Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.52 no.3 Texcoco Abr./Mai. 2018
Food Science
Physical and nutritional characterization of yucca fruits (Yucca mixtecana)
1Consejo Nacional de Ciencia y Tecnología. Hornos 1003 Colonia Noche Buena; 71230. Santa Cruz Xoxocotlán, Oaxaca, México.
2Instituto Politécnico Nacional. Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional unidad Oaxaca. Departamento de posgrado e investigación. Hornos 1003 Colonia Noche Buena; Santa Cruz Xoxocotlán, Oaxaca, México; c.p.71230; fax (55) 9515170610
Fruits from some species of yucca are consumed as dietary complement in the arid and semiarid zones of México, although their nutritional information is unknown, which is why it is difficult to understand their nutritional contribution. Yucca mixtecana is distributed in the state of Oaxaca, México, but the fruits together with the rest of the plant are not used. The objective of this study was to evaluate the nutritional properties of mature fruits of Y. mixtecana through a descriptive statistical analysis. The variables in mature fruits of Y. mixtecana were: 1) the partial chemical composition (raw protein, method AOAC 960.52; total sugars, norm NMX-F-132; and lipids, method AOAC 823.03); 2) some specific nutritional properties (total phenols, Folin-Ciocalteu method; and saponins, afrosymmetric method). The content of raw protein (RP) was 2.29 % and of total sugars 1.70 % (dry base). The content of total phenols was 0.63 mg g-1 equivalents of gallic acid, and of saponins 0.01 mg g-1. The concentration of total sugars was lower than the fruits of other species of Opuntia (11 %) and of Prosopis alba (2.70 %). The protein content was higher than that of the fruit of Opuntia analyzed (0.39 %), but lower than the fruit of P. alba (7.70 %). The high concentration of RP and the low concentration of total sugars in the fruits of Y. mixtecana, compared to most fruits, make it a fruit with great dietary potential.
Key words: nutritional composition; physical characteristics; saponins; total phenols; desert fruits
Los frutos de algunas especies de yucas se consumen como complemento dietario en las zonas áridas y semiáridas de México, pero la información nutrimental se desconoce, por lo cual es difícil saber su aporte nutricional. Yucca mixtecana se distribuye en el estado de Oaxaca, México, y los frutos, al igual que el resto de la planta, no se aprovechan. El objetivo de este estudio fue evaluar las propiedades nutritivas de frutos maduros de Y. mixtecana mediante un análisis estadístico descriptivo. Las variables en frutos maduros de Y. mixtecana fueron: 1) la composición química parcial (proteína cruda, método AOAC 960.52; azúcares totales, norma NMX-F-132; y lípidos, método AOAC 823.03); 2) algunas propiedades nutritivas particulares (fenoles totales, método de Folin-Ciocalteu; y saponinas, método afrosimétrico). El contenido de proteína cruda (PC) fue 2.29 % y el de azúcares totales fue 1.70 % (base seca). El contenido de fenoles totales fue 0.63 mg g-1 de equivalentes de ácido gálico y el de saponinas fue 0.01 mg g-1. La concentración de azúcares totales fue menor que los frutos de otras especies de Opuntia (11 %) y de Prosopis alba (2.70 %). El contenido proteico fue superior al del fruto de Opuntia analizados (0.39 %), pero inferior al fruto de P. alba (7.70 %). La alta concentración de PC y la baja concentración de azúcares totales en los frutos de Y. mixtecana, relativos a la mayoría de las frutas, los convierten en un fruto con gran potencial dietético.
Palabras clave: composición nutricional; características físicas; saponinas; fenoles totales; frutos del desierto
Introduction
Most of the rural population in México (close to 24 million people) lives in arid and semiarid zones, and 60 % of them under dietary poverty. The inclusion of new foods can contribute to improving the nutritional status of these populations, as well as broaden the offer of agro products susceptible to be commercialized.
Within the arid zones of México, the Yucca genus is the second most important in distribution and diversity, after the Agave genus (Flores-Hernández et al., 2011), and it is made up of around 47 species distributed primarily in arid or semiarid zones of México (Piña, 1979). These species are used as material for construction, prime material in the rope-making industry, fuel, and source of food, mainly the inflorescences and, less frequently, the fruits or dates (Granados-Sánchez and López-Ríos, 1998; Aguirre, 2008).
Among the chemical compounds present in the Yucca genus, those of highest interest are the saponins that are used in products with surfactant properties (Balandrin, 1996), in foods as additives (Jensen and Elgaard, 2001; Aoun et al., 2003), cosmetics (Balandrin, 1996), and as prime material for products with pharmacological, hormonal precursors, anti-cancer and anti-viral activity (Man et al., 2010; Saleem et al., 2010; Son et al., 2012). There is not a complete use of the fruits from different species of yucca as a dietary complement, as additive in functional foods or as a source of molecules with biological activity.
Despite the potential to use the fruits, there is no information referring to the physical and nutritional characteristics for any species of the Yucca genus; thus, it is difficult to analyze their contribution as part of the intake in human nutrition. Therefore, the objective of this study was to evaluate the nutritional properties of mature fruits of Yuca mixtecana through a descriptive statistical analysis.
Materials and Methods
Plant material
Mature fruits of Y. mixtecana were selected based on their color and texture. The fruits were classified by color, uniform yellow in the mesocarp and yellow with dark spots in the epicarp; firmness of the mesocarp and ease of separation from the stem; all these physical characteristics indicate maturity. The harvest was performed inside the live collection of the Dr. Cassiano Conzatti botanical garden at the Research Center for Integral Regional Development (Centro de Investigación para el Desarrollo Integral Regional, CIIDIR- IPN) Oaxaca unit, México.
Morphological characterization
The physical variables of the fruit were determined in the second state of maturation (mature fruit) and for their quantification, the contour of the fruit was sought to be nearly cylindrical, where the width corresponds to the dimension of the radius in the central part of the cylinder.
The epicarp, endocarp and mesocarp were removed through mechanical dissection, to later quantify their weight (humid base). The mesocarp removed was used to determine the nutritional properties.
Color quantification
The color of the mesocarp was determined with a MiniScan EZ colorimeter (HunterLab, Reston Eter, USA) and the results were recorded in a CIE La*b*scale. The parameter L represents the luminosity of the sample from 0 to 100 (highest luminosity); the coordinate a* represents the change of color from red (+) to green (-), and the coordinate b* represents the change in color from yellow (+) to blue (-).
The parameters of color hue (h° and C*) were calculated with Equations 1 and 2. The parameters of color hue range from 0° (pure red color), 90° (pure yellow color), 180° (pure green color), to 270° (pure blue color) (Sant´Anna et al., 2013).
Titrable acidity
This evaluation was performed through potentiometry (HANNA HI-98172, USA) according to the Mexican Norm NMX-F-102 (2010). The result was expressed in mg of equivalents of citric acid per 100 g of endocarp (EAC 100 g-1).
Total solids
The total solids were quantified through refractometry (Westover RHB-32ATC, USA) based on the Mexican Norm NMX-F-112-NORMEX-2010 (2010). The content of total solids was expressed as degrees Brix.
Moisture
Moisture was quantified with the AOAC modified method 934.06 (1996) in 5 g of fresh mesocarp. The temperature for vacuum drying was 105 °C (Shell lab SVAC1, EUA), with intervals of quantification of 24 h (30 min of cooling in desiccator), until the variation was lower than 0.01 g (Denver Instrument TP-1114), and the weight was expressed in g of water per g of dry solid (gH20 gss -1).
Total ashes
The content of inorganic matter was quantified with the AOAC method 923.03 (2005), through muffle (Thermolyne Furnace 1400, USA) and the content of ashes was expressed as % of inorganic compounds (dry base).
Raw protein (RP)
The quantification of RP was performed with the AOAC method 960.52 (1995), using micro-digestion and distillation equipment (Foss Tecator 2100 Kjetlec, USA), and a conversion factor of 6.25 was used to express the content of RP as the % of protein (dry base).
Determination of reducing and total sugars
The content of total sugars was determined according to the Official Mexican Norm NMX-F-312-NORMEX-2016 (2016) and was expressed as the % of sugars (dry base).
Content of lipids
The content of total lipids was determined with the AOAC method 991.36 (2006) and it was expressed as % of lipids (dry base).
Content of total raw fiber (RF)
The content of RF was determined with the methodology by Olvera et al. (1993) and it was expressed as the % of RF (dry base).
Content of total phenols
The content of total phenols was determined through the Folin-Ciucalteu technique of spectrophotometry (spectrophotometer BBC Cintra 4040, USA), with the methodology described by Almaraz-Abarca et al. (2004) and gallic acid was used as standard of reference. The total phenols value was expressed as mg of equivalents of gallic acid per g of dry-base sample (EAG g ss -1).
Content of saponin
The content of saponins was determined through the afrosymmetrical method reported by Guzmán et al. (2013) and 10 g of fresh mesocarp were macerated in distilled water (1:3 v/v), for 15 min at - 25 °C. The content of saponins was expressed as mg of saponins per g of dry-base sample (ST gss -1).
Profile of fatty acids (FA)
The evaluation of the FA profile was carried out through the identification and quantification of its functionalization (methyl-esters) and the functionalization, according to the methodology by Martínez et al. (1999). For every 100 mL of ether extract from the fruits, 800 µL of chloroform-methanol solution (J. T. Baker) were added in a proportion of 2:1 v/v. To 200 µL of this solution, 1 mL of HCl-methanol (J. T. Baker) were added in a concentration of 1 N. The reaction took place at 80 °C during 20 min, 200 µL of distilled water were added, and the methyl-esters were extracted through a liquid-liquid extraction with chromatograph hexane (Fermont); in order to remove the residues from the polar solution, anhydrous sodium sulfate (J. T. Baker) was used. The solution was decanted and evaporated under a current of nitrogen; the methyl-esters were suspended in 1.4 mL of chromatographic hexane (Fermont) before their injection.
The methyl-esters were identified in a gas chromatograph with a flame ionization detector (Perkin-Elmer Clarus 580, USA) and an Elite-SMS-30 capillary column (30 m x .32 x 0.25 μm; Perkin-Elmer), a “split” injection relation of 100:1 was used, and the temperature of injection and of the detector was 220 °C, and of the oven 186 °C in isothermal method of 16 min. The volume of injection was 2 μL of sample. The identification was carried out by comparison of the retention times with regards to standards of reference (Supelco fatty acid methyl esters, C4 - C24).
To quantify the FA profile, hexadecane was used (Sigma-Aldrich) as internal standard, according to Equation 3 and Equation 4.
wheremCx: FA concentration in the sample (mg 100 mg-1 of ether extract), Ve: volume of solvent.
where Ci: FA concentration in the final extract (mg mL-1), Cs: concentration of the internal standard in the extract (mg mL-1), Ax: area of the compound of interest; As: area of the internal standard
Determination of minerals
This determination was carried out through plasm atomic emission spectrophotometry (Termo Electron Corporativo IRIS INTREPID II XSP Duo, USA). The quantification of potassium was accomplished through spectrophotometry and potassium dihydrogen orthophosphate was used (Sigma-Aldrich) as standard. The results were expressed in mg of the element by 100 g of dry solid.
Size of the sample
The size of the sample was determined with Equation 4 (Levin and Rubin, 2004). For each element of the sample, the determinations were performed by triplicate.
where N: total fruits in the population; za 2 = 2.57, for α = 0.01; p: possibility of obtaining information from the sample (0.5); q: possibility of not obtaining information from the sample (0.5); d: margin of maximum percentage error admitted (0.5)
Results and Discussion
Data analysis
According to the total number of fruit counted in the plants from the live collection, a sample of seven fruits per plant was determined, for a total of 35 fruits analyzed, without taking into account the number of replicas defined.
Morphological characterization
The Y. mixtecana fruits (Figure 1) have capsular shape, fine and thin epicarp, soft and slightly yellow mesocarp, which surround capsular tissue where the seeds are found (black matte color of tear shape).
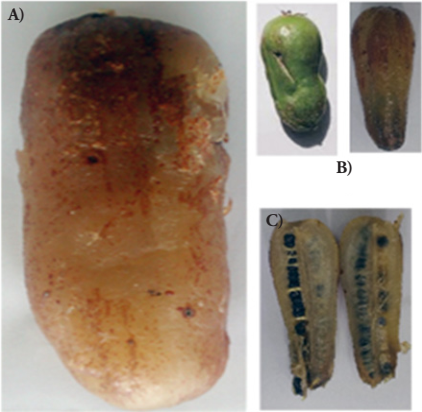
Figure 1 Yucca mixtecana fruits. A) Mature fruits; B) fruits in the first and third state of maturation; C) transversal view of the fruit.
The fruits are climacteric and at least three states of maturation were observed. The most evident was the third state characterized by accentuated darkening of the epicarp and mesocarp. In the first state of maturation, the epicarp and mesocarp are of green color and compact texture; the epicarp is strongly adhered. The fruit stem is difficult to separate from the plant.
In the second state of maturity, the epicarp is slightly adhered and the firmness of the mesocarp decreases without losing its consistency. The peduncle is easily separable from the plant, causing the fall of the fruit with ease. The color of the mesocarp changes toward uniform yellow. According to the methodology used to evaluate color, the mesocarp and the epicarp tend to exhibit a luminous color and tending toward reddish tones (Table 1).
Table 1 Parameters of fruit color.
| Tejido | Segundo estado de madurez | Tercer estado de madurez | ||||
|---|---|---|---|---|---|---|
| L* | C* | h° | L* | C* | h° | |
| Epicarpio | 49.80 | 25.78 | 1.01 | 39.52 | 22.48 | 0.96 |
| Mesocarpio | 55.59 | 44.95 | 0.82 | 46.32 | 38.62 | 0.84 |
In the third state of maturity, the mesocarp acquires a darker coloration (Table 1), the adherence of the epicarp is similar to the second state of maturity and the consistency is soft losing its structure in the mesocarp. The epicarp and the mesocarp lose luminosity although they continue exhibiting a characteristic reddish shade.
The gustatory sensation of the mesocarp is sweet with astringent retaste compared to the epicarp, which exhibits a neutral taste.
Physical characteristics
Most of the weight of the fruit is constituted by the mesocarp (Table 2), which represents a fraction of the weight similar to the endocarp. The main source of proteins in the human diet is tissues of animal origin or leguminous plants (peanuts, chickpeas, soy and others), although their consumption has inconveniences related to the quality of the proteins, and with the presence of diverse compounds with unwanted or harmful biological activity for human health (growth hormones, pesticides, presence of aflatoxins, among others).
Table 2 Physical properties of fresh Yucca mixtecana fruits.
| Peso (g) | 100.49 ± 19.73 |
| Ancho (cm) | 5.68 ± 0.50 |
| Largo (cm) | 11.70 ± 3.72 |
| Biomasa del epicarpio (g) | 10.10 ± 0.45 |
| Biomasa del mesocarpio (g) | 49.48 ± 10.42 |
| Biomasa del endocarpio (g) | 40.90 ± 12.74 |
| Humedad (gH2O gss -1) | 31.50 ± 1.78 |
| Sólidos totales, mesocarpio | 11.10 ± 0.70 |
| Acidez titulable (EAC 100 g-1) | 0.03 ± 0.00 |
† The results are the average of three replicas
The daily protein intake recommended for human diets varies from 16.2 g to 65 g d-1 (Latham, 2002), which can be satisfied adequately by the intake of fruits which can cover 3 % to 13.5 % of the recommended daily intake for human beings.
Composition of the nutritional variables of the mesocarp
Raw protein (RP)
The content of RP of the fruits (Table 3) is higher compared to most of the fruits of frequent consumption, whose average content is close to 1 % (Silva, 1994), with the exception of the fruits from Persea americana (avocado) which have 2.1 % of RP (Silva, 1994).
Total reducing sugars
The content of total reducing sugars (Table 3) is noticeably lower than what is found in most of the fruits of frequent consumption and varies from 6 to 22 % (Silva, 1994; Latham, 2002). Therefore, its incorporation into the daily intake as energetic complement would not be significant.
Total lipids
The content of lipids from the Y. mixtecana fruit (Table 3) indicates that it has non-oleaginous characteristics, with quite lower values than other fruits with high lipid content; for example, P. americana and Oleae europeae, with average values of 20 % (Silva, 1994).
Profile of fatty acids (FA)
The predominant FA were palmitic 42.40 mg 100 mg-1 of ethereal extract, oleic 39.40 mg 100 mg-1, and stearic 18.10 mg 100 mg-1 of ethereal extract.
The diets whose rate of concentration between unsaturated and saturated acids is higher than the unit (called Mediterranean diets) were correlated with a decrease in the risk of cardiovascular diseases, as well as alterations in generation of energy in mitochondria (Siri-Tarino et al., 2010). Even so, diets with a more balanced rate of FA concentration have positive correlation with better metabolic rates of energetic generation and use, and with lower rates of fractional oxidation of biological substrates caused by the excess consumption of oxygen post work (BØrsheim et al., 2006).
Our results show that the concentration of saturated fats is higher than the unsaturated, although the proportion of the latter is close to that one found in fruits of Helianthus annuus, Arachis hypogaea and Cocos nucifera. The Y. mixtecanafruit could be an important source of energy. The World Health Organization recommends consuming diets with lipid profiles where the poly-unsaturated acids should constitute 6 to 11 % of the total lipids ingested, but the saturated acids should be less than 10 % of the total (WHO, 2003; FAO, 2010).
Phenolic compounds
The content of total phenols (Table 4) was comparable to what was reported for fruits of Prosopis alba, 0.6 EAG g ss -1 (González-Galán et al., 2008), and higher to that of diverse Opuntia species, 0.153 EAG g ss -1 (Aquino et al., 2012).
Table 4 Total phenols and saponins in dry fruits of Yucca mixtecana.
| Fenoles totales (EAG g ss -1) | Saponinas (ST gss -1 ) |
|---|---|
| 0.63 ± 0.03 | 0.01 ± 0.00 |
† The results are the average of three replicas.
Our results show that the content of phenols and saponins is low so their anti-nutritious effect is minimal. The biological activities of phenolic compounds of foods are like vasodilators, anti-carcinogenic, modulators of the anti-inflammatory response, bactericides, and stimulators of the immune response (Jiang and Dusting, 2003). The inhibition of the oxidative action of free radicals (Owen et al., 2000) and the modulation of pro-carcinogenic mutagenic genes and inductors of inflammation processes (Bravo, 1998) are outstanding.
The following are some of the anti-nutrition aspects regarding the intake of phenolic compounds: decrease of the rates of protein adsorption, inactivation of digestive enzymes (Martínez-Valverde et al., 2000), and decrease of mineral bioavailability, primarily iron and zinc (Hurrell et al., 1997; Martínez-Valverde et al., 2000). The biological activity of the phenolic compounds depends on the concentration, type of phenolic compound and bioavailability (Jiang and Dusting, 2003; Galati and O´Brien, 2004).
Content of saponins
The content of saponins in fruits of Y. mixtecana is sensibly lower than the one reported in the epicarp and the mesocarp of Prosopis alba, 0.80 mg g-1 of dry solid (González-Galán et al., 2008), as well as in fruits of various species of the Opuntia genus related to the habitats where the Yucca genus thrives (0.70 ST gss -1) (Aquino et al., 2012).
The aglycone (sapogenin) and glucoside molecules of the saponins are related to unwanted or harmful effects for human and animal nutrition. The glucoside molecules cause the formation of foam in the digestive system, while the aglycone molecules are related to biological activity (Forturbel, 2003; Güclü-Üstündağ and Mazza, 2007). The biological activity derived from sapogenings (acid hydrolysis of the saponins) is related to hypo-cholesterolemic (López et al., 1993), anti-mycotic (Zamilpa et al., 2002), anti-viral (Aquino et al., 1991), anti-cancer (Sung et al., 1995), hypoglycemic (Kato et al., 1995), anti-trombotic (Zhang et al., 1999), diuretic (Silva et al., 2005), anti-inflammatory (da Silva et al., 2002), and molluscicide (Abdel-Gawad et al., 1999) effects. There is no information referring to the maximum mean intake of saponins recommended, although a high intake is not advised (Southonh et al., 1988).
Minerals
The fruits of Y. mixtecana (Table 5) are rich in magnesium and calcium. The mineral concentration in fruits has a significant correlation with the concentration of these in the soil, the water regime which the plant is subject to during the period of fructification, the weather and the genotype (Vig et al., 2003; Barceló and Poschenrieder, 2003; Rooney, et al., 2006). Therefore, the inferences that refer to the contribution of the Y. mixtecana fruits to the daily diet should be carried out within a multifactorial framework. Among the minerals quantified from the fruit, only iron and calcium are considered essential (James and Schofield. 1999). The consumption of yucca fruits supplements partially the recommended consumption of iron, of 21 to 48 mg d-1 and calcium, 400 to 700 mg d-1.
Conclusions
The nutritional composition evaluated in the fruits of Yuca mixtecana indicate that they can be considered as nutritional complements to the diet, since the moderate consumption of the fruit can contribute the nutritional requirements of proteins and fibers necessary in a balanced diet.
The relation between fatty acids indicates a higher proportion of saturated acids, which is advised against in the diet for humans. However, the concentration of total lipids in the yucca fruits is low compared to most foods.
The concentration of phenolic compounds and saponins is not a limiting factor in the intake of this fruit. The low concentrations of these compounds improve the sensorial characteristics and ensure low toxicity.
Literatura Citada
Abdel-Gawad M, M M., M. El-Sayed., and E S. Abdel-Hameed. 1999. Molluscicidal steroidal saponins and lipid content of Agave decipiens. Fitoterapia 70: 371-381. [ Links ]
Aguirre A, A. 2008. La útil Yucca. Ideas para el Cambio 1: 46-49. [ Links ]
Almaraz-Abarca N., Ma. da G., Campos, A., Ávila-Reyes, N., Naranjo-Jiménez, J., Herrera-Corral, L., S. González-Valdez. 2004. Variability of antioxidant activity among honeybee-collected pollen of different botanical origin. Interciencia 29: 574-578. [ Links ]
AOAC (Association of Official Analytical Chemists). 1995. Method 960.52: Microchemical determination of nitrogen. Micro-Kjeldahl method. Official Methods of Analysis of AOAC International. 12 th ed. Association of Official Analytical Chemists, Arlington. 1.12.07 p. [ Links ]
AOAC (Association of Official Analytical Chemists). 1996. Method 934.06: Moisture in Dried Fruits method. Official Methods of Analysis of AOAC International. 13 th ed. Association of Official Analytical Chemists, Arlington. 2.15.04 p. [ Links ]
AOAC (Association of Official Analytical Chemists). 2005. Method 923.03: Filth in grain products and brewers. Official Methods of Analysis of AOAC International. 18 th ed. Association of Official Analytical Chemists, Arlington. 32.2.02 p. [ Links ]
AOAC (Association of Official Analytical Chemists). 2006. Method 991.36: Fat (Crude) in meat and meat products. Official Methods of Analysis of AOAC International. 18 th ed. Association of Official Analytical Chemists, Arlington. 4.10.05 p. [ Links ]
Aoun M., G. Amiand, P. Garres, and P. Boide. 2003. Food supplement used in feed formulations in ruminants. WO Patent 03/056935 A1. [ Links ]
Aquino B, E N., Y. Chavarría M J., L Chávez S., R., I Guzmán G., E., R. Silva H., y I. Verdalet G. 2012. Caracterización fisicoquímica de siete variedades de tuna (Opuntia spp.) color rojo-violeta y estabilidad del pigmento de las dos variedades con mayor concentración. Investigación y Ciencia 20: 3-10. [ Links ]
Aquino R., C., Conti, F., de Simone, N., Orsi, C. Pizza C., and M. Stein. 1991. Antiviral activity of constituents of Tamus communis. J. Chemother. 3: 305-309. [ Links ]
Balandrin M., F. 1996. Commercial utilization of plant-derived saponins: An overview of medicinal, pharmaceutical, and industrial applications. Adv. Exp. Med. Biol. 404: 1-14. [ Links ]
Barceló, J., and C. Poschenrieder. 2003. Phytoremediation: principles and perspectives. Contrib. Sci. 2: 333-344. [ Links ]
BØrsheim, E., C K. Lawrence, and W M. Peral. 2006. Differential effects of dietary intake of palmitic acid and oleic acid on oxygen consumption during and after exercise. Metabolism 55:1215-1221. [ Links ]
Bravo, L. 1998. Polyphenols: Chemistry, dietary sources, metabolism and nutritional significance. Nutr. Rev. 56: 317-333. [ Links ]
Da Silva B, P., C. de Sousa A., M. Silva G., P. Mendes T., and P. Parente J. 2002. A new bioactive steroidal saponin from Agave attenuate. Z. Naturforsch. 57: 423-428. [ Links ]
Flores-Hernández, A J., A. Hernández-Herrera, H., Madinaveitia-Rios, L., M. Valenzuela N, Murillo-Amador, O., Rueda-Puente, J., L. García H, y H G. Ortiz-Cano. 2011. Evaluación de la población natural y hábitat de palma azul (Yucca rigida) en Mapimí, Durango, México. Trop. Subtrop. Agroecosys. 14: 315-321. [ Links ]
Food and Agriculture Organization. 2010. Fats and fatty acids in human nutrition: report of an expert consultation. In: Food and Agriculture Organization (ed). FAO Food and Nutrition Paper 91. pp: 43 - 53. [ Links ]
Forturbel, R. 2003. Problemática de la producción y comercialización de Chenopodium quinoa W. (Chenopodiaceae), debido a la presencia de saponinas. Ciencia Abierta 21: 1-10. [ Links ]
Galati, G., and J. O´Brien P. 2004. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer proprieties. Free Radic. Biol. Med. 37: 287-303. [ Links ]
Gobierno de los Estados Unidos Mexicanos, Secretaria de Economía (SE). 2010. Norma Mexicana NMX-F-102-NORMEX-2010. Determinación de acidez titulable en alimentos-método de ensayo (prueba). Diario Oficial de la Federación, México. 4 p. [ Links ]
Gobierno de los Estados Unidos Mexicanos, Secretaria de Economía (SE). 2010. Norma Mexicana NMX-F-112-NORMEX-2010. Alimentos-determinación de solidos solubles-método refractométrico. Diario Oficial de la Federación, México. 3 p. [ Links ]
Gobierno de los Estados Unidos Mexicanos, Secretaria de Economía (SE). 2010. Norma Mexicana NMX-F-312-NORMEX-2016. Determinación de reductores directos y totales en alimentos. Diario Oficial de la Federación, México. 5 p. [ Links ]
González-Galán A, A. Duarte-Correa C., M. de Abreu P.,y Ma de F. Piccolo-Barcelos. 2008. Caracterización química de la harina del fruto deProsopis spp. procedente de Bolivia y Brasil. Arch. Latinoam. Nutr. 58: 309-315. [ Links ]
Granados-Sánchez D., y G. López-Ríos. 1998. Yucca "Izote" del desierto. Rev. Chapingo Serie Cienci. Fores. Ambiente 4: 179-192. [ Links ]
Güclü-Üstündağ, O., and G. Mazza. 2007. Saponins: properties, applications and processing. Crit. Rev. Food Sci. Nutr. 47: 231-258. [ Links ]
Guzmán B, D., L. Cruz J., A. Alvarado, y P. Mollinedo. 2013. Cuantificación de saponinas en muestras de Cañihua chenopodium Pallidicaule Aellen. Rev. Bol. Quim. 30: 131-136. [ Links ]
Hurrell, R., M. Reddy M., and D. Cook J. 1997. Influence of polyphenol containing beverage on iron absorption. In: Proceedings of a European Cost Concerted Action Scientific Workshop (ed). Polyphenols in Foods. Aberdeen Press, Netherlands. pp: 169-172. [ Links ]
James, V., and P E. Schofield. 1999. Human Energy Requirements: A Manual for Planners and Nutritionists. Oxford University Press. Oxford. pp: 42-54. [ Links ]
Jensen, J., and T. Elgaard. 2001. Natural feed additive, a method of preparing said feed additive, a feed mixture containing the feed additive as well as a method of breeding farm animals. EP Patent 129,627 A1. [ Links ]
Jiang, F., and J. Dusting G. 2003. Natural phenolic compounds as cardiovascular therapeutics: potential role of their anti-inflammatory effects. Curr. Vasc. Pharmacol. 1: 135-156. [ Links ]
Kato, A., T. Miura, and T. Fukunaga. 1995. Effects of steroidal glycosides on blood glucose in normal and diabetic mice. Biol. Pharm. Bull. 18: 167-168. [ Links ]
Latham, M. C. 2002. Nutrición Humana en el Mundo en Desarrollo. Roma. Organización de las Naciones Unidas para la Agricultura y la Alimentación, ONU-FAO (Ed.). Roma, 480 p. [ Links ]
Levin R, I., and D S. Rubin. 2004. Estadística para Administración. 7a ed. Pearson Education, México. pp: 235-268. [ Links ]
López, R E S., O Ruiz, Madel R. Fusco, y A. Sosa. 1993. Aislameinto de un glicósido flavonoide y de una saponina del “mastuerzo” (Coronopus didymus Sm., Brassicaceae). Acta Farm. Bonaer. 12: 101-104. [ Links ]
Man, S., W. Gao, Y. Zhang, L. Hoang, and V. Lio. 2010. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 81: 703-714. [ Links ]
Martínez S, C J., C. Vinay, R. Brieva, and H. García. 1999. Lipase-catalyzed interesterification (acidolysis) of corn oil and conjugated linoleic acid in organic solvents. Food Biotechnol. 13: 183-193. [ Links ]
Martínez-Valverde I, Ma de JPeriago, y G. Ros. 2000. Significado nutricional de los compuestos fenólicos de la dieta. Arch. Latinoam. Nutr. 50: 1-19. [ Links ]
Owen R., W A. Giacosa, W. Hull, R. Haubner, G. Würtele, B. Spiegelhalder, and H. Bartsch. 2000. Olive-oil consumption and health: the possible role of antioxidants. Lancet. Oncol. 1: 107-112. [ Links ]
Olvera N., C., A. Martínez P., y E. Real de León. 1993. Manual de Técnicas para Laboratorio de Nutrición de Peces y Crustáceos. Organización de las Naciones Unidas para la Agricultura y la Alimentación, ONU-FAO (Ed.). México. 220 p. [ Links ]
Piña I, L. 1979. Algunas especies de Yucca del noreste de México. Cactáceas y Suculentas Mex. 19: 1-7. [ Links ]
Rooney C, P., J. Zhao F., and P. McGrath S. 2006. Soil factors controlling the expression of copper toxicity to plants in a wide range of European soils. Environ. Toxicol. Chem. 25: 726-732. [ Links ]
Saleem, M., M. Nazir, M. Ali, H. Hossain, Y. Lee, N. Riaz, and A. Jabbar. 2010. Antimicrobial natural products: an update on future antibiotic drug candidates. Nat. Prod. Rep. 27: 238-254. [ Links ]
Sant’anna V, P., D Gurak L., D Marczak I., and C. Tessaro. 2013. Tracking bioactive compounds with colour changes in foods-A review. Dyes Pigments 98: 601-608. [ Links ]
Silva G, M., M de Souzab A., S. Larab L., P. Mendesa T., P da Silvaa B., G. Lopesb A., C. Caruso-Nevesb., and P, Parentea J. 2005. A new steroidal saponin from Agave brittoniana and its biphasic effect on the Na+-ATPase activity. Z. Naturforsch 60: 121-127. [ Links ]
Silva P, C. 1994. Composición y evaluación de los componentes químicos de la plata (Persea americana Mill) durante su maduración. Alimentos. Rev. Soc. Chil. Tecnol. Aliment. 19: 88-102. [ Links ]
Siri-Tarino P, W. Sun Q., Hu F., and R M. Krauss. 2010. Saturated fatty acids and risk of coronary heart disease: modulation by replacement nutrients. Curr. Atheroscler. Rep. 12: 384-90. [ Links ]
Son S X, M., Y Li, W Fan., P Cheng, L Lio, and F Li. 2012. Effect and mechanism of AR-6 in experimental rheumatoid arthritis. Clin. Exp. Med. 10: 113-121. [ Links ]
Southon S, J., R. Wright A., S. Price K., J. Fairweather-Tait, and R. Fenwick G. 1988. The effect of three types of saponin on iron and zinc absorption from a single meal in the rat. Br. J. Nutr. 59: 389- 396. [ Links ]
Sung M., K., W. Kendall C., and V. Rao A. 1995. Effect of saponins and Gypsophila saponin on morphology of colon carcinoma cells in culture. Food Chem. Toxicol. 33: 357-366. [ Links ]
Vig, K., M. Megharaj, N. Sethunathan, and R. Naidu. 2003. Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: a review. Adv. Environ. Res. 8: 121-135. [ Links ]
World Health Organization. 2003. Diet, nutrition and the prevention of chronic diseases: report of a Joint WHO/FAO Expert Consultation. In: World Health Organization (ed). WHO Technical Report Series, No. 916. Geneva. pp: 54-132. [ Links ]
Zamilpa, A., J. Tortoriello, V.Navarro, G. Delgado, and L. Álvarez. 2002. Five new steroidal saponins from Solanum chrysotrichum leaves and their antimycotic Activity. J. Nat. Prod. 65: 1815-1819. [ Links ]
Zhang, J., Z. Meng, M. Zhang, A. Ma, S. Xu, and I. Kodama. 1999. Effect of six steroidal saponins isolated from Anemarrhenae rhizoma on platelet aggregation and hemolysis in human blood. Clin. Chim. Acta 289: 79-88. [ Links ]
Received: May 2017; Accepted: December 2017











 texto em
texto em 


