); the source of calcium in T2 and T3 was agricultural gypsum. The evaluated variables were plant height, chlorophyll content, numbers and nutritional composition of leaves, weight and volumetric weight of the tuber. The viability of the microorganisms in the rhizosphere was determined with selective agars for each type of microorganism. There were no statistical differences regard on chlorophyll between treatments; the control significantly exceeded in height compared to the plants from T2 to T4; the number of tubers (51.5 %) and yield (49.4 %) from T3 was significantly higher than T1. At the biocontrol in the tuber for Streptomyces T3 was better (9 %) than T1 (32.14 %). T3 had a higher yield and better standard quality.
Keywords: Solanum tuberosum L.; productivity; microbial consortium; biofertilizer
Como alternativa al uso excesivo de fertilizantes sintéticos, los agricultores adoptan el uso de microorganismos promotores de crecimiento en plantas, para potenciar el crecimiento de raíces, fortalecer mecanismos naturales de reacción a enfermedades e insectos y aumentar la producción. El objetivo de este estudio fue evaluar el potencial del yeso agrícola en combinación con Bacillus cereus, B. subtilis, Pseudomonas fluorescens y Trichoderma harzianum como promotor de crecimiento vegetal. Su aplicación al suelo permitirá seleccionar la combinación más productiva, con calidad y rendimiento mayores del cultivo. El diseño fue totalmente al azar, con tres tratamientos aplicados al cultivo de papa (Solanum tuberosum L.) en condiciones de casa sombra y riego por goteo. Los tratamientos fueron: testigo (T1), Trichoderma harzianum combinado con 40 kg ha-1 calcio (T2), B. cereus, B. subtilis, Pseudomonas fluorescens y Trichoderma harzianum combinados con 40 kg ha-1 calcio (T3); la fuente de calcio en T2 y T3 fue yeso agrícola. Las variables evaluadas fueron altura de planta, contenido de clorofila, composición nutrimental de hoja y número, peso y peso volumétrico del tubérculo. La viabilidad de los microorganismos en la rizósfera se determinó con agar selectivo para cada tipo de microorganismo. En el clorofila no hubo diferencias estadísticas entre los tratamientos; el testigo superó significativamente en altura a las plantas de T2 a T4; el número de tubérculos (51.5 %) y rendimiento (49.4 %) de T3 fue significativamente mayor que T1. En el biocontrol en tubérculo contra Streptomyces T3 fue mejor (9%) que T1 (32.14 %). T3 tuvo rendimiento mayor y calidad estándar mejor.
Palabras claves: Solanum tuberosum L.; productividad; consorcio microbiano; biofertilizante
Introduction
Conventional agriculture developed in recent decades has characterized by the intensive use of fertilizers and pesticides to increase agricultural production. Such activities degrade soils and alter their physical, chemical and biological properties because most of them are highly toxic, thus altering microbial communities and pollute soils, surface, and underground water (Jiménez, 2011).
The current alternative to optimize crops is biological products or biofertilizers. The addition into productive systems of selected organisms for their functions in various biological processes. Among the most valuable elements in the production of these biofertilizers are microorganisms that promote plant growth, known as PGPM (Plant Growth-Promoting Microorganism), isolated from diverse environments, with the potential ability to positively affect plants growth (Elein et al., 2005; Bashan et al., 2014).
Pseudomonas fluorescens is among the most used PGPM, which stimulates plant growth through antibiotics production, thereby preventing plant diseases by other pathogenic bacteria and fungi. It will also accelerate seeds germination and plant growth by the synthesis of hormones, such as auxins, gibberellins and cytokinins, and other substances such as amino acids and specific growth promoters (Uribe et al., 1999).
Some bacteria are versatile and present several mechanisms, such as Bacillus subtilis, which produces auxins that promote tomato growth and induce systemic resistance to Fusarium oxysporum; this causes wilting and roots rotting (Gupta et al., 2000).
Different species of Trichoderma are used to control pathogenic fungi in the soil, mainly from the Phytophthora, Rhizoctonia, Sclerotium, Colletotrichum, Pythium and Fusarium genera, and also have a growth promoting effect due to the production of phytohormones and phosphates solubilization (Cubillos-Hinojosa, 2009).
Certain microorganisms, typically from the rhizosphere, favor root development, atmospheric N fixation, soil P solubilization, production of organic acids and secondary metabolites that act similarly to phytohormones, so they directly influence nutrients availability and the stimulation of plant growth. This is the case of Pseudomonas spp. and Trichoderma spp. (Puente et al., 2010, Cano, 2011). Biofertilizers based on these microorganisms notably minimize the environmental impact produced by chemical fertilizers and improve crop yield; therefore, they limit the use of toxic products (Hernandez-Leal et al., 2011; Patiño-Flores and Sanclemente-Reyes, 2014).
Soil quality is defined by its ability to maintain a natural or modified ecosystem, sustain plant and animal productivity, or improve water and air quality, and contribute to human health and habitability. Microbial processes that occur in it influence the quality of the soil; therefore, the permanence of microbial community structure can be an indicator of the degradation or soil impoverishment (Abril, 2003).The microorganisms express a variety of functions and biochemical versatility, which includes oxidation, reduction, and precipitation of soil elements (Atlas, 1984).
One of the Ca source currently used due to its low cost and abundance is the agricultural gypsum (Ca2SO4). This is recommended as fertilizer (source of Ca and S), an amendment that facilitates the displacement of Na from the exchange sites, as an enhancer of physical impediments (crusting or compaction), and a temporary acidulant, which favor the development of microorganisms (Gambaudo, 2006). In the first case it contributes S and Ca; in the second case, it facilitates the displacement of the Na from the exchange sites and improves physical impediments (crusting and compaction) (Gambaudo, 2006).
The need for mechanisms that increase field productivity has driven the search for alternative methods to chemical control for agricultural diseases, with less environmental, human and health risks. This is now a great challenge for agriculture and its development. The objective of this study was to evaluate the potential of the agricultural gypsum, combined with growth promoting microorganisms (B. cereus, B. subtilis, Pseudomonas fluorescens and Trichoderma harzianum) applied to soil, by means of microbiological, nutrimental analysis and physiological and yield variables in potatoes.
Materials and methods
The experimental treatments took place at the Experimental Center and Technology Transfer (CETT-910), from the Instituto Tecnológico de Sonora (Block 910 of the Yaqui Valley, Sonora, Mexico). The experimental design was completely randomized with three treatments, a useful experimental area of 2 m2 and 10 repetitions per treatment (chosen at random). The experimental units were 30 (Table 1). The means comparison or multiple range test was evaluated via the LSD method (p≤0.05), with the Statgraphics Centurión XVI software.
Table 1 Treatments evaluated with microorganisms that promote plant growth combined with agricultural gypsum in the cultivation of potatoes (Solanum tuberosum L.) in shaded greenhouse.
| Tratamiento | Descripción |
| T1 | Testigo |
| T2 | 40 kg ha-1 de Ca + Trichoderma harzianum |
| T3 | 40 kg ha-1 de Ca + (Bacillus subtilis, Bacillus cereus, Pseudomonas fluorescens, Trichoderma harzianum) |
The sowing was done with seed-tubers, of the Atlantic variety, in a five tubers per linear meter density, in beds separated 1.50 m and length of 30 m, in shade house. The plants were fertilized with 250-100-250 N: P: K, with urea, potassium nitrate and agricultural phosphoric acid; and weekly irrigated by dripping.
The treatments included a 108 CFU mL-1 per m2 mixed with 0.5 kg application of agricultural gypsum (equivalent to 40 kg of Ca per ha), these were applied to 150 m2 of the crop, in six doses, from the emergence, every 15 d via the irrigation system.
Evaluated physiological and performance variables
Plant height
The height (cm) of the plants was weekly measured with a flexometer, from the base of the stem to the apex, starting at the first application of the treatments.
Chlorophyll content index
The chlorophyll content index was measured weekly on the physiologically mature leaf, from 11 am to 2 pm, with Spad 502 (Minolta), and was reported in chlorophyll units (UC).
Yield and number of tubers
The total yield was the weight of the tubers per treatment then extrapolated to t ha-1. The number of tubers represented the experimental units per treatment.
Incidence of Fusarium and Streptomyces
The incidence of two of the most common pathogenic microorganisms was determined. Their identification was through the damage they produced, following the quality manual “Sampling and analysis of potatoes in the field” (PEPSICO Alimentos México, 2014). All tubers from the experimental units were inspected; the infected and healthy ratio was obtained and reported as an incidence percentage.
Leaves nutrimental analysis
The macronutrients (N, P, K, Ca and Mg) and micronutrients (Mn, Zn, Fe and Cu) concentrations were determined in a composite plant tissue sample, at flowering, with a spectrophotometer (DR2100) following the HACH method (Alcántar and Sandoval, 1999) with modifications adjusted to the nature of the samples.
Rhizosphere microbiology
The viable counts were made at the beginning, in the middle and at the end of the cultivation cycle. A sample of each experimental unit was used to form a composite sample by treatment. The 10-4, 10-5 and 10-6 dilution technique was used for plate casting, by triplicate, in media for B. subtilis and B. cereus (mannitol-egg yolk-polymyxin agar, MYP), T. harzianum (potato dextrose agar) and P. fluorescens (isolation agar of Pseudomona F); for bacteria were kept at 30 °C and for fungi at 25 °C. The count was made after 24-48 h and 120 h, respectively, with a manual colony counter; the results were reported as CFU g-1 of soil (Pepper and Gerba, 2004).
Quality test of potato frying
The frying quality was evaluated with the AGROBO laboratory method based on the potato quality sampling manual and field analysis (PEPSICO Alimentos México, 2014).
Results and discussion
Height
The height in T1, except of the first week, was higher than T2 and exceeded T3 only in week 5 (Table 2).
Table 2 Effect of microorganisms and agricultural gypsum on the height (cm) of potato plants (Solanum tuberosum L.).
| Tratamientos | †S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 |
| Testigo | 19a | 43b | 61b | 76b | 87b | 86b | 86b | 86b | 93b |
| 40 kg ha-1 de Ca Trichoderma harzianum | 12a | 31a | 45a | 56a | 71a | 72a | 73a | 70a | 73a |
| 40 kg ha-1 de Ca + (Bacillus subtilis, Bacillus cereus, Pseudomonas fluorescens, Trichoderma harzianum) |
18a | 42b | 60b | 73b | 78a | 81 ab | 82ab | 79ab | 85b |
Means with different letters in a column are statistically different (LSD; p≤0.05). †S: weeks.
The plants in T2 and T3 could be the highest due to the effect of the micro-organisms from the Bacillus genus, growth promoters (Izzeddin and Medina, 2011) applied in the treatments, with greater roots growth and absorbent hairs due to the presence of T. harzianum (Calvo and Zuñiga, 2010; Jiménez et al., 2011), and by the solubilization of phosphate groups by the presence of P. fluorescens (Molina-Romero et al., 2015). But T1 and T3 showed higher growth than T2.
Chlorophyll content index
T2 had higher chlorophyll rates than control at weeks 3, 6 and 7 and greater than T3 only at week 8 (Table 3).
Table 3 Effect of microorganisms and agricultural gypsum on the chlorophyll content units in potato plants (Solanum tuberosum L.).
| Tratamientos | Unidades de clorofila | |||||||
| S†1 | S†2 | S†3 | S†4 | S†5 | S†6 | S†7 | S†8 | |
| Testigo | 42a | 38a | 37a | 41a | 41a | 34a | 34a | 34a |
| 40 kg ha-1 de Ca + Trichoderma harzianum | 43a | 40a | 40b | 41a | 43a | 37b | 38b | 37b |
| 40 kg ha-1 de Ca + (Bacillus subtilis, Bacillus cereus, Pseudomonas fluorescens, Trichoderma harzianum) |
43a | 40a | 41b | 42a | 42a | 36ab | 36ab | 34a |
Averages with different letters in a column are statistically different (LSD; p≤0.05). †S: weeks.
All values were between 43 and 34 chlorophyll units (Table 3). This could be because fertilization with N was adequate. The decrease in the values towards the end was due to the senescence of the crop. Giletto et al. (2010) compared the green index of potato crops regard the N content in the plants; they confirmed that adequate fertilization (30 to 50) with N is associated with the increase in chlorophyll units.
Variables of the yield
Number of tubers
The highest yields in the number of tubers were obtained at T2 and T3, the latter exceeded by 51.5 % the control and was slightly higher than T2 (47 %) (Figure 1).
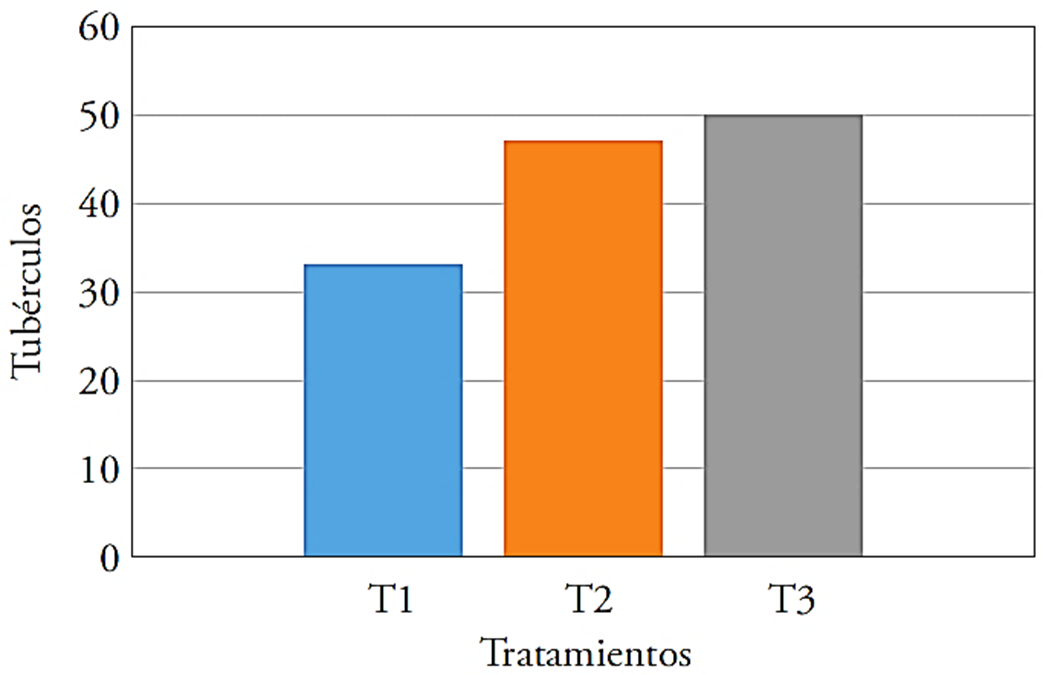
Figure 1 Number of tubers per treatment in evaluated potato crop, T1: control, T2: 40 kg ha-1 of Ca + T. harzianum, T3: 40 kg ha-1 of Ca + Bacillus subtilis, Bacillus cereus, Pseudomonas fluorescens, Trichoderma harzianum.
The favorable effect of T. harzianum, B. subtilis and P. fluorescens on the development was evident with the higher number of tubers in T2 and T3. This effect is because microorganisms produce growth hormones, which favor the root system development and improve nutrition (Cubillos-Hinojosa et al., 2009). Pozo (1997) noted that the presence of growth hormones increased tuberization; so that T1, without inoculation, showed a smaller number of tubers.
Yield
T3 showed higher yield (26.5 t ha-1) and surpassed by 49.4 % the control (Figure 2). The yield in T3 was the highest. In this case, the action of the growth promotion of T. harzianum was also demonstrated (Cubillos-Hinojosa et al., 2009). Puente et al. (2010) noted that B. cereus, B. subtilis and P. fluorescens are used for their direct promoter functions, based on their production of phytohormones and P solubilizers that increase crop yield.
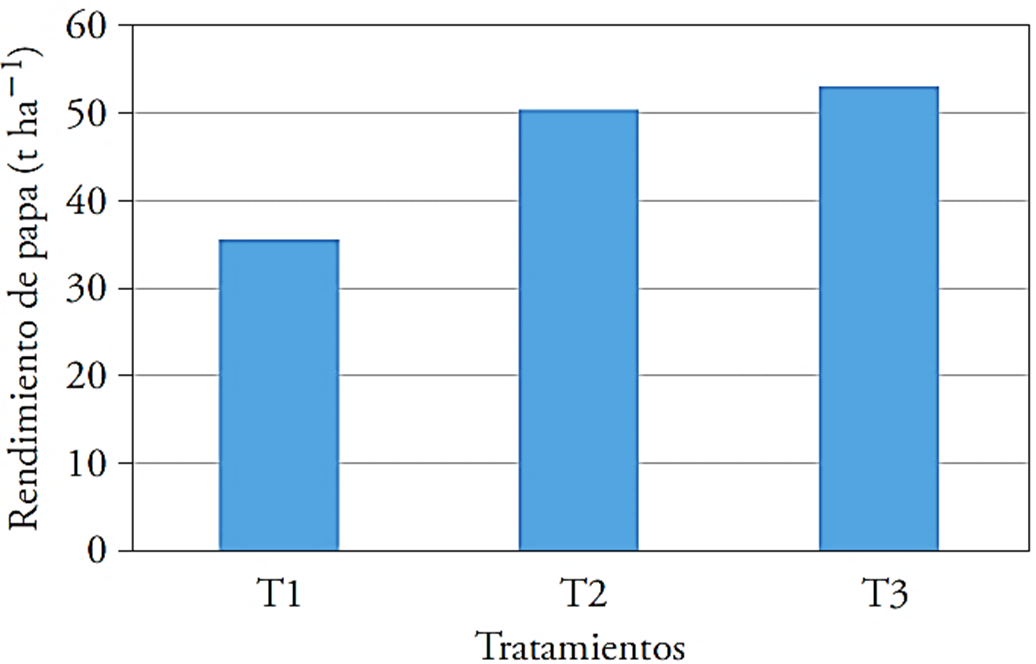
Figure 2 Effect of microorganisms and agricultural gypsum on potato yield (t ha-1). T1: control 1, T2: 40 kg ha-1 of Ca + T. harzianum, T3: 40 kg ha-1 of Ca + Bacillus subtilis, Bacillus cereus, Pseudomonas fluorescens, Trichoderma harzianum.
The incidence of Fusarium and Streptomyces in the harvested tuber of the experimental units was higher in T1, with an incidence percentage for pathogens with 32.14 % and 1 %, each. On the contrary, T3 was the one that had less incidence with only 9 % for Streptomyces and without Fusarium presence (Figure 3).
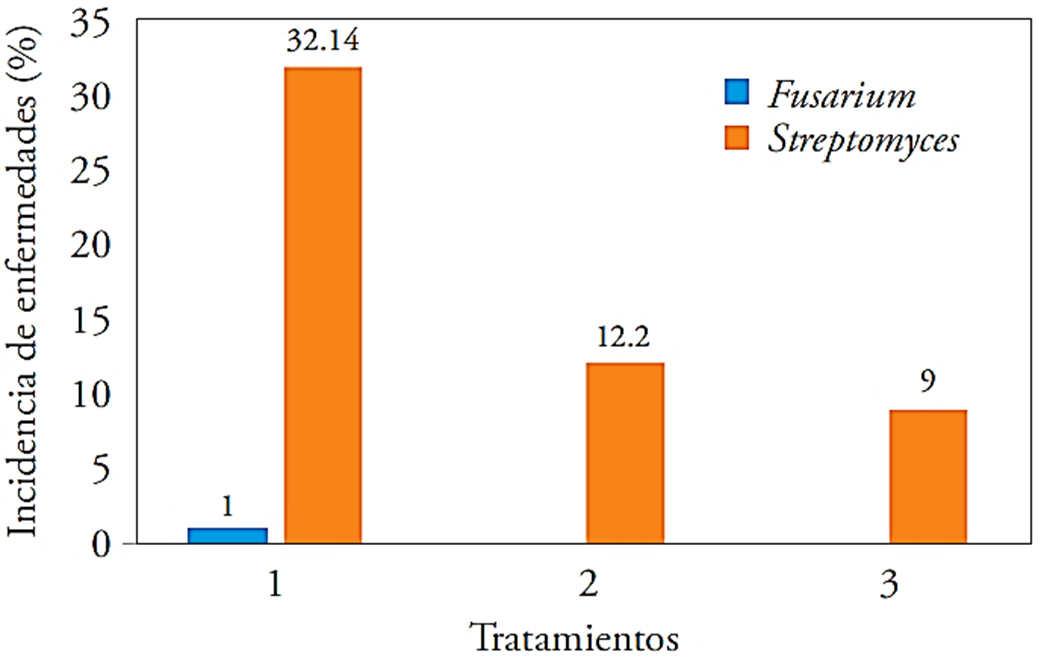
Figure 3 Fusarium and Streptomyces incidence in three treatments applied to the potato crops. 1: control, 2: 40 kg ha-1 of Ca + T. harzianum, 3: 40 kg ha-1 of Ca + B. subtilis, B. cereus, P. fluorescens, T. harzianum.
The effects of the applied microorganisms in the treatments were positive respect to the control, which no received microorganisms; this had a better quality or lower incidence of Streptomyces or Fusarium. The microorganisms in the T3 acted as antagonists for these pathogens. This can be compared with studies where the bacilli are used as antagonists against microorganisms’ precursors of diseases as shown by Sharga and Lyon (1998), when using B. subtilis to inhibit Pectobacterium carotovorum growth that causes soft rot in potatoes. Ezziyyani et al. (2004) used Trichoderma harzianum as biocontrol against Phytophthora capsici causing the “sadness” in green pepper cultivation, obtaining results of up to 65 % inhibition in pots and 80 % at greenhouse. To all the above, it is to be added that Pseudomonas spp. has also been shown to have antagonistic power for various pathogenic microorganisms, as pointed out by Walsh et al. (2001).
The macro and micronutrients in leafs were quantified in values close to the referenced averages. The Cu contents were superior to those documented by Jones et al. (1991), due to preventive treatment with Cu(OH)2 for possible diseases in potato crops. At T3 P concentration of was higher than the standard values, this is due to the fact that P. fluorescens produces P solubilizing metabolites that make it available for the plant. Katiyar and Goel (2003) indicated that the presence of Pseudomonas increases P concentrations because it solubilizes inorganic phosphates. In the N case, the solubilizing action of the microorganisms allowed its absorption and efficient transport, which led to tuber filling, high productivity and its concentration in leaf tissue at levels documented by Jones et al. (1991) and Gilleto et al. (2010); in this case the green index in units of chlorophyll, was 40 (Table 4).
Table 4 Effect of the application of microorganisms and agricultural gypsum on the nutritional content of potatoes leafs (Solanum tuberosum L.).
| Tratamiento | N | P | K | Ca | Mg | Cu | Fe | Zn | Mn |
| (%) | (ppm) | ||||||||
| Testigo | 2.4 | 0.20 | 6.1 | 1.32 | 0.75 | 232 | 20 | 24 | 80 |
| 40 kg ha-1 de Ca + T. harzianum | 3.09 | 0.32 | 5.7 | 1.19 | 1.22 | 48 | 132 | 32 | 280 |
| 40 kg ha-1 de Ca + (B. subtilis, B. cereus, P. fluorescens, T. harzianum | 3.84 | 0.68 | 6.5 | 1.76 | 1.17 | 182 | 44 | 88 | 160 |
| Referencia † | 3.0-4 | 0.25-0.4 | 6.00-8.0 | 1.5-2.5 | 0.70-1.0 | 7.0-20 | 40-100 | 30-200 | 30-250 |
Rhizosphere soil microbiological analysis
The viable count of the microflora from each treatment was assessed at 50 and 90 d from the emergence, and the number of microorganisms per g of dry weight was determined. This showed that environmental and nutrient conditions were acceptable for optimal development and prevalence in rhizospheric soil (Table 5).
Table 5 Viable microorganisms directly applied to soil with potato culture, at 50 and 90 days after emergence (CFU g-1 of dry soil).
| Tratamiento | Cuenta de microorganismos viables (UFC g-1 de suelo) | |||
| B. Subtilis | B. Cereus | Pseudomona sp. | Trichoderma sp. | |
| Testigo | † | † | † | † |
| 40 kg ha-1 de Ca + T. harzianum | † | † | † | 1.0×104 |
| ††1.0×104 | ||||
| 40 kg ha-1 de Ca + (B. subtilis, B. cereus, P. fluorescens, T. harzianum) |
7.9×104 ††1.02×105 |
2.0×103 ††6×105 |
3.0×105 ††2.9×106 |
1.0×104 ††1.0×104 |
† Not detected; †† count at 90 days.
The microorganism populations in the treatments in this study were similar to those reported by González et al. (1999), quantified in rhizosphere. These were of 4.3×105±17 460 (microorganisms g-1 of dry soil) of potatoes treated with conidia of T. harzianum.
Reinoso et al. (2006) indicated that bacteria of the Bacillus genus are part of a group with an important inhibitory effect on the growth of P. carotovorum, which is the causal agent of soft potato rot. Sharga and Lyon (1998) obtained similar results for the inhibition of this pathogen with Bacillus subtilis. Therefore, it is important that these microorganisms are present in the rhizosphere.
Literatura citada
Abril A. 2003. ¿Son los microorganismos edáficos buenos indicadores de impacto productivo en los ecosistemas? Ecología Austral 13: 195-204. [ Links ]
Alcántar G., G., y M. Sandoval V. 1999. Manual de Análisis Químico de Tejido Vegetal. Publicación Especial 10. Sociedad Mexicana de la Ciencia del Suelo. Chapingo, México. 156 p. [ Links ]
Atlas, R. M. 1984. Diversity of microbial communities. In: K. C. Marshall (ed). Advances in Microbial Ecology 7: 1-47. Plenum Press, New York, USA. [ Links ]
Bashan, Y., L. E. de-Bashan, S. R. Prabhu, and J. P. Hernandez. 2014. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (19982013). Plant Soil 378: 1-33. [ Links ]
Calvo P., y D. Zúñiga. 2010. Caracterización fisiológica de cepas de Bacillus spp. aisladas de la rizósfera de papa (Solanum tuberosum). Ecol. Apl. 9: 31-39. [ Links ]
Cano, M. A. 2011. Interacción de microorganismos benéficos en plantas: micorrizas, Trichoderma spp. y Pseudomonas spp. Revista UDCA Actualidad & Divulgación Científica. 14: 15-31. [ Links ]
Cubillos-Hinojosa J., L. Mejía, y N. Valero. 2009. Trichoderma harzianum como promotor del crecimiento vegetal del maracuyá (Passiflora edulis var. flavicarpa Degener). Agron. Colomb. 27: 81-86. [ Links ]
Elein T., A., Á. Leyva, y A. Hernández. 2005. Microorganismos benéficos como biofertilizantes eficientes para el cultivo del tomate (Lycopersicon suculentum Mill). Rev. Colomb. Biotecnol. 7: 47-54. [ Links ]
Ezziyyani M., C. Pérez-Sánchez, A. Sid-Ahmed, M. E. Requena, y M. E. Candela. 2004. Trichoderma harzianum como biofungicida para el biocontrol de Phytophthora capsici en plantas de pimiento (Capsicum annuum L.). An. Biol. 26: 35-45. [ Links ]
Gambaudo, S. 2006. Calidad del yeso natural para uso agrícola. Instituto Nacional de Tecnología Agropecuaria Estación Experimental Agropecuaria Rafaela. Información Técnica de Cultivos de Verano. Campaña 2006. Pub. Miscelánea 6: 110-113. [ Links ]
Giletto C., M, C. Díaz, J. E. Rattín, H. E. Echeverría, and D. O. Caldiz. 2010. Green index to estimate crop nitrogen status in potato processing varieties. Chil. J. Agric. Res. 70: 142-149. [ Links ]
González S., L. Rodríguez, C. Arjona, A. Puerta, y M. Fonseca. 1999. Efecto de la aplicación de Trichoderma harzianum R. sobre la composición cuantitativa de bacterias, hongos y actinomicetos de la rizósfera de Solanáceas y su influencia en el crecimiento vegetativo. Invest. Agrar. Produc. Invest. Veg. 14: 297-30. [ Links ]
Gupta, V.P., H., Bochow, S. Dolej and I. Fischer. 2000. Plant growth-promoting Bacillus subtilis strain as potential inducer of systemic resistance in tomato against Fusarium wilt. Z. Pflanzenk. Pflanzen. 107: 145-154. [ Links ]
Hernández-Leal, T. I., G. Carrión, y G. Heredia. 2011. Solubilización in vitro de fosfatos por una cepa de Paecilomyces lilacinus (Thom) Samson. Agrociencia 45: 881-892. [ Links ]
Izzeddin N., y L. Medina. 2011. Efecto del control biológico por antagonistas sobre fitopatógenos en vegetales de consumo humano. Salus 15: 8-12. [ Links ]
Jiménez C., N. S. de Albarracin, G. Altuna, y M. Alcano. 2011. Efecto de Trichoderma harzianum (Rifai) sobre el crecimiento de plantas de tomate (Lycopersicon esculentum L.). Rev. Fac. Agron. LUZ 28: 1-10. [ Links ]
Jones, Jr. J. B., B. Wolf, and H. A. Mills. 1991. Plant Analysis Hanbook. A practical sampling, preparation, analysis, and interpretation guide. Micro-Macro Publishing, Inc., Athens, Georgia, USA. pp:184-185 [ Links ]
Katiyar V., and R. Goel. 2003. Solubilization of inorganic phosphate and plant growth promotion by cold tolerant mutants of Pseudomonas fluorescens. Microbiol. Res. 158: 163-168. [ Links ]
Molina-Romero D., M. Bustillos-Cristales, O. Rodríguez-Andrade, Y. Morales-García, Y. Santiago-Saenz, M. Castañeda-Lucio, y J. Muñoz-Rojas. 2015. Mecanismos de fitoestimulación por rizobacterias, aislamientos en América y potencial biotecnológico. Biológicas 17: 24-34. [ Links ]
Patiño-Torres, C. O., y O. E. Sanclemente-Reyes. 2014. Los microorganismos solubilizadores de fósforo (MSF): una alternativa biotecnológica para una agricultura sostenible. Entramado 10: 288-297. [ Links ]
Pepper I., L., and C. P. Gerba. 2004. Environmental Microbiology a Laboratory Manual. 2a. ed. Elsevier Academic Press. USA, 197 p. [ Links ]
PEPSICO Alimentos México. 2014. Muestreo y análisis de papa en campo. 7a ed. México. pp: 31. [ Links ]
Pozo C., M. 1997. Tuberización, tamaño de la semilla y corte de tubérculos. Producción de Tubérculos-semillas de Papa Manual de Capacitación. Fascículo 2.3 Centro Internacional de la Papa. [ Links ]
Puente M., J. García, E. Rubio, y A. Perticari. 2010. Microorganismos promotores del crecimiento vegetal empleados como inoculantes en trigo. INTA EEA Rafaela, Pub. Miscelánea 116: 39-44. [ Links ]
Reinoso P., R. Casadesús, S. García, P. Gutiérrez, y R. Álvarez. 2006. Aislamiento, selección e identificación de bacterias del género Bacillus antagonistas de Pectobacterium carotovorum. Fitosanidad 10: 187-191. [ Links ]
Sharga B., and G. Lyon. 1998. Bacillus subtilis BS 107 As an Antagonist of Potato Blackleg and Soft Rot Bacteria, Can. J. Microbiol. 44: 777-783. [ Links ]
Uribe D., E. Ortiz, M. Portillo, G. Bautista y J. Cerón. 1999. Diversidad de Pseudomonas fluorescentes en cultivos de papa de la region cundiboyacense y su actividad antagonista in vitro sobre Rhizoctonia solani. Rev. Colomb. Biotecnol. 2: 50-58. [ Links ]
Walsh U., F., J. P. Morrisey, and F. O’Gara. 2001. Pseudomonas for biocontrol of phytopathogens: from functional genomics to commercial exploitation. Curr. Opin. Biotech. 12: 289-295. [ Links ]
Received: March 2017; Accepted: July 2017











 texto em
texto em 


