Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.52 no.4 Texcoco Mai./Jun. 2018
Crop Science
Flavor components and ascorbic acid content in native and commercial hybrid tomatoes (Solanum lycopersicum L.)
1 Coordinación Académica Región Altiplano Oeste, Universidad Autónoma de San Luis Potosí. 78600. Carretera Salinas-Santo Domingo No. 200, Salinas, San Luis Potosí. (edgar.berrospe@uaslp.mx)
2 Colegio de Postgraduados. 56230. Km. 36.5 Carretera México-Texcoco, Montecillo, Texcoco, Estado de México.
Tomatoes are commercially important worldwide for the ways it is consumed and its nutritional value. Due to its demand, cultivars with improved agronomic attributes, resistance to mechanical damage and longer shelf life have been developed. In certain cases, this has been achieved in detriment of organoleptic or nutritional quality, which native populations maintain. For this study, out of a collection of 600 native populations 13 were selected for their suitable agronomic characteristics for greenhouse cultivation, adequate yield and fruit size. Our objective was characterizing their chemical attributes related to taste and assessing their ascorbic acid content and compared it to two commercial hybrids. Our hypothesis was that the ascorbic acid and citric acid content, pH and total soluble solids in the fruits confer significant differences to the flavor. The experimental design was completely randomized, with 15 treatments and four repetitions. The data analysis was multivariate. In four stages of fruit maturity, pH, ascorbic acid, citric acid and total soluble solids content were quantified. The flavor components and the ascorbic acid content allowed three groups identification, one commercial hybrid with low ascorbic acid content, another with three native samples and the “Daniela” hybrid, classified as very sweet; and the one that included the “Sun7705” hybrid and five native samples classified as sweet, three native samples with neutral flavor and two tasteless. This confirms that native populations have potential to produce fruit for fresh or processed consumption and gourmet dishes.
Key words: ascorbic acid; pH; Solanum lycopersicum L.; titratable acidity; total soluble solids
El jitomate es comercialmente importante en el mundo por sus formas de consumo y valor nutricional. Debido a su demanda, cultivares con atributos agronómicos mejorados, resistencia a daños mecánicos y mayor vida de anaquel se han desarrollado. En ciertos casos, esto se ha logrado con detrimento de la calidad organoléptica o nutrimental, pero las poblaciones nativas las mantienen. Para este estudio, de una recolecta de 600 poblaciones nativas se seleccionaron 13 con características agronómicas adecuadas para su cultivo en invernadero, producción y tamaño de fruto aceptables, con el objetivo de caracterizar atributos químicos relacionados al sabor y conocer el contenido de ácido ascórbico del fruto, respecto a dos híbridos comerciales. La hipótesis fue que el contenido de ácido ascórbico, ácido cítrico, pH y sólidos solubles totales de los frutos confieren diferencias significativas al sabor. El diseño fue completamente al azar, con 15 tratamientos y cuatro repeticiones. El análisis de datos fue multivariado. En cuatro estados de madurez de fruto se cuantificó pH y contenido de ácido ascórbico, ácido cítrico y sólidos solubles totales. Los componentes del sabor y el contenido de ácido ascórbico permitieron identificar tres grupos. Uno con los híbridos comerciales con contenido menor de ácido ascórbico, otro con tres recolectas y el híbrido Daniela, clasificados como muy dulces; y el que incluyó el híbrido Sun7705 y cinco recolectas clasificadas como dulces, tres con sabor neutro y dos insípidas. Esto confirma que las poblaciones nativas poseen potencial de fruto para consumo fresco o procesado y componente de platillos gourmet.
Palabras clave: Solanum lycopersicum L.; sólidos solubles totales; pH; acidez titulable; ácido ascórbico
Introduction
The Andean region of Peru is recognized as the origin center of the tomato (Solanum lycopersicum L.) and the Mesoamerican region as the domestication center. In Mexico, tomato is used and consumed since pre-Columbian time and is worldwide distributed to the rest of the world since the Spanish colonization (Roman et al., 2013, Bergougnoux, 2014; Blanca et al., 2015). Currently, it is consumed because of its nutrient content (Carrera et al., 2007; San Martín-Hernández et al., 2012). In Mexico, fresh per capita consumption was 14.66 kg (FAOSTAT, 2015), higher than the USA (9.35 kg) where it is mainly consumed industrialized (30.51 kg).
Tomato is produced all year round and it is considered a source of antioxidants, minerals and vitamins (Adalid et al., 2010). Humans does not synthesize ascorbic acid (vitamin C), due to L-glucono-1,4-lactone oxidase presence; therefore, about 90 % of the ascorbic acid intake comes from the consumed fruits and vegetables, such as tomato (Tsaniklidis et al., 2014). Ascorbic acid is essential for human growth, development and reproduction, as it improves Fe absorption, decreases blood cholesterol levels, prevents veins thrombosis formation, increases organismic immunity and acts against infections (Zhang et al., 2011). This vitamin content in tomato fruits varies from just under 5 to more than 100 mg 100 g-1 which depends on its production system, the technology in the production, the agroclimatic zone it’s grown in, their cultivar, the maturity stage of the fruit and the species.
The tomato’s industrialization has led to a decrease in the number of cultivars and their organoleptic and nutritional quality (Adalid et al., 2010). These nutritional attributes are maintained in the native populations. The presence and use of different shapes and sizes of native tomato fruits is documented from the northern Mexico states to the Yucatan Peninsula in the southeast; in addition, its consumption is preferred compared to commercial hybrids (Álvarez-Hernández et al., 2009; Rodríguez et al., 2009; Carrillo and Chávez, 2010, Pacheco-Triste et al., 2014). Native populations are important because of their genetic variability, which results of conscious or unconscious selection by indigenous communities. Currently, there are collections of native tomatoes that allow taking advantage of and conserving genetic variability and generating new cultivars. For this reason, the agronomic attributes in their productive behavior, in field and protected conditions, have been characterized and confirmed their genetic diversity (Vásquez-Ortiz et al., 2010; Carrillo-Rodríguez et al., 2013; Bonilla-Barrientos et al., 2014; San Juan-Lara et al., 2014). The Colegio de Postgraduados, through its Program for Use and Conservation of the Native Tomato Diversity in Mexico, has around agronomically studied 600 populations. Still, the flavor characterization and the ascorbic acid (vitamin C) content of the fruits are poor. The objective of the study was to characterize the development of some chemical attributes related to flavor and the ascorbic acid content of the fruit of 13 native collections compared to two commercial hybrids, produced in greenhouse. Our hypothesis was that the ascorbic acid, citric acid and total soluble solids content and pH of the fruits of the commercial hybrids are higher than that of the native populations, regardless of their maturity at harvest time.
Materials and Methods
Thirteen tomato collections were evaluated in this study; these were selected out from 600 collected from different producing areas from Mexico. The 13 collections stood out in their adaptation to greenhouse cultivation, their health, plant morphology and fruit shape and size. The results were compared with those of the commercial hybrids SUN7705© of Nunhems, a “saladette” fruit type, and Daniela© from Hazera Genetics©, with a “ball” fruit type. Collections from Puebla state were identified as 16, A, B1, BR, and R, those from Guerrero as 34, 35, and 38, those from Oaxaca as 48 and 49, and as L, 83 and 96 those from Campeche, Yucatán and Estado de México, each. The collected ones and commercial hybrids showed indeterminate growth.
The evaluated fruits were obtained from plants developed in a greenhouse (19° 27’ 42.45” N, 98° 54’ 32.58” W and 2241 m altitude) 500 m2 tunnel type, with a milky white plastic cover. The planting started in 200 cavities polystyrene germination trays with peatmoss and agrolite mixture (3:1 v/v) as substrate. The seeds remained in acidulated water (pH 5.5) for 12 h, and then one per cell placed in seedbeds and covered with the same substrate until the cavities were filled. The nurseries were watered twice a day with 25 % Steiner solution (Steiner, 1984) prepared with calcium nitrate (Haifa®), potassium nitrate (Haifa®), magnesium sulfate (Ultrasol®), monopotassium phosphate (Ultrasol®), sulfuric acid (Procom®) and chelated micronutrients (TradeCorp®), pH 6.5 and electrical conductivity of 2.5 mS. Thirty one days after sowing (das) they were transplanted to 10 L capacity black polyethylene bags (30 cm diameter and 40 cm high), in a substrate based on red volcanic rock (tezontle) of 5±2 mm particle size. The culture bags were placed in a double row with 1.0 m between rows to obtain a density of 3.5 plants per m2. From the transplant day to 65 das the nutritive solution concentration was increased by 25 % every 8 d until reaching a 100 % concentration (Steiner, 1984). During the production stage, eight daily 345 mL irrigations were programmed, concentrating five irrigations between 12:00 and 14:00 h, averaging a total of 2.75±0.25 L per plant daily. Forty das lateral buds were eliminated and a tutor placed on each plant; by pruning the floral clusters, the six largest well-developed flowers cluster were left.
During harvest, between 07:00 and 08:00 h, fruits were obtained from four physiological stages of maturation: ripe green, changing, pink, red-firm and red-ripe (Batu, 2004), taking the first fruit of each floral cluster from clusters one to five, as they appeared in the plant. These were harvested; avoiding mechanical damage they were labeled and placed in plastic containers for immediate analysis in the laboratory.
The fruits were washed with distilled water and dried. The following was then assessed from the fruit’s juice: soluble solids total content (SST in °Brix) with method 983.17 of the AOAC (1990) in a digital refractometer (ATAGO series A56280 of ATAGO, Co., USA, LTD Pr-32α, with a scale of 0 to 32 %). The content of citric acid (AC) was measured with method 942.15 of the AOAC (1990), and pulp pH with a potentiometer (Corning, USA, model 12) following method 964.24 of the AOAC (1990). In the pulp, ascorbic acid concentration (AA) was quantified with method 967.21 from the AOAC (1990), which is based on extraction with oxalic acid and oxidation of 2, 6-dichlorophenol-indophenol, the results were calculated as mg AA 100 g-1.
The experimental design was completely randomized with 15 treatments (four pots per treatment) and four repetitions (four double rows), and each fruit as the experimental unit. The physiological stage of reference was red-firm. Analyzes included a cluster type, via the groups means of weighted pairs with Euclidean distances method, a Chernoff type graph (Chernoff, 1973) and a canonical discrimination. The analyzes were made based on the nutritional and flavor attributes of the fruits to characterize the groups with the SAS System( ver. 9.0 programs (SAS Institute, Inc., Cary, NC, USA) and STATISTICA® ver. 7.1 (StatSoft, Inc., Tulsa, Oklahoma, USA).
Results and Discussion
Based on the determination of the content of AA, TSS, CA and pH, three groups were identified, with three units distance, similar in AA content and flavor in a firm red stage, which is what the final consumer acquires. Commercial hybrids “Daniela” and “Sun7705” and the natives A, BR, L and R formed group I. Natives 16, 34, 38, 48, 49, 83, 96 and B1 formed group II and native 35 formed group III (Figure 1).
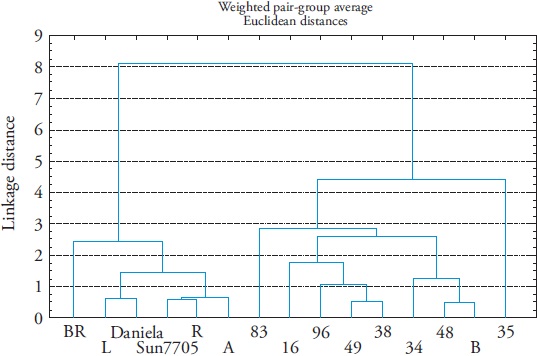
Figure 1 Dendrogram of fruit quality at red-firm stage from native populations and two commercial hybrids of tomato (Solanum lycopersicum L.).
We obtained the Chernoff graph taking into account the AA content as the main attribute. This character represented the width of the face, the ears elevation represented the TSS, the eccentricity of the forehead reflected the concentration of CA and the height of the forehead the pH (Figure 2). This graph showed that group III had a high content of AA and TSS characteristic compared to the other two groups, and, therefore, considered as sweet. The native collections fruits from group II had lower AA content compared to group I and lower TSS concentration (except for natives 83 and 16), with minimal variations in CA content and pH, so they were considered medium sweet and not acidic. The fruits from group I had the lowest AA amount, similar sweetness to group II and variable CA content and pH, so that its flavor was variable respect to the native harvest.

Figure 2 Chernoff faces of nutrimental attributes from fruits at red-firm stage from native populations and two commercial hybrids of tomato (Solanum lycopersicum L.). AA: ascorbic acid, TSS: total soluble solids, pH: hydrogen potential and CA: citric acid.
Discriminant canonical analysis (DCA) generated four variables based on the chemical and nutritional attributes. These canonical variables explained 100 % of the data variability. Canonical variable one (Can1, 45.73 %) was related to the pH of the fruits, Can2 (34.69 %) with the SST content, Can3 (12.77 %) with the AA content and Can4 (6.81 %) with the AC content.
The Can1: Can2 graphic relation showed four similar groups in pH and TSS; the hybrids “Daniela”, “Sun7705” and native 38 integrated the first group and characterized by its fruits with high pH and low TSS content. The second group consisted of natives 34, 48, 49 and 96, from which their fruits were acidic and their TSS content similar to the first group. Natives A and BR formed the third group, which had acid fruits with higher TSS content, compared to the first two groups. Natives L and R were grouped in a fourth group, due to their slight acid fruits and slightly higher TSS than the third group. The natives 16, 35, 83 and B1 were not grouped because they had different pH and SST (Figure 3A).
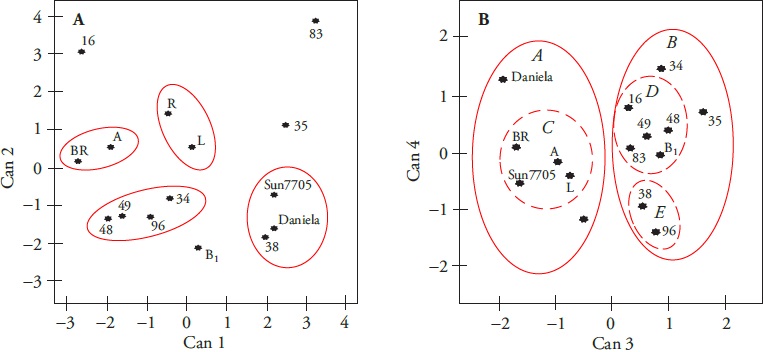
Figure 3 List of canonical variables from thirteen native populations and two commercial hybrids of tomato (Solanum lycopersicum L.). Can1 (45.73 %): pH, Can2 (34.69 %): total soluble solids, Can3 (12.77 %): ascorbic acid, and Can4 (6.81 %): citric acid.
The Can3 and Can4 relation showed two results. The first was the presence of two groups (A and B). Grup A was characterized by its low AA content, which contained the Daniela and Sun7705 hybrids and the A, BR, L and R collects. This corresponds with the dendrogram described above. B was characterized by AA content and contained collects 16, 34, 35, 38, 48, 49, 83, 96 and B1. Within this group, three subgroups (C, D and E) were identified and showed relation between the AA and the CA content. Subgroup C (collections A, BR, L and Sun7705 hybrid) had low AA content and intermediate CA concentration. In contrast, subgroup D (collects 16, 48, 49, 83 and B1) had high AA concentration and slightly higher CA than subgroup C. Its high AA content and low CA (Figure 3B) characterized subgroup E (collects 38 and 96); this implies differences in its flavor.
The commercial hybrids and the R collection from group I showed moderate and stable acidity compared with the rest of the group (Daniela 5.17, Sun7705, 5.00 and R 4.83). Collects A and BR showed decreased pH (from 4.53 and 4.43 to 4.83 and 4.90) (Figure 4A). Group II, consisting on collects 16, 38, 48, 49, 83 and 96 showed pH stability during fruit ripening. Collect 16 had the lowest pH (4.63) and 38 the highest (5.03). From the group, B1 collect increased its pH (from 4.83 in the green-ripe state to 5.27 in the red-firm state) with maturation. Only collect 34 exhibits an irregular pH change during maturation (Figure 4B). Collect 35 (group III) decreased its pH to 4.77 in the breaking maturation stage, later it increased to 5.13 in the red mature stage (Figure 4C).
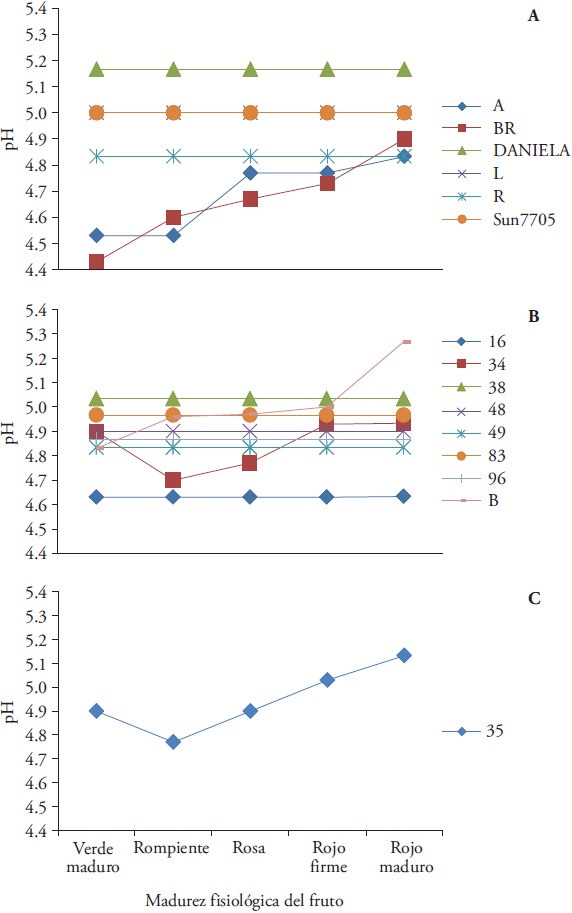
Figure 4 pH during tomato fruit (Solanum lycopersicum L.) maturation from 13 native collects and two commercial hybrids. A) Group I: Daniela and Sun7705 hybrids and A, BR, L and R, B collects). Group II: collects 16, 34, 38, 48, 49, 83, 96 and B1 and C) Group III: collect 35.
Crisanto-Juárez et al. (2010) obtained pH between 3.63 and 4.30 in a tomato collects from Oaxaca. Shah et al. (2015) and Vinha et al. (2014) reported values between 4.3 and 4.6 in commercial hybrids, and placed the exocarp as the anatomical tissue that mainly influences the fruit’s acidity. Commercial hybrids and native harvests had less acid fruits when obtained in hydroponic production systems.
At group I, Daniela and Sun7705 hybrids showed 0.39 and 0.68 % CA; this evidenced the difference with the saladette type Sun7705 hybrid and the ball type Daniela, despite maintaining stable concentration during ripening. Collects L, R and A also showed stable content (0.56, 0.52 and 0.39 %) during maturation. Collect A showed similar content to the Daniela hybrid. The exception from the group was the BR collect, due to its higher CA concentration (90.12 % at the green-ripe stage which decreased similarly to the Sun7705 hybrid, 0.70 %, up to the mature red stage) regard to the rest of the group (Figure 5A).
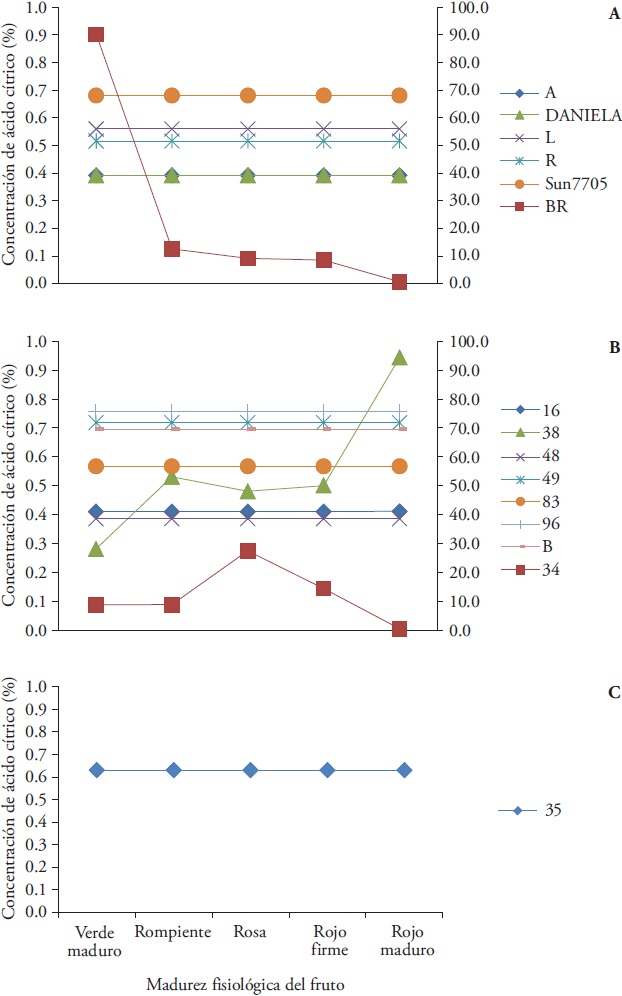
Figure 5 Citric acid (%) during fruit ripening from thirteen native collects and two commercial hybrids of tomato (Solanum lycopersicum L.) grouped by fruit type according to nutritional attributes. A) Group I: Daniela and Sun7705 hybrids and collecs A, BR, L and R, B1, Group II: collects 16, 34, 38, 48, 49, 83, 96, and B1, and C) Group III: collect 35. Collect 34 and BR referenced in secondary vertical axis.
In group II, collects 16, 48, 49, 83, 96 and B showed stable CA values during maturation (0.41, 0.39, 0.72, 0.57, 0.76 and 0.70 %), differentiating collect 38 by its progressive CA increase of up to 0.94 % during mature red stage. In addition, in this group collect 34 presented irregular CA values during maturation (more than 8 % CA during the first four stages and subsequent decrease) (Figure 5B). Collect 35 (group III) showed stable CA (0.63 %) during maturation (Figure 5C).
Breksa et al. (2015) and Kaur et al. (2013) determined CA between 0.28 and 0.63 % in commercial tomato hybrids. The CA values of the Daniela and Sun7705 commercial hybrids were within that range. In contrast, the native collects had higher CA. The CA is a triprotic bio-compound that significantly modifies fruits pH; this depends on its relation with other present minerals and compounds. In this study, the correlation between the CA content and pH was negative (r =-0.48); which indicates that the pH will decrease as the CA increase. This was the case with most of the collects, with the exception of L, R, 16, 48 and 83.
In group I, Daniela hybrid was the only one that showed TSS variability, with a lower level (4.80 °Brix) in a changing stage and higher (6.37 °Brix) at the red-mature stage; the rest of the collects from this group kept a stable TSS content (Figure 6A).

Figure 6 Total soluble solids during fruit ripening from thirteen native collects and two commercial hybrids of tomato (Solanum lycopersicum L.) grouped by fruit type according to nutritional attributes. A) Group I: Daniela and Sun7705 hybrids and collects A, BR, L and R, B) Group II: collects 16, 34, 38, 48, 49, 83, 96 and B1 and C) Group III: collect 35.
In Group II, collect 83 at the red-mature stage was notable for its TSS content of 8.5 °Brix. Collects 38 and 49 showed similar development among them, with a TSS increase from the breaking stage (5.73 °Brix) and without change until the red-firm stage. Collects 16, 34, 48, 96 and B1 showed no changes in the TSS during ripening (6.13, 5.90, 4.76, 6.10 and 5.14 %) (Figure 6B).
Group III (collect 35) showed 7.03 % TSS during maturation and was the native collect with the highest TSS concentration respect to group A and most group B members. Collect 83 was the only one that exceeded it at the red-firm and red-soft stages (Figure 6C). The TSS content varies between 3.2 to 9.3 % (Kaur et al., 2014; Vinha et al., 2014; Breksa et al., 2015; Shah et al., 2015) and the results in our study are within that interval.
Taste can be defined as the sum of the interactions between taste receptors, the olfactory system, texture in the mouth and visual appearance (Mathieu et al., 2009). In the case of tomatoes, taste receptors react to sugars concentration, organic acids and glutamate (Klee, 2010). Hogendoorn et al. (2010) reported that tomato fruits with high TSS concentrations (10.6 %) and CA of 4 % were highly preferred (95 %) by panelists. These fruits tasted sweet (85 %), acid (85 %) and intense (89 %). Thus, collects A, 16 and 83 and the Daniela hybrid were classified as very sweet, the Sun7705 hybrid and the collects L, R, 35, 38 and 48 were classified as sweet, collects B, 49 and 96 with neutral flavor and collect BR and 34 as insipid. These results must be confirmed by sensory analysis.
The AA content at group I from half of the group (collect L, R and hybrid Sun7705) was not stable. The highest concentrations occurred at the red-mature stage; but, collects A, BR and hybrid Daniela kept constant AA content (4.89, 5.12 and 6.46 mg 100 g−1) (Figure 7A).
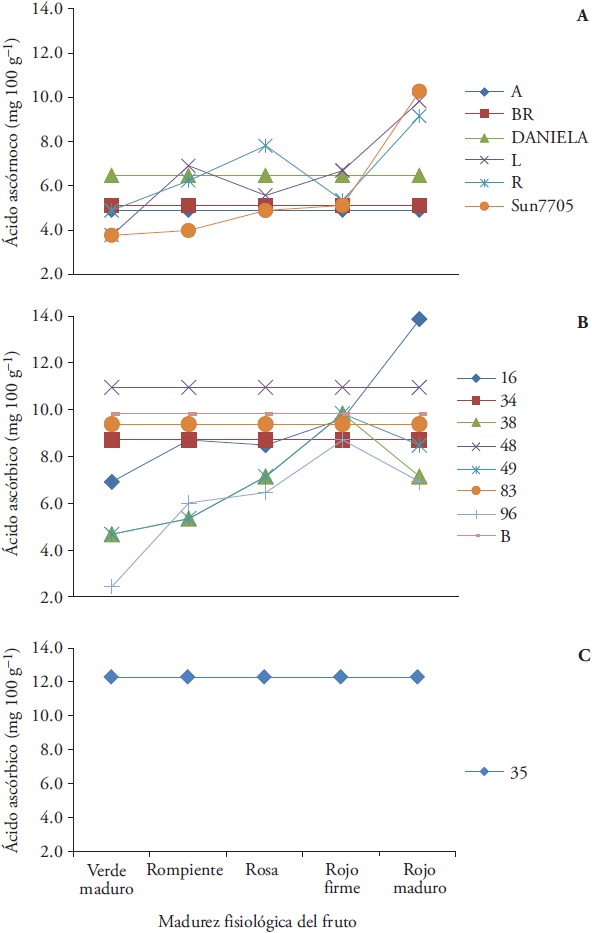
Figure 7 Ascorbic acid content in fruit from thirteen native collects and two commercial hybrids of tomato (Solanum lycopersicum L.), during ripening, grouped by fruit type according to nutritional attributes. A) Group A: Daniela and Sun7705 hybrids and collects A, BR, L and R, B) Group B: collects 16, 34, 38, 48, 49, 83, 96 and B1 and C) Group C: collect 35.
AT group II, collects 34, 48, 83 and B1 (8.70, 10.95, 9.38 and 9.82 mg 100 g-1) kept their AA stable during maturation. On the contrary, the native collects 38, 49 and 96 tended to increase their AA content, from the green-ripe to the red-firm stage, and decreased to 7.14, 8.48 and 6.91 mg 100 g-1 at the red-mature stage. A particular case of the group was collect 16, which increased its AA content to 13.86 mg 100 g-1 in the red-ripe stage. Thus, this collect was the one that contained the largest AA in the entire group (Figure 7B). Therefore, Group II showed the highest AA contents respect to group I.
The collect 35 showed stability in the AA concentration (12.29 mg 100 g-1) during maturation; it only exceeded the collect 16 in the red-mature stage (Figure 7C).
In native collects the AA contents were quantified between 6.1 and 16.1 mg 100 g-1 (Crisanto-Juaréz et al., 2010; Gaspar-Peralta et al., 2012). Some cultivars have shown AA contents of up to 50.21 mg 100 g-1 (Kaur et al., 2013). These values are affected by factors such as production technology, species, cultivation and maturity (Lee and Kader, 2000).
The National Health Institute (Instituto Nacional de Salud, 2016) recommends an AA daily intake of 90 and 75 mg day-1 for men and women over 19 years. According to the results in this study, 100 g of fruit from the Sun7705 hybrid and the L and R collects would provide 11.41, 10.9 and 10.2 % of the recommended daily intake (IDR), collects 16, 48, B and 83 would contribute 15.4, 12.2, 10.9 and 10.4 % IDR and group III would contribute 13.7 % IDR.
The AA metabolic pathway directly depends on the glucose-6P concentration (Smirnoff et al., 2001) and the AA content seems to correlate with the sugar content (Massot et al., 2010). In the present investigation, the correlation of AA content with the TSS was not significant.
Conclusions
The ascorbic acid, citric acid, and total soluble solids content and pH clustered three groups among the fruits of the 16 collects. But, four groups were identified based on the flavor, they included: very sweet, which included three collects and the Daniela hybrid, sweet, which included five collects and the Sun7705 hybrid, neutral, which included three collects and tasteless, which included two collects. Thus, native populations showed potential for their fresh or processed production and as gourmet dish component.
Hybrid Sun7705 and native collect 16, 35, 48, B, L, 83 and R showed the highest AA content and collects 16 and 35 for nutraceutical purposes.
Literatura Citada
Adalid, A. M., S. Roselló, and F. Nuez. 2010. Evaluation and selection of tomato accessions (Solanum section licopersicum) for content of lycopene, ((-carotene and ascorbic acid. J. Food Comp. Anal. 23: 613-618. [ Links ]
Álvarez-Hernández, J. C, H Cortez-Madrigal, y I. García-Ruiz. 2009. Exploración y caracterización de poblaciones silvestres de jitomate (Solanaceae) en tres regiones de Michoacán, México. Polibotánica 28: 139-159. [ Links ]
A.O.A.C. 1990. Association of Official Analytical Chemists. Official Method of Analysis. Ed. Washington. [ Links ]
Batu, A. 2004. Determination of acceptable firmness and color values of tomatoes. J. Food Eng. 61: 471-475. [ Links ]
Bergougnoux, V. 2014. The history of tomato: from domestication to biopharming. Biotechnol. Adv. 32: 170-189. [ Links ]
Blanca, J., J. Montero-Pau, C. Sauvage, G. Bauchet, E. Illa, M. José D., D. Francis, M. Causse, E. van der Knaap, and J. Cañizares. 2015. Genomic variation in tomato, from wild ancestors to contemporary breeding accesions. BMC Genomics 16: 257. [ Links ]
Bonilla-Barrientos, O., R. Lobato-Ortiz, J. J. García-Zavala, S. Cruz-Izquierdo, D. Reyes-López, E. Hernández-Leal, y A. Hernández-Bautista. 2014. Diversidad agronómica y morfológica de tomates arriñonados y tipo pimiento de uso local en Puebla y Oaxaca, México. Rev. Fitotec. Mex. 37: 129-139. [ Links ]
Breksa, A. P., L. D. Robertson, J. A. Labate, B. A. King, and D. E. King. 2015. Physicochemical and morphological analysis of ten tomato varieties identifies quality traits more readily manipulated through breeding and traditional selection methods. J. Food Comp. Anal. 42: 16-25. [ Links ]
Carrera, P. M., X. Gao, and K. L. Tucker. 2007. A study of dietary patterns in the Mexican-American population and their association with obesity. J. Ame. Dietetic Assoc. 107: 1735-1742. [ Links ]
Carrillo R., J. C., y J. L. Chávez S. 2010. Caracterización agromorfológica de muestras de tomate de Oaxaca. Rev. Fitotec. Mex. 33: 1-6. [ Links ]
Carrillo-Rodríguez, J. C., J. L. Chávez-Servia, G. Rodríguez-Ortiz, R. Enríquez-del Valle, y Y. Villegas-Aparicio. 2013. Variación estacional de caracteres agromorfológicos en poblaciones nativas de jitomate (Solanum lycopersicum L.). Rev. Mex. de Cienc. Agric. 6: 1081-1091. [ Links ]
Chernoff, H. 1973. The use of faces to represent points in K-dimensional space graphically. J. Am. Stat. Assoc. 68: 361-368. [ Links ]
Crisanto-Juaréz, A., A. M. Vera-Guzmán, J. L. Chávez-Servia, y J. C. Carrillo-Rodriguez. 2010. Calidad de frutos de tomates silvestres (Lycopersicum esculentum var. ceraciforme Dunal) de Oaxaca México. Rev. Fitotec. Mex. 33: 7-13. [ Links ]
FAOSTAT (Food and Agriculture Organization of the United Nations). 2015. Food and Agriculture Organization of the United Nations, Statistic Division. USA. http://faostat3.fao.org/browse/G1/*/E (Consulta: junio 2015). [ Links ]
Gaspar-Peralta, P., J. C. Carrillo-Rodríguez, J. L. Chávez-Servia, A. M. Vera-Guzmán, y I. Pérez-Léon. 2012. Variación de caracteres agronómicos y licopeno en líneas avanzadas de tomate (Solanum lycopersicum L.). ((YTON 81: 15-22. [ Links ]
Hogendoorn, K., F. Bartholomaeus, and M. A. Keller. 2010. Chemical and sensory comparison of tomatoes pollinated by bees and by a pollination wand. J. Econ. Entomol. 103: 1286-1292. [ Links ]
Kaur, C., S. Walia, S. Nagal, S. Walia, J. Singh, B. B. Singh, S. Saha, B. Singh, P. Kalia, S. Jaggi, and Sarika. 2013. Functional quality and antioxidant composition of selected tomato (Solanum lycopersicon L.) cultivars grown in Northern India. LWT Food Sci. Tech. 50: 139-145. [ Links ]
Klee, H. J. 2010. Improving the the flavor of fresh fruits: genomics, biochemistry and technology. New Phytol. 187: 44-56. [ Links ]
Lee, S. K., and A. A. Kader. 2000. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 20: 207-220. [ Links ]
Massot, C., M. Génard, R. Stevens, and H. Gautier. 2010. Fluctuations in sugar content are not determinant in explaining variations in vitamin C in tomato fruit. Plant Physiol. Bioch. 48: 751-757. [ Links ]
Mathieu, S., V. Dal Cin, Z. Fei, H. Li, P. Bliss, M. G. Taylor, H. J. Klee, and D. M. Tieman. 2009. Flavour compounds in tomato fruits: identification of loci and potential pathways affecting volatile composition. J. Exp. Bot. 60: 325-337. [ Links ]
National Institute of Health. 2016. Datos sobre vitamina C. Office of Dietary Supplements. Department of Health and Human Services. https://ods.od.nih.gov/pdf/factsheets/VitaminC-DatosEnEspanol.pdf (Consulta: julio, 2017). [ Links ]
Pacheco-Triste, I. A., J. L. Chávez-Servia, y J. L. Carrillo-Rodríguez. 2014. Relación entre variación ecológica-orográfica y variabilidad morfológica de tomate (Solanum lycopersicum L.) en Oaxaca. Rev. Mex. Agroecosist. 1: 28-39. [ Links ]
Rodríguez G., E., D. Vargas C., J. J. Sánchez G., R. Lepiz I., A. Rodríguez C., J. A. Ruiz C., P. Puente O., y R. Miranda M. 2009. Etnobotánica de Solanum lycopersicum var. Cerasiforme en el Occidente de México. Nat. Desarro. 7: 46-59. [ Links ]
Roman, S., C. Ojeda-Granados, y A. Panduro. 2013. Genética y evolución de la alimentación de la población en México. Rev. Endocrinol. Nutr. 21: 42-51. [ Links ]
San Juan-Lara, F., P. Ramírez-Vallejo, P. Sánchez-García, M. Livera-Muñoz, M. Sandoval-Villa, J. C. Carrillo-Ródriguez, y C. Perales-Segovia. 2014. Variación en características de interés agronómico dentro de una población nativa de tomate (Solanum lycopersicum L.). Rev. Fitotec. Mex. 37: 159-164. [ Links ]
San Martín-Hernández, C., V. M. Ordaz-Chaparro, P. Sánchez-García, M. T. B. Colinas-Leon, y L. Borges-Gómez. 2012. Calidad de tomate (Solanum lycopersicum L.) producido en hidroponia con diferentes granulometrías de tezontle. Agrociencia 46: 243-254. [ Links ]
Shah, K., M. Singh, and A. C. Rai. 2015. Bioactive compounds of tomato fruits from transgenic plants tolerant to drough. LWT Food Sci. Technol. 61: 609-6014. [ Links ]
Smirnoff, N., P. L. Conklin, and F. A. Loewus. 2001. Biosynthesis of ascorbic acid in plants: a renaissance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52: 437-467. [ Links ]
Steiner, A. A. 1984. The universal nutrient solution.Sixth International Congress on Soiless Culture. ISOSC.Proceedings. The Netherlands. pp: 633-649. [ Links ]
Tsaniklidis, G., C. Delis, N. Nikoloudakis, P. Katinakis, and G. Aivalakis. 2014. Low temperature storage affects the ascorbic acidmetabolism of cherry tomato fruits. Plant Physiol. Biochem. 84: 149-157. [ Links ]
Vásquez-Ortiz, R., J. C. Carrillo-Rodríguez, y P. Ramírez-Vallejo. 2010. Evaluación morfo-agronómica de una muestra del jitomate nativo del centro y sureste de México. Nat. Desarro. 8: 49-64. [ Links ]
Vinha, A. F., S. V. P. Barreira, A. S. G. Costa, R. C. Alves, and M. B. P. P. Olvera. 2014. Organic versus conventional tomatoes: Influence on physochemical parameters, bioactive compounds and sensorial atributes. Food Chem. Toxicol. 67: 139-144. [ Links ]
Zhang, Y., H. Li, W. Shu, C. Zhang, and Z. Ye. 2011. RNA interference of a mitochondrial APX gene improves vitamin C accumulation in tomato fruit. Sci. Hort. 129: 220-226. [ Links ]
Received: August 2016; Accepted: September 2017











 texto em
texto em 


