Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.51 no.5 Texcoco Jul./Ago. 2017
Crop Science
DL50 AND GR50 determination with gamma rays (60CO) on in vitro Laelia autumnalis protocorms
1Universidad Michoacana de San Nicolás de Hidalgo (UMSNH). Paseo de la Revolución esquina con Berlín, Colonia Emiliano Zapata, 60180. Uruapan, Michoacán. (selene451a@hotmail.com), (fernandezpavia@hotmail.com), (apalacios56@gmail.com), (codigogenetico@gmail.com).
2Colegio de Postgraduados Campus Puebla. Boulevard Forjadores de Puebla Núm. 205, Santiago Momoxpan, 72760. Municipio de San Pedro Cholula. (palopez@colpos.mx).
3Instituto Nacional de Investigaciones Nucleares. Carretera México-Toluca s/n. La Marquesa, 52750. Ocoyoacac, México. (eulogio.delacruz@inin.gob.mx).
Laelia autumnalis is an orchid with high ornamental value in Mexico. Yet, their marketing is restricted because no commercial quality varieties exist. Mutagenesis radiation is an effective method for generating varieties. These depend on the radiosensitivity of the radiated tissues. The aim of the study was to determine the lethal and mean reductive dose to gamma rays in L. autumnalis protocorms treated with 0, 5, 10, 15, 20, 25, 30, 35, 40, 45 and 50 Gy. The experimental design was completely random, with three repetitions and 500 to 700 protocorms as the experimental unit. Fory five days after irradiation (DAR) survival rates photosynthetic protocorms, protocorms with pro-meristems and leaves, and protocorm fresh matter were quantified. At 214 DAR we evaluated the number of leaves and roots, root length, leaf and pseudo-bulbs, width and length, and, fresh biomass and seedling biomass we evaluated in 15 seedlings per treatment. The data were analyzed with ANOVA, Pearson correlation, Tukey test (p≤0.05) and linear regression. Radiation with 5 to 20 Gy did not affect protocorms survival, doses between 20 and 30 Gy stimulated the chlorophyll presence. The length of the seedlings from the 5 Gy treatment increased 32 %. From 40 Gy fresh biomass (44 %) and leaf width (25 %) decreased. The correlation between radiation levels and protocorms survival (-0.91), leaf formation (-0.90), weight of fresh matter of protocorms (-0.69) seedling (-0.56) and seedling length (-0.57) were highly significant. The LD50 for protocorm survival and the mean reductive dose for leaf formation were 53 and 28 Gy, each. The radiation dose to induce variability in Laelia autumnalis protocorm is between 28 and 53 Gy.
Key words: Laelia autumnalis; breeding; mutagenesis; ionizing radiation; orchids
Laelia autumnalis es una orquídea con valor ornamental alto en México, pero su comercialización se restringe porque las variedades con calidad comercial no existen. La mutagénesis con radiación es un método efectivo para generar variedades y depende de la radiosensibilidad de los tejidos. El objetivo del estudio fue determinar las dosis letal y reductiva media a los rayos gamma en protocormos de L. autumnalis tratados con 0, 5, 10, 15, 20, 25, 30, 35, 40, 45 y 50 Gy. El diseño experimental fue completamente al azar, con tres repeticiones y 500 a 700 protocormos como unidad experimental. A los 45 d después de la irradiación (ddi) se cuantificaron los porcentajes de supervivencia, protocormos fotosintéticos, protocormos con promeristemos, con hojas, y materia fresca de protocormos. A los 214 ddi, en 15 plántulas por tratamiento, se evaluó el número de hojas y raíces, longitud de plántulas, pseudobulbos y raíces, anchura de hojas y pseudobulbos, materia fresca y biomasa de plántula. Los datos se analizaron con ANDEVA, correlación de Pearson, prueba de Tukey (p≤0.05), y regresión lineal. La radiación con 5 a 20 Gy no afectó la supervivencia de los protocormos, dosis de 20 a 30 Gy estimularon la presencia de clorofila. La longitud de plántulas se aumentó 32 % con 5 Gy. A partir de 40 Gy se redujo la materia fresca (44 %) y anchura de hojas (25 %). La correlación entre los niveles de radiación y la supervivencia de protocormos (-0.91), la formación de hojas (-0.90), el peso de materia fresca de protocormos (-0.69) y plántula (-0.56) y la longitud de plántula (-0.57) fue altamente significativa. La DL50 para la supervivencia de protocormos y la dosis reductiva media para formación de hojas fueron 53 y 28 Gy, respectivamente. La dosis de radiación para inducir variabilidad en protocormos de Laelia autumnalis está entre 28 y 53 Gy.
Palabras clave: Laelia autumnalis; mejoramiento genético; mutagénesis; radiación ionizante; orquídeas
Introduction
The orchid Laelia autumnalis is native to Mexico, has inflorescences with showy purple flowers in various shades, shapes, sizes and essences. This species has been used as an ornamental species since prehispanic times by different indigenous groups. Nevertheless, it has never been subject to a breeding program. Wild specimens are illegally traded in local and regional markets from diverse communities in Mexico at very low prices (Beltran-Rodriguez et al., 2012). The generation of new and improved varieties can increase its value and acceptance of this species in domestic markets and constitute an economic advantage in an export market.
Improvement by mutagenesis is one of the most efficient methods to generate new plant cultivars (Aros et al., 2012; Kazi, 2015), and ornamental plants, particularly orchids (Kikuchi, 2000; Luan et al., 2012; Lee et al., 2015), anthurium (Puchooa, 2005), and chrysanthemums (Yamaguchi et al., 2008; Kumar et al., 2012). If the use of ionizing radiation techniques is combined with in vitro culture, time and costs for the development of new cultivars are reduced (Yunus et al., 2013), a continuous large-scale production of plants can be obtained, as well as maintain and multiply mutant individuals (Barakat and El-Sammack, 2011; Angeles-Espino et al., 2013) Also, the somaclonal variation that can occur during micropropagation offers the possibility to increase the frequency of gene and point mutations, which are important for genetic improvement (Estrada-Basaldua et al., 2011). The in vitro culture is essential for asymbiotic seed germination of orchids, which in their habitat require the symbiosis with mycorrhizal fungi for that process (Verma et al., 2014).
Applying ionizing radiation in plants has improved a wide range of characters such as architecture, performance, flowering, and tolerance to biotic and abiotic stress (Kon et al., 2007). The main advantage is its ability to change one or few characters in an adequate cultivar without changing the rest of the genotype (Patil et al., 2015). The efficiency of this radiation is high because about 89 % of the plant varieties obtained by mutagenesis were developed with physical mutagens, such as X rays, gamma rays and fast neutrons. Gamma rays are within the most mutagens used in cultivated species (grains, fruits and ornamentals), because with this technique 60 % of plant varieties mutants has been developed (Kon et al., 2007).
Mutagens alter DNA and cause point mutations, deletions and chromosomal aberrations (Tanaka et al., 2010) so that new individuals have significant changes in their genomic structure (Emmanuel and Levy, 2002). The mutagenesis technique is particularly efficient in plants with long juvenile periods before flowering and seed production (Predieri, 2001), as well as in native ornamental plants with limited genetic variability (Lee et al., 2008).
Physical mutagens, particularly gamma radiation, are tiny particles of ionizing radiation having a high capacity of penetrable energy on biological tissues (Sadhukhan et al., 2015). It’s wavelength is 10 nm (Kitano et al., 2015). Its biological effect is based on the interaction with atoms or molecules, particularly water, in the cell to produce free radicals and make changes in bases and breaks in hydrogen bonds between complementary strands of DNA (Kovács and Keresztes, 2002). These radicals can damage or modify major components of plant cells and change their morphology, anatomy, biochemistry and physiology, depending on the radiation dose (Wi et al., 2007). These effects include changes in cell structure and plant metabolism (Kovács and Keresztes, 2002), such as dilation of the thylakoid membranes, alteration in photosynthesis, modulation of the antioxidant system and accumulation of phenolic compounds (Wi et al., 2005). The cell nucleus is the organelle most affected by ionizing radiation (Pavan et al., 2013).
The radiation effect on cell tissues is divided into three phases. The physical or initial phase lasts only a split second, the chemical phase lasts a few seconds and the biological stage in which the time scale varies from tens of minute to decades (Pavan et al., 2013).
The interval in which the appearance of useful mutations in breeding programs is favored is the median lethal dose (LD50) or mean reductive dose (GR50), This is the amount of radiation absorbed by the population to which 50 % survives or the growth is reduced by 50 %, so it is important to know this dosage range (Ángeles-Espino et al., 2013) before starting a breeding program assisted by mutagenesis. The LD50 is unique for each species, genotype and even in different tissues of the same plant. For example, in callus generated from ligulate flowers of Chrysanthemum morifolium cv. Delistar White with a DL50 of 0.5 Gy, changes in the flower´s shape and the number of flowers per inflorescence were obtained (Barakat et al., 2010). The LD50 of Tricyrtis hirta embryogenic callus was determined at 20 Gy and variants with dwarfism, with more green thin leaves and increased number and size of flowers were obtained (Nakano et al., 2010). In seeds of Moluccella laevis the DL50 of 25 Gy increased the number of flowers per stem (Minisi et al., 2013). For leaf explants of Torenia fournieri the DL50 was 63 Gy and 72 Gy in diploid and polyploid plants, both cases obtained mutants with red leaves (Chanchula et al., 2015).
The radiosensitivity of tissues to gamma radiation is not known for the orchid’s native to Mexico, nor has the optimal dose for variants of ornamental importance been determined. Therefore, the objective of this study was to determine the DL50 and GR50 with gamma rays (60Co) in L. autumnalis protocorms cultured in vitro. Our hypothesis was that there is an optimal dose to generate variants of L. autumnalis from irradiated protocorms.
Materials and Methods
This research was conducted at the in vitro Plant Tissue Laboratory of the Facultad de Agrobiología of the Universidad Michoacana de San Nicolas de Hidalgo, located in Uruapan, Michoacán, México (19° 23’ 41’’ N and 102° 03’ 31’’), from May 2014 to March 2015. Protocorms irradiation was conducted at the Gamma Irradiator Department of the Instituto Nacional de Investigaciones Nucleares (ININ), located at Ocoyoacac, State of Mexico, Mexico.
Plant material
In this study, powder seeds from six fruit obtained via manual autofecundation from L. autumnalis plants, from the germplasm bank of the National Plant Genetic Resources System Collection, were used.
Culture medium
Culture medium with the Murashige and Skoog (1962) (MS) mineral salts was used, without phytohormones and supplemented with sucrose (30 g L-1), myoinositol (100 mg L-1), thiamine (0.4 mg L-1) and agar (6 g L-1). The pH was adjusted to 5.7±0.1 with NaOH 1N. 20 mL of this medium were served in 100 mL glass bottles and autoclaved for 15 min at 1.2 kg cm-2 pressure and 121 °C.
Aseptic culture establishment
For planting, 33 sample seeds (20 mg each) were placed in 10 mL syringes, and disinfection with commercial sodium hypochlorite 15 % v/v (6 % active ingredient) for 15 min was performed. In a laminar flow hood disinfectant solution was removed and three rinses with sterile water were performed. The powder seeds of each sample were suspended in 0.5 mL of sterile water and placed in a vial with culture medium. Their incubation was carried out in a 16/8 h light/dark photoperiod and photosynthetically active radiation of 45 μE m-2 s-1 provided by 75 W white fluorescent light lamps.
Irradiation with 60Co gamma rays
Forty-five days after sowing (das), the obtained protocorms were treated with ten irradiation doses (5, 10, 15, 20, 25, 30, 35, 40, 45 and 50 Gy) plus a control treatment without irradiation. The experimental design was completely randomized with 11 treatments and three repetitions, the experimental unit was a vial with protocorms obtained from germination of 20 mg of seeds. The amount fluctuated between 500 and 700 protocorms. One day after irradiation (46 das), the protocorms of each bottle were transferred to fresh culture medium. At 90 das, these were transferred to Petri dishes in order to facilitate their evaluation. Throughout all this time, the development of the protocorms was divided into three phenological stages: photosynthetic protocorms, protocorms with pro-meristems and protocorms with leaves. These phases were observed simultaneously in all the experimental units because the seedlings development in L. autumnalis is an asynchronous process.
At 135 das, protocorms were subcultured in 250 mL plastic containers with 50 mL of fresh basal medium and incubated in the already described environmental conditions.
Variables evaluated in protocorms
In the protocorms, we assessed the percentages of survival, photosynthetically active, and with pro-meristems and leaves. Each experimental unit (bottle) was divided into quadrants to facilitate counts. In each quadrant, the number of living, dead, photosynthetic, with pro-meristems and leafs protocorms were counted. With the addition of protocorms at each phenological stage, the total protocorm number per experimental unit was obtained. This was used as denominator to obtain the survival percentage, percentage of photosintetic protocorms, with pro-meristems and leaves as follows: survival percentage per experimental unit = (number of living protocorms per vial/total number of protocorms per flask) x 100. The same procedure was used for each variable.
Protocorm fresh matter. The total protocorms per experimental unit were weighed on an analytical balance (OHAUS®) with 0.01 mg readability.
These variables were assessed 45 d after irradiation (90 das).
Variables evaluated in the seedlings
We assessed the seedling, root and pseudobulbs length. The distance between the base and the apex of the seedling, pseudobulbs and root with digital vernier was measured.
Leaves and pseudobulb width. It was measured in the intermediate area of each organ with a digital vernier.
Number of leaves and roots. It was quantitated directly on each seedling.
Seedlings fresh mass (mg). Each seedling was weighed on a digital analytical balance (OHAUS®) with 0.01 mg readability.
Seedling biomass. Each seedling was dried in an oven (FELISA), at 70 °C for 72 h. The dry seedlings were then weighed into digital analytical balance (OHAUS®) with a 0.01 mg readability.
The assessment of these variables was performed in five seedlings per repetition, a total of 15 per treatment, at 214 d after irradiation (259 das).
Statistical analysis
An ANOVA was performed on the data obtained at 45 and 214 days after radiation, the Pearson correlation coefficient was calculated and the Tukey test was carried out for means comparison between treatments. These were performed with the SAS software version 9.0 (Statistical Analysis System, 2002).
In order to assess the magnitude of the response of each variable to the radiation doses a linear regression model was evaluated. However, only the survival and leaves emergence variables adjusted to this model, thus, the LD50 and GR50 determination was performed only for these variables.
Results and Discussion
Radiation effect on protocorm development
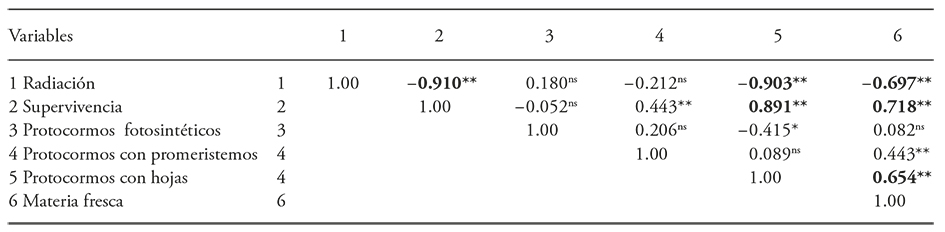
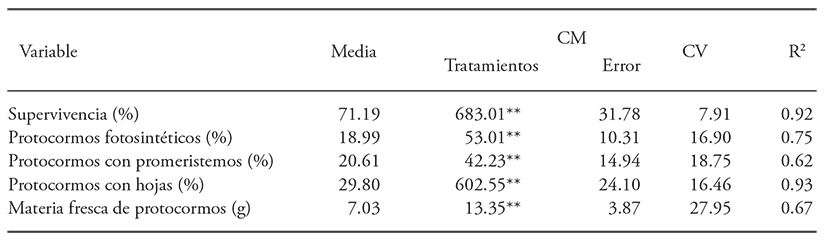
The analysis of variance revealed that the radiation affected the protocorm’s survival, the presence of photosynthetic protocorms, protocorm with pro-meristems, with leaves and fresh protocorm matter (Table 1).
Table 1 Analysis of variance for the 60Co gamma radiation effect on survival and development of Laelia autumnalis protocorms
**p≤0.01; ns = not significant.
Radiation at low doses (5 to 20 Gy) did not affect the survival of protocorms compared to the control treatment, although there was a reduction of live protocorms between 17.6 % and 46.4 % in the tissues treated with 25 to 50 Gy (Figure 1A). Doses between 20 and 30 Gy stimulated chlorophyll presence in the protocorms, which was noted as a change from the light yellow coloration to green (Figure 1B), statistically not differents to those of 45 Gy. The fresh material accumulation was similar between the protocorms of the control treatment and those treated with radiation levels, but different between those irradiated at 5 and 10 Gy regard those treated with 35 Gy, the first with 69 % and 66 % more accumulation of fresh matter (Figure 1C). A similar response was observed for the promeristems formation, which was statistically similar in irradiated treatments and the control, but almost twice in the irradiated protocorms at 20, 30 and 35 Gy, compared to the 50 Gy (Figure 1D).
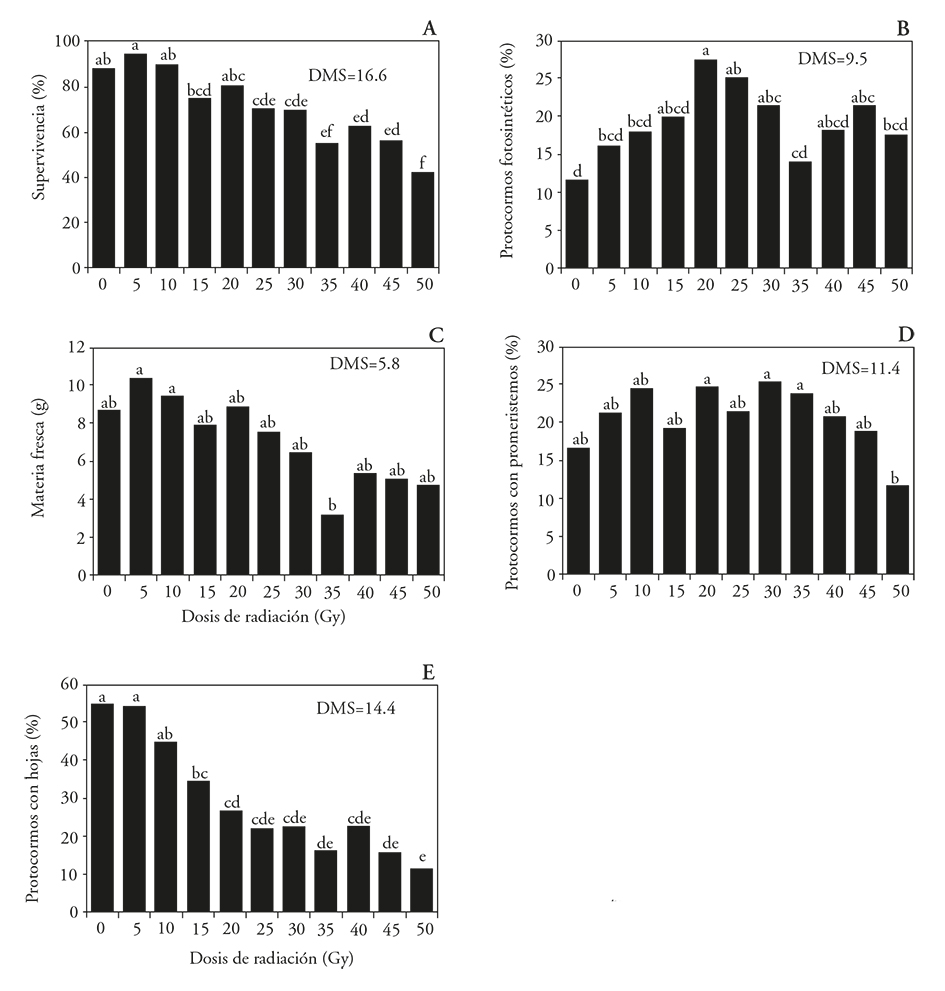
Figure 1 Effect of 60Co gamma radiation on the survival and development of Laelia autumnalis protocorms. A) Protocorms survival, B) photosynthetic protocorms, C) fresh matter protocorm, D) protocorms with promeristems and E) protocorms with leaves. LSD: Least Significant Difference. In each figure, different letters show significant differences (Tukey; p≤0.05).
The formation of protocorms with leaves did not differ (p>0.05) between the non-radiated seedlings and those treated with 5 and 10 Gy. From 15 Gy on, protocorms with leaves formation was reduced (p≤0.05), as the irradiation dose increased (Figure 1E). The explants development accelerated at low radiation doses (radio-stimulation), but decreased with the increasing gamma radiation doses, as in Chrysanthemum morifolium in vitro callus (Soliman et al., 2014).
Although our investigation did not carried out assessments at molecular level, it is known that tissue damage with increasing gamma radiation dose occurred, even though the cells have a DNA-repair mechanism that acts when exposed to any source of radiation. Nevertheless, if the intensity of the radiation source is too high, this mechanism cannot repair any damaged cells, which die as is the case in ginger plants (Zingiber officinale) (Yoon et al., 2014). Free radicals that cause metabolic disorders are produced in surviving cells (Pavan et al., 2013), the gene expression pattern is modified (Corthals et al., 2000), which regulates certain metabolic pathways and defense systems (Zolla et al., 2003), causing quantitative and qualitative changes in the total soluble protein content (Corthals et al., 2000). These proteins have an important role in signal transduction in antioxidant defense, antifreeze, heat shock, anti-pathogenesis, and osmolytes synthesis, which are essential for the functions and growth of plants (El-Fiki et al., 2015). These alterations at cellular level, caused either by physiological or physical malfunctions, which include chromosome damage and reduced survival as the radiation dose increases, are reported in Jatropha curcas L. (Dhakshanamoorthy et al., 2011), as well as in four commercial varieties of Lycopersicon esculentum (Ramirez et al., 2006), Withania somnifera (Bhosale and More, 2014), Orthosiphon stamineus (Kiong et al., 2008) and Chrisanthemun (Yamaguchi et al., 2009).
The correlation coefficients were negative and highly significant between the levels of applied radiation and protocorms survival (-0.91), protocorms with leaves (-0.90) and fresh protocorm matter (-0.69). There was also a positive correlation between survival and protocorms with leaves (0.89) and between fresh protocorm matter (0.71) and the formation of protocorms with leaves and protocorm fresh matter (0.65) (Table 2).
Radiation effect on seedling development
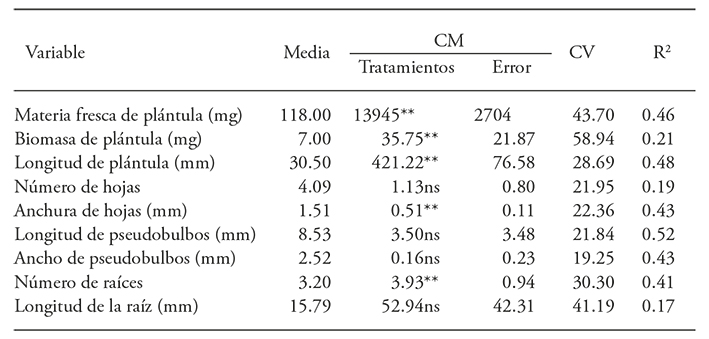
The ANOVA revealed that the radiation influenced fresh matter, biomass and length seedling, roots and leaves width, but did not affect the number of leaves, pseudobulbs length and width and number of roots (Table 3).
Table 3 Analysis of variance for the 60Co gamma radiation effect on Laelia autumnalis seedling development.
**p≤0.01; ns: not significant.
The length of the seedlings increased 32 % relative to the unirradiated treatment when radiation at k5 Gy was used. This suggests a radio-stimulant growth effect at low radiation doses; at 35, 40 and 50 Gy seedlings were 31, 50 and 42 % smaller than the unirradiated seedlings (Figures 2A and 3). The greatest fresh material accumulation (189 and 176 mg) was recorded in seedlings treated with 5 and 10 Gy. This was statistically similar to the control and treated with 15 Gy, but superior to other treatments (Figure 2B). The width of the leaves decreased by 25 and 29 % with doses of 40 and 50 Gy as compared to control: the roots number decreased by 37% with 45 Gy (Figure 2C and D). Biomass accumulation was similar in all seedlings treatments, except in those treated with 5 and 15 Gy, which accumulated almost twice the biomass than those irradiated with 45 Gy (Figure 2E). The biomass decreased by the increase in the radiation dose was observed in Arabidopsis seedlings that inhibit carbohydrate transport when treated with radiation dosages greater than 50 Gy (Bondada and Oosterhuis, 2003), because of damage and disorientation in the grana of the thylakoids, as well as increased accumulation of starch granules in the chloroplasts. These ultrastructural changes were not observed in the chloroplasts of cells irradiated with low doses (Wi et al., 2007).
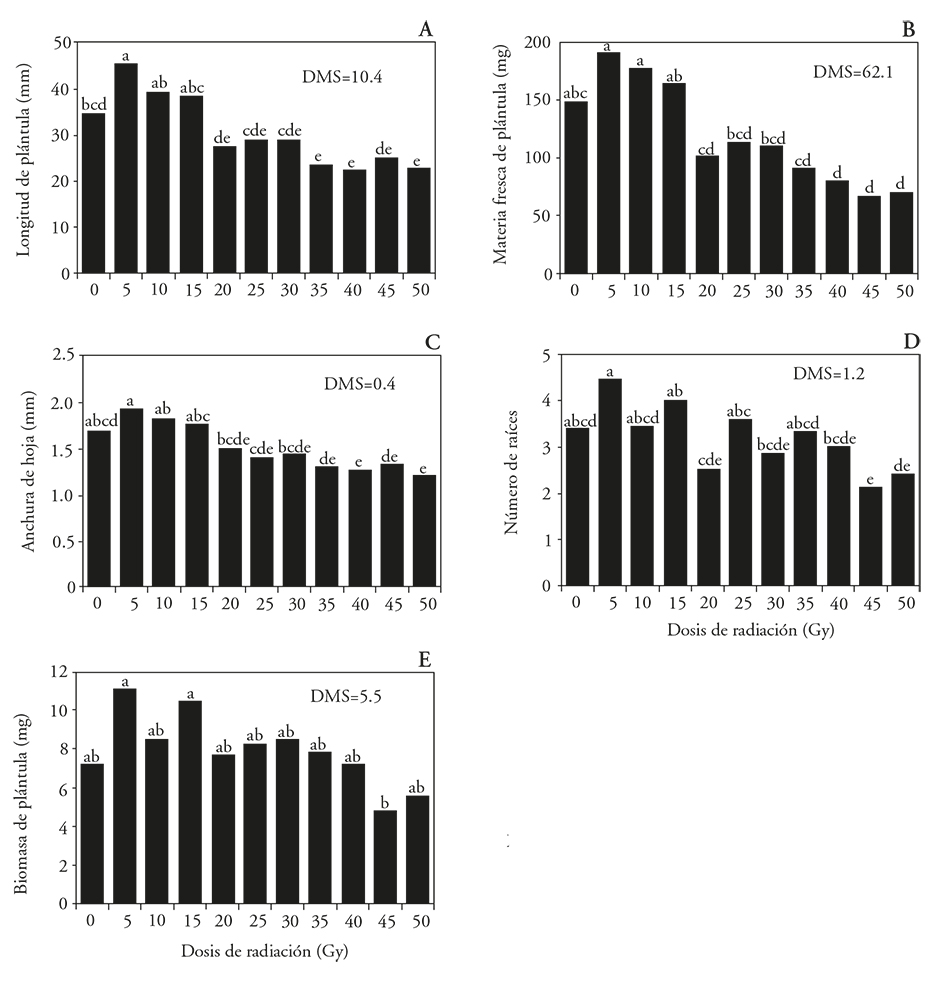
Figure 2 Effect of 60Co gamma radiation on L. autumnalis seedlings from 60Co irradiated protocorms. A) Length, B) fresh matter, C) leaf width, D) number of roots and E) seedling biomass. LSD: Least Significant Difference. In each figure, different letters show significant differences (Tukey; p≤0.05).

Figure 3 Seedlings of Laelia autumnalis from protocorms irradiated with 60Co gamma rays at different radiation doses.
In contrast to the radioinhibition phenomenon, the hormesis observed at 5 Gy dose on L. autumnalis seedlings growth is described alike to the stimulatory effect of any growth factor on an organism (Szarek, 2005). This is attributed to a change in the hormone signaling network in plant cells or an increase in the antioxidant capacity of the cells in order to overcome stress factors such as fluctuations of light intensity and temperature in growth conditions (Kim et al., 2004). In addition, radiation accelerates the rate of cell division and actives auxin, this phenomenon has been reported in Gypsophilla paniculata in which, when calluses were irradiated with 1 Gy increased shoot length (3.4 cm) was obtained (Barakat and El-Sammack, 2011). In L. esculentum there is a radio-stimulant interval between 5 and 25 Gy for plant height (Ramírez et al., 2006); the gamma radiation on seeds of Triticum durum increased between 32 and 75 % the number and length of roots when irradiated with 20 Gy (Melki and Marouani, 2010). In the regeneration of embryogenic callus of Heliconia psittacorum irradiated with 60Co gamma radiation, the 30 Gy dose showed the highest percentage of regenerated callus (80.8 %), 20.8 % more than in the unirradiated treatment and 74.8 % more than in callus treated with 75 Gy (6 %) (Urrea and Ceballos, 2005).
The correlation coefficients were negative and highly significant between the different levels of applied radiation and the seedlings fresh matter (-0.57), seedling length (0.56), leaf width (-0.52), number of roots (-0.40) and length of pseudobulbs (-0.40) (Table 4). This confirms a negative correlation between the energy absorbed per unit mass in the applied dose and the growth and development variables of the plants (Giovannini et al., 2015). In Arabidopsis this inhibition is attributed to the arrest of the cell cycle at the G2/M phase during the division of somatic cells or by genome damage (Preuss and Britt, 2003). On chrysanthemums this is attributed to the generation of chromosomal aberrations and the loss of the cells proliferative capacity (Patil et al., 2015) and damage to the genes involved in the morphogenesis of true leaves, as in Rumex obtusifolius leaves irradiated with 500 Gy (Kitano et al., 2015). In Agave tequilana the absorbed radiation produces mutations in the cell’s DNA and alter their normal development as the radiation dose increases (Ángeles-Espino et al., 2013).
Table 4 Correlation of the 60Co gamma radiation effect on seedling development of Laelia autumnalis.
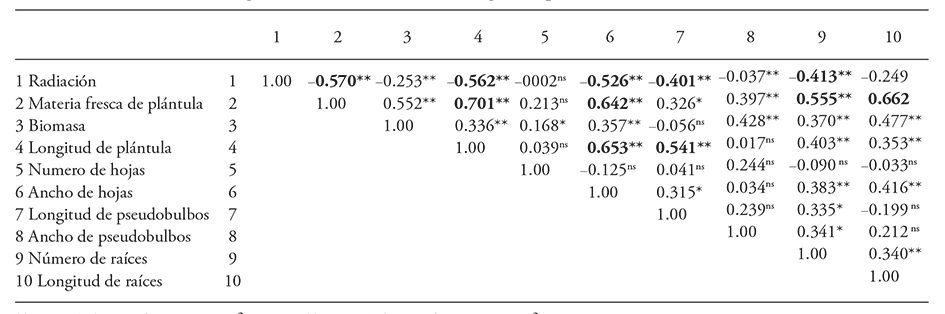
**p≤0.01; *≤0.05; ns: not significant.
There were positive correlation coefficients between fresh matter and seedling length (0.70), root length (0.66), leaf width (0.64) and roots number (0.55). Significant correlation coefficients were also present between the length of seedling and width leafs (0.65) and pseudobulb length (0.54).
Determination of LD50 and GR50
The linear regression model showed the best adjustment to explain the radiation effect on the protocorms survival (Table 5). The protocorm and seedlings fresh matter showed a tendency to decrease as the radiation dose increased. However, they presented a low R2 value (0.48 and 0.35) and, therefore, these variables were not considered for the calculation of the GR50.
Table 5 Regression analysis for protocorm survival and leaf emergency in Laelia autumnalis protocorms irradiated with 60Co gamma radiation.
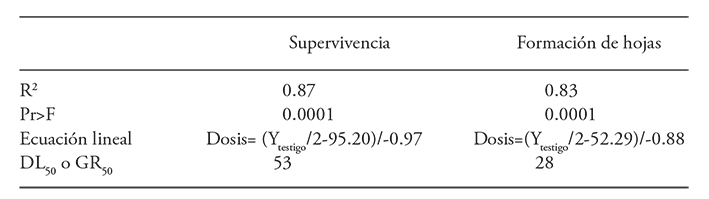
The average lethal dose for the protocorms survival was 53 Gy (Figure 4), which is considered as the dose that favors useful mutations development. The average growth reduction dose (GR50) for forming protocorm leaves was 28 Gy (Table 5 and Figure 4).
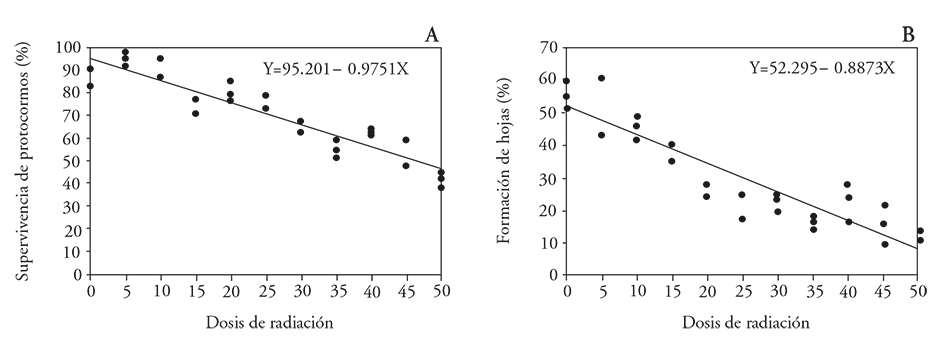
Figure 4 Effect of 60Co gamma radiation on Laelia autumnalis orchid protocorms. A) Protocorms survival rate and B) leaf formation in protocorms.
The LD50 for L. autumnalis differs to that reported for Alstroemeria aurea G. rhizomes irradiated with gamma rays at doses between 0 and 40 Gy, and LD50 was determined at 40 Gy; this dose reduced survival explants in 50 %, and might be used for breeding purposes (Aros et al., 2012). The GR50 for the percentage of seed germination in two varieties of rice (Oryza sativa L.) irradiated with 60Co gamma radiation from 20 to 200 Gy, was 89 and 188 Gy in the JGL and Vijetha varieties (Pavan et al., 2013). In strawberries shoots irradiated with doses between 30 to 325 Gy the survival LD50 was 177 Gy (Murti et al., 2013); long bean seeds (Vigna sesquipedalis) irradiated with 300 to 800 Gy had a LD50 for survival between 600 and 800 Gy, and GR50 for plant height was between 400 and 500 Gy (Kon et al., 2007).
Based on those data, it is confirmed that the DL50 and GR50 are specific to each species, genotype and even size and type of the irradiated tissue, and that the sensitivity to radiation depends on the nature and extent of chromosomal damage (Kiong et al., 2008; Pavan et al., 2013). Besides, it varies depending on the DNA content, size and number of chromosomes (Sparrow et al., 1961), and upton water content in the irradiated plant material, since the radiation interacts with molecules, particularly water, to produce free radicals in the cells. These radicals modify various important compounds in plants cells, because of which, seeds tolerate higher LD50 due radiation doses compared to succulent tissues (Yunus et al., 2013).
The LD50 determined for L. autumnalis seedlings confirms that this species is highly sensitive to gamma radiation. This sensitivity is also observed for solar radiation, which is attributed to the fact that L. autumnalis is an epiphytic species that lives under tree canopies. In epiphytic orchids high solar radiation intensities reduce photosynthetic capacity and affect gas exchange (Stancato et al., 2002).
The average reductive dose of 28 Gy for L. autumnalis protocorms to form leaves is similar to that of Agave fourcroides, in which, a GR50 of 20 Gy for fresh weight was identified. Besides, weight of fresh callus material was an excellent indicator of radiosensitivity (González et al., 2007). In A. tequilana explants irradiated with gamma rays at doses up to 50 Gy the average reductive dose was between 20 and 25 Gy for seedlings formation and 15 to 25 Gy for callus formation. These doses are likely to induce favorable mutations for selection and increased breeding purposes (Ángeles-Espino et al., 2013). However, the GR50 determined for leaves formation for L. autumnalis protocorms is high when compared with the results in Etlingera elatior explants irradiated with 60Co gamma rays from 0 to 140 Gy, where the DL50 for survival explants was determined to be at 10 Gy (Yunus et al., 2013). Also, in nard tubers (Polianthes tuberosa L.) irradiated with gamma ray doses, between 0 to 30 Gy, LD50 was 9.09 Gy for the survival of acclimated seedlings from in vitro cultured shoots; this was not the case for the tubers established in vivo, for which a LD50 of 25.91 Gy for the survival of plants was determined (Estrada-Basaldua et al., 2011).
Conclusions
The 60Co gamma radiation exercised an inversely proportional effect on growth and development of L. autmnalis seedlings. Low doses stimulate seedling growth, high doses inhibit protocorms survival, leaf formation and seedling fresh material.
Irradiation dose between 28 and 53 Gy may be used in breeding programs of L. autumnalis in order to promote mutations that favor variants of ornamental importance
Literatura Citada
Ángeles-Espino A., J. A. Valencia-Botín, G. Virgen-Calleros, C. Ramírez-Serrano, L. Paredes-Gutiérrez, y S. Hurtado-De la Peña. 2013. Determinación de la dosis letal (DL50) con 60Co en vitroplantas de Agave tequilana var. Azul. Rev. Fitotec. Mex. 36: 381-386. [ Links ]
Aros D., S. Valdés, E. Olate, and R. Infante. 2012. Gamma irradiation on Alstroemeria aurea G. in vitro rhizomes: an approach to the appropriate dosage for breeding purposes. Rev. FCA UNCUYO 44: 191-197. [ Links ]
Barakat M., N., and H. El-Sammak. 2011. In vitro mutagenesis, plant regeneration and characterization of mutants via RAPD analysis in baby’s breath Gypsophila paniculata L. Austr. J. Crop Sci. 5: 214-222. [ Links ]
Barakat M. N., R. S. Abdel-Fattah, M. Badr, and M. G. El-Turky. 2010. In vitro mutagenesis and identification of new variants via RAPD markers for improving chrysanthemum morifolium. Afr. J. Agric. Res. 5: 748-757. [ Links ]
Beltrán-Rodríguez, L. A., B. Martínez-Rivera, y A. P. Maya 2012. Etnoecología de la flor de catarina - Laelia autumnalis (La Llave & Lex. Lindl.) (orchidaceae) en una comunidad campesina al sur del estado de Morelos, México: conservando un recurso y preservando saberes populares. Etnobiología 10: 1-17. [ Links ]
Bondada B., R., and D. Oosterhuis M. 2003. Morphometric analysis of chloroplast of cotton leaf and fruiting organs. Biol. Plantarum 47: 281-284. [ Links ]
Bhosale R., S., and A. More D. 2014. Effect of gamma radiation on seed germination, seedling height and seedling injury in Withania somnifera, (L.) Dunal. Int. J. Life Sciences 2: 226-228. [ Links ]
Chanchula N., T. Taychasinpitak, A. Jala, T. Thanananta, and S. Kikuchi. 2015. Radiosensitivity of in vitro cultured Torenia fournieri Lind. from Thailand by α-ray irradiation. Int. Trans. J. Eng. Manag. Sci. Tech. 6: 157-164. [ Links ]
Corthals G. L., S. P. Gygi, R. Aebersold, and S. D. Patterson. 2000. Identification of proteins by mass spectrometry. In: Rabilloud, T. (ed). Proteome Research: Two-Dimensional Gel Electrophoresis and Identification Methods. Springer Verlag Berlin Heidelberg. pp: 197-231. [ Links ]
Dhakshanamoorthy D., R. Selvaraj, and A. L. A. Chidambaram. 2011. Induced mutagenesis in Jatropha curcas L. using gamma rays and detection of DNA polymorphism through RAPD marker. C. R. Biologies 334: 24-30. [ Links ]
El-Fiki A., G. El-Metabteb, S. Abdel-Hadi, and M. Adly. 2015. Androgenesis induced in Nicotiana alata and the effect of gamma irradiation. Not. Sci. Biol. 7: 66-71. [ Links ]
Emmanuel E., and A. A. Levy. 2002. Tomato mutants as tools for functional genomics. Curr. Opin. Plant. Biol. 5: 112-117. [ Links ]
Estrada-Basaldua J. A., M. E. Pedraza-Santos, E. De la Cruz-Torres, A. Martínez-Palacios, C. Sáenz-Romero, y J. L. Morales-García. 2011. Efecto de rayos gamma 60Co en nardo (Polianthes tuberosa L.). Rev. Mex. Cienc. Agríc. 3: 445-458. [ Links ]
Giovannini A., V. Scariot, M. Caser, A. Buttafava, A. Mansuino, G. G. Ghione, M. Savona, M. E. Sabatini, D. Carboner, and A. Balestrazzi. 2015. Mutation breeding using gamma rays to increase seed germination in Rosa hybrida. Acta Hortic. 1087: 373-378. [ Links ]
González O., G., S. Alemán G., M. Garriga, R.Ortíz, and C. De la Fe . 2007. Radio sensi tivity to gamma rays (60Co) in shoot tips of henequen. Biot. Veg. 7: 115-117. [ Links ]
Kazi N. A. 2015. Mutation breeding in flower crops. A. J. M. S. 3: 228-230. [ Links ]
Kikuchi K., O. 2000. Orchid flowers tolerance to gamma-radiation. Radiat. Phys. Chem. 57: 555-557. [ Links ]
Kim J. H., M. H. Baek, B. Y. Chung, S. G. Wi, and J. S. Kim. 2004. Alterations in the photosynthic pigments and antioxidant machineries of red pepper (Capsicum annuum L.) seedlings from gamma-irradiated seeds. J. Plant Biol. 47: 314-321. [ Links ]
Kiong L., A. P., A. G. Lai, S. Hussein, and A. Rahim H. 2008. Physiological responses of Orthosiphon stamineus plantles to gamma irradiation. Am.-Eurasian J. Sustain. Agric. 2: 135-149. [ Links ]
Kitano S., A. Miyagi , Y.Oono, Y.Hase, I. Narumi, M. Yamaguchi, H.Uchimiya , and M.Kawai-Yamada. 2015. Metabolic alterations in leaves of oxalate-rich plant Rumex obtusifolius L. irradiated by gamma rays. Metabolomics 11: 134-142. [ Links ]
Kon E., O.Haruna A ., S. Shaharudin, and N. M. Ab M. 2007. Gamma radiosensitivity study on long bean (Vigna sesquipedalis). Am. J. Appl. Sci. 4: 1090-1093. [ Links ]
Kovács E., and A. Keresztes. 2002. Efect of gamma and UV-B/C radiation on plant cells. Micron 33: 199-210. [ Links ]
Kumar B., S. Kumar, and M.Thakur. 2012. In vitro mutation induction and selection of chrysanthemum (Dendranthema grandiflora Tzelev) lines with improved resistance to Septoria obesa Syd. International J. Plant Res. 2: 103-107. [ Links ]
Lee G. J., S. C. J. Chung, I. S. Park, J. S. Lee, J. B. Kim, D. S. Kim, and S. Y. Kang. 2008. Variation in the phenotypic features and transcripts of color mutants of chrysanthemum (Dendranthema grandiflorum) derived from gamma ray mutagénesis. J. Plant Biol . 51: 418-423. [ Links ]
Lee Y. M., Y. D. Jo, H. J. Lee, Y. S. Kim, D. S. Kim , J. B. Kim , S. Y. Kang , and S. H. Kim. 2015. DNA damage and oxidative stress induced by proton beam in Cymbidium hybrid. Hort. Environ. Biotechnol. 56: 240-246. [ Links ]
Luan L. Q., N. H. Phuang U., and V. T. Thu H. 2012. In vitro mutation breeding of Paphiopedilum by ionization radiation. Sci. Hort. 144: 1-9. [ Links ]
Melki M., and A. Marouani. 2010. Effects of gamma rays irradiation on seed germination and growth of hard wheat. Environ. Chem. Lett. 8: 307-310. [ Links ]
Minisi F. A., M. E. El-mahrouk, M. E. F. Rida, and M. N. Nasr. 2013. Effects of gamma radiation on germination, growth characteristics and morphological variations of Moluccella laevis L. Am.-Eurasian J. Agric. Environ. Sci., 13: 696-704. [ Links ]
Murashige T., and F. Skoog. 1962. A revised medium for rapid growth and biossays whit tobacco tissue cultures. Physiol. Plant. 15: 473-497. [ Links ]
Murti H., R., H. Y. Kim H., and Y. Yeoung R. 2013. Effectiveness of gamma ray irradiation and ethyl methane sulphonate on in vitro mutagenesis of strawberry. Afr. J. Biotechnol. 12: 4803-4812. [ Links ]
Nakano M. et al. 2010. Morphological variation in Tricyrtis hirta plants regenerated from heavy ion beam-irradiated embryogenic calluses. Plant Biotechnol. 27: 155-160. [ Links ]
Patil U. H., G. N. Deshmukh, and N. A. Kazi. 2015. Mutation breeding in chrysanthemum (Dendranthema grandiflora T.). Asian J. Multidiscipl. Studies 3: 25-27. [ Links ]
Pavan K., D. et al. 2013. Gamma radiosensitivity study on rice (Oryza sativa L.). Asian J. Plant Sc. Res. 3: 54-68. [ Links ]
Predieri S. 2001. Mutation induction and tissue culture in improving fruits. Plant Cell Tiss. Org. 64: 185-210. [ Links ]
Preuss S. B., and A. B. Britt. 2003. A DNA-damage-induced cell cycle checkpoing in Arabidopsis. Genetics 164: 323-334. [ Links ]
Puchooa D. 2005. In vitro mutation breeding of Anthurium by gamma radiation. Int. J. Agri. Biol. 7: 11-20. [ Links ]
Ramírez R. et al. 2006. Estudio de radiosensibilidad y selección del rango de dosis estimulantes de rayos X en cuatro variedades de tomate (Lycopersicon esculentum Mill). Cult. Trop. 27: 63-67. [ Links ]
Sadhukhan R., K. Swathi, D. Sarmah, and T. Mandal. 2015. Effect of different doses of gamma rays on survivability and rooting ability in chrysanthemum (Chrysanthemum morifolim Ramat.). J. Crop Weed 11: 62-65. [ Links ]
Soliman T.., S.Lv , H.Yang, B. Hong, N. Ma, and L.Zhao . 2014. Isolation of flower color and shape mutations by gamma radiation of Chrysanthemum morifolium Ramat cv. Youka. Euphytica 199: 317-324. [ Links ]
Sparrow A. H., R. L. Cuany, J. P. Miksche, and L. A. Schairer. 1961. Some factors affecting the responses of plants to acute and chronic radiation exposures. Radiat. Bot. 1: 10-34. [ Links ]
Stancato C., G., P. Mazzafera, and M. S. Buckeridge. 2002. Effects of light stress on the growth of the epiphytic orchid Cattleya forbesii Lindl. X Laelia tenebrosa Rolfe. Rev. Bras. Bot. 25: 229-235. [ Links ]
Statistical Analysis System. 2002. Institute Inc., SAS/STAT. User´s Guide, versión 9.0. Carey, N. C. [ Links ]
Szarek S. 2005. Use of concept of hormesis phenomenon to explain the law of diminishing returns. Part II. EJPAU Serie Econ. 8: 1-61. [ Links ]
Tanaka A., N. Shikazono, andY. Hase . 2010. Studies on biological effects of ion beams on lethality, molecular nature of mutation, mutation rate, and spectrum of mutation phenotype for mutation breeding in higher plants. J. Radiat. Res. 51: 223-233. [ Links ]
Urrea A. I., y S. M. Ceballos. 2005. Empleo de las radiaciones gamma en la inducción de variabilidad genética en Heliconia psittacorum. Actual Biol. 27: 17-23. [ Links ]
Verma J., K. Sharma, K. Thakur, J. K. Sembi, and S. P. Vij. 2014. Study on seed morphometry of some threatened Western Himalayan orchids. Turk. J. Bot. 38: 234-251. [ Links ]
Wi S. G., B. Y. Chung , J. H. Kim, M. H. Baek, D. H. Yang , J. W. Lee, and J. S. Kim . 2005. Ultrastructural changes of cell organelles in Arabidopsis stem after gamma irradiation. J. Plant Biol . 48: 195-200. [ Links ]
Wi S. G ., B. Y. Chung , J. S. Kim , J. H. Kim , M. H. Baek , J. W. Lee , andY. S. Kim . 2007. Effects of gamma irradiation on morphological changes and biological responses in plants. Micron 38: 553-564. [ Links ]
Yamaguchi H., A. Shimizu A., K. Degi, and T. Morishita. 2008. Effects of dose and dose rate of gamma ray irradiation on mutation induction and nuclear DNA content in chrysanthemum. Breeding Sci. 58: 331-335. [ Links ]
Yamaguchi H ., A. Shimizu A., Y. Hase , K. Degi , A. Tanaka, andT. Morishita . 2009. Mutation induction with ion beam irradiation of lateral buds of chrysanthemum and analysis of chimeric structure of induced mutants. Euphytica 165: 97-103. [ Links ]
Yoon A., R., R. Kamaludin, A. Tajudin, K. Hazmi Z., A. Bakar D., A. Nezhadahmadi, and F. Golam. 2014. The contribution of muslim scientists in botanical science: Studies on the using of gamma rays for ginger plants (Zingiber Officinale). Stem Cell 5: 88-94. [ Links ]
Yunus F., M., M. Azizi A., M. Kadir A., S. Daud K., and A. Rashid A. 2013. In vitro mutagenesis of Etlingera elatior (Jack) and early detection of mutation using RAPD markers. Turk. J. Biol. 37: 716-725. [ Links ]
Zolla L., A. M. Timperio, W. Walcher, and C. G. Huber. 2003. Proteomics of light harvesting proteins in different plant species. Analysis comparison by liquid chromatography-electrospray ionization mass spectrometry. Photosystem II. Plant Physiol. 13: 198-214. [ Links ]
Received: March 2016; Accepted: May 2016











 texto em
texto em