Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.51 no.4 Texcoco Mai./Jun. 2017
Crop science
Isolation and characterization of rhizobacteria associated with rice crops (Oryza sativa L.) in Norte de Santander (Colombia)
1Semillero de Investigación Biotecnología para la Agricultura y la Alimentación, SIBAA. Universidad Francisco de Paula Santander. Cúcuta, Colombia.
2Departamento de Biología, Grupo de Investigación en Ciencias Biológicas “Majumba”. Universidad Francisco de Paula Santander. Cúcuta, Colombia.
The plant-growth promoting rhizobacteria are recognized and studied for their beneficial effects on several crops. Strains of the Azotobacter and Pseudomonas genera were isolated from rhizospheric soil where rice crops (Oryza sativa L.) are grown, from 10 farms located in the Zulia River’s irrigation district, Norte de Santander, Colombia. The bacterial isolation was obtained from granular soil and serial soil dilutions, which were planted on Ashby and King B agar, for Azotobacter and fluorescent Pseudomonas, respectively. Forty-two isolates were stored in vials with a sterile saline solution (0.85 % NaCl) in a refrigerator at 4 °C and were deposited in the Bank of Strains of the Applied Biology Research Laboratory of the Universidad Francisco de Paula Santander, Cúcuta, Colombia. The capacity to solubilize inorganic phosphate, to fix biological nitrogen, and to synthesize indoleacetic acid and siderophores were determined in all strains, in order to select isolates with potential to develop microbial inoculum. With a hierarchical clustering of the medians of the four activities, four isolations were selected because they presented the closest values to the controls designed as promising strains in the four activities, according to the similarity dendrogram. These four strains were identified as Pseudomonas putida (RzA027 and RzA035), Azotobacter chrococcum (RzA040), and Azotobacter tropicalis (RzA042), by amplification of the 16S rDNA gene.
Key words: Azotobacter; Pseudomonas putida; phosphates solubilization; biological nitrogen fixation; indoleacetic acid; siderophores
Las rizobacterias promotoras de crecimiento vegetal son reconocidas y estudiadas por sus efectos benéficos en varios cultivos. Cepas de los géneros Azotobacter y Pseudomonas se aislaron de suelo rizosférico de cultivos de arroz (Oryza sativa L.) de 10 fincas en el distrito de riego del río Zulia, Norte de Santander, Colombia. El aislamiento bacteriano se hizo a partir de gránulos de suelo y diluciones seriadas de suelo, sembradas en agar Ashby para Azotobacter y King B para Pseudomonas fluorescentes, respectivamente. Cuarenta y dos aislamientos se conservaron en viales con solución salina estéril (0.85 % NaCl) en refrigeración a 4 °C y se ingresaron al Banco de Cepas del Laboratorio de Investigaciones en Biología Aplicada de la Universidad Francisco de Paula Santander, Cúcuta, Colombia. Para seleccionar aislamientos con potencial para el desarrollo de inoculantes microbianos, en todas las cepas se determinó su capacidad de solubilización de fosfato inorgánico, fijación biológica de nitrógeno, síntesis de ácido indoloacético y sideróforos. Con un conglomerado jerárquico de las medianas de las cuatro actividades se seleccionaron cuatro aislamientos por presentar los valores más cercanos a los testigos diseñados como cepas promisorias en las cuatro actividades según el dendrograma de similitudes. Estas cuatro cepas se identificaron como Pseudomonas putida (RzA027 y RzA035), Azotobacter chrococcum (RzA040) y Azotobacter tropicalis (RzA042) mediante amplificación del gen 16S ADNr.
Palabras clave: Azotobacter; Pseudomonas putida; solubilización de fosfatos; fijación biológica de nitrógeno; ácido indolacético; sideróforos
Introduction
ice (Oryza sativa L.) is one of the most important crops and a major source of food for the world population. In order to meet the demand for this cereal over the next decade, it will be necessary to produce 8 to 10×106 Mg more than the current production (Seck et al., 2012). Therefore, rice production must be increased in the same crop area and face climate change, which implies applying strategies such as improving soil quality and the precise management of agricultural practices (Uribe-Vélez, 2011; García de Salamone et al., 2012). Applying chemical fertilizers is an effective way to provide the soil with nutrients, but an excessive contribution has a negative short -and long- term effect on the environment -such as the emission of nitrous oxide into the atmosphere and nitrate pollution of water-, because they are dispersed by the surface runoff or become insoluble forms that plants cannot assimilate (Halpern et al., 2014).
Norte de Santander is a Colombian department with an important agricultural production, but its conventional production model has not changed for more than 40 years in regions such as the Zulia River irrigation district. As in the rest of the country, this is set apart by monoculture, excessive tillage, and the continuous use of synthetic fertilizers and pesticides, which affect the physical and chemical properties of these soils (Cuevas, 2012). This results in an ecological imbalance of the agroecosystem, with a decrease in the population of beneficial organisms, an increase of weed, phytopathogens and pests, soil acidification, reduction of organic matter content and reduction of the biological activity of beneficial microorganisms (Cañón et al., 2009). This group of microorganisms includes plant growth promoting rhizobacteria (PGPR), which stand out for their beneficial effects on plants and, consequently, on ecosystems by various mechanisms, including: biological nitrogen fixation, solubilization of phosphates and micronutrients, production of phytohormones and other metabolites associated with biocontrol of pathogens (such as antibiotics and siderophores), production of chitinases, and induction of resistance in plants (Ahemad and Khan, 2012; Son et al., 2014).
Microorganisms adapted to a specific region can be more adequate in the selection of isolates, for the development of inoculants destined to regional crops, because they could be more competitive than foreign or induced bacterium (Karagöz et al., 2012). The development of biofertilizers should be initiated with the isolation of relevant microorganisms and the description of the mechanisms associated with the promotion of plant growth (Vanegas et al., 2011). The isolation and identification of strains of rhizobacterium in rice (De Souza et al., 2012; Sahoo et al., 2013), corn (Zea mays L.) (López-Ortega et al., 2013), vegetables (Coriandrum sativum L. and Lattuca sativa L.) (Cárdenas et al., 2013), and banana (Musa paradisiaca) (Andrade et al., 2014) have been subject to research. These strains were characterized according to plant growth promoting activities such as: indoleacetic acid synthesis, solubilization of phosphates, biological nitrogen fixation, and production of siderophores. At the end of the process, microorganisms with beneficial effects on crops are selected. Therefore, the objective of this research was to isolate bacterium associated with rice plants cultivated in soils of the Zulia River irrigation district (Norte de Santander), to determine their potential as promoters of plant growth, and to select the ones that are more promising for the development of biofertilizers that increase the yield of regional crops.
Materials and Methods
Isolation of rhizobacterium
Sampling
Samples of rhizospheric soil from rice crops were taken in ten plots in the following areas: El 52 (area 1), Matecaña (area 2, 3, and 10), Limoncito (area 4), San Roque (area 5 and 6), Gorgona (areas 7 and 8) and Buena Esperanza (area 9); these areas are located in the Zulia River irrigation district (Norte de Santander). This district is located between 68 and 120 m altitude. It has a warm humid climate with temperatures between 27 and 34 °C, and a relative humidity of 70-90 %. According to Holdrige life zones, this region is classified as a tropical humid forest (bh-T) (IGAC, 2006). The sampling areas were crossed in zigzag and 10 1-kg subsamples were taken from each plot (20-cm deep areas). This sampling was carried out 50-80 d after sowing. The samples were processed in the Applied Biology Research Laboratory of the University Francisco de Paula Santander.
Isolation of Azotobacter genus bacteria
The isolation was carried out spreading 10 to 30 rhizospheric granular soil on Ashby agar (Aquilanti et al., 2004). Cultures were incubated at 32 °C until an abundant growth of viscous colonies with yellowish, green or brown pigment appeared around the soil grains. These colonies were cultured on new Ashby agar until pure cultures were obtained. Gram staining was performed in the isolations, to confirm the development of Gram-negative cells and cysts characteristic of this genus.
Isolation of Pseudomonas genus bacteria
The isolation was developed using the serial dilution methodology of rhizospheric soil in a saline solution (0.85 % NaCl). King B agar plates (King et al., 1954) were inoculated with 100 µl of 10-2 to 10-7 dilutions, and incubated at 32 °C until colonies surrounded with a soluble pigment formation appeared. The colonies were spread in a new agar King B until a pure culture was obtained. Later, Gram staining was performed to confirm the development of Gram-negative bacilli. The colonies on King B agar were exposed to ultraviolet light to observe the fluorescent pigment presence, a distinctive trait of the species of this genus.
The isolations were preserved using the method of vials under refrigeration with a sterile saline solution (0.85 % NaCl) (Sarmiento et al., 2013).
Description of rhizobacteria isolates according to plant growth promoting activities
Activation of microorganisms
Isolates preserved in a sterile saline solution were activated in Ashby and King B agar, for Azotobacter and Pseudomonas genera, respectively; they were incubated at 32 °C until the colonies grew. Plant growth promoting activities were carried out using a quantitative evaluation of the viable cultures obtained.
Preparation of bacterial inocula
From one strain of each isolation, an inoculum was prepared in 50 mL of DYGS culture broth (Radwan et al., 2005) and incubated 24 h at 32 °C. A 10 mL suspension of this culture was transferred to a tube and centrifuged for 10 min at 4124 g. The supernatant was discarded, and 10 mL of a sterile phosphate buffer solution (0.06 M and pH 7.0) was added, in order to resuspend the cells and adjust the cell concentration to O.D.605nm=0.5 in a Spectroquant NOVA60 spectrophotometer. The cell suspensions obtained from each isolate were used to determine indoleacetic acid (AIA) production, solubilization of phosphates, biological nitrogen fixation, and siderophore synthesis.
Determination of indoleacetic acid (AIA) production
One-hundred µL were taken from the adjusted cell suspension, and they were inoculated into 25 mL of DYGS culture broth, plus a supplement of 0.15 g L-1 tryptophan and 0.2 g L-1 NH4Cl. Cultures were incubated in the dark at 120 rpm and 32 °C. Each isolate was inoculated in three replicates and an uninoculated control was set up under the same conditions. After 72 h of incubation, 10 mL of the microbial broth were centrifuged for 10 min at 4124 g. Indolic compounds were determined by adding 1 mL of the supernatant and 4 mL of the Salkowsky reagent (5 mL of FeCL3·6H2O [0.5M] in 250 mL of HCLO4 [35 %] for a 500 mL volume), until a pink coloration was obtained. The samples’ absorbance was recorded at 525 nm in the Spectroquant NOVA60 spectrophotometer. The concentration of indole compounds was calculated using the linear regression equation of the calibration curve constructed with seven concentrations of indoleacetic acid, AIA (25, 50, 100, 150, 200, 250 and 300 mM) (Cárdenas et al., 2010).
Determination of phosphate solubilization
The determination of available P was carried out according to the Bray II method, adapted for bacterial cultures (Cárdenas et al., 2013). 100 µL were taken from the cell suspension -adjusted to a O.D.605nm=0.5 from each isolation, as described above- and inoculated into 25 mL SRS broth (Sundara-Rao and Sinha, 1963), with tricalcium phosphate as an insoluble P source. Cultures were incubated for 48 h at 30 °C at 120 rpm. Each isolation was inoculated in three replicates and an uninoculated control was set up under the same conditions. After incubation, 10 mL of microbial culture were centrifuged at 4124 g for 10 min. The available P was extracted from 10 mL of supernatant, plus 10 mL of the extraction solution (1.11 g of NH4F and 25 mL of HCl [4N] in a volume of 1 L), and stirred 1 min. Then 1 mL of this solution was taken and 9 mL of a coloring solution were added (5 mL of solution A and 2 mL of solution B in 200 mL of distilled water) (Solution A: 30 g (NH4)6MO7O24·4H2O in 100 mL of distilled water and 0.728 g of K(SbO)C4H4O6·½H2O in 160 mL of distilled water. Solution B: 3.05 g of ascorbic acid in 25 mL of distilled water.) The absorbance (605 nm) was recorded in a spectrophotometer 15 min after the reaction. The available P concentration (µg of P2O5 mL-1) -which indicates the phosphate solubilization capacity- was calculated using the linear regression equation of the calibration curve constructed from concentrations of KH2PO4: 5, 10, 20, 30, 40 and 50 µg mL-1.
Evaluation of biological nitrogen fixation
Nitrogen (N) fixation by rhizobacteria isolated strains was determined according to a modified version of the micro-Kjeldahl method (Kuss et al., 2007). From the adjusted cell suspension to a O.D.605nm=0.5 (from each isolation, as described above), 1000 µL were inoculated into vials with 10 mL of a semi-solid Ashby medium for Azotobacter sp. and semi-solid JMV medium for Pseudomonas sp. The JMV medium had the following composition (g L-1): Mannitol 5.0; KH2PO4 0.6; MgSO4·7H2O 0.2; NaCl 0.1; CaCl2·2H2O 0.2; micronutrient solution 2 mL (g L-1: CuSO4·5H2O 0.04; ZnSO4·7H2O 1.20; H3BO3 1.40; Na2MoO4·H2O 1.00; MnSO4·H2O 1.18); 2 mL of Bromothymol blue (0.5 % solution in 0.2N KOH); 4 mL of FeEDTA (1.64 % solution); 1 mL of vitamin solution (10 mg of biotin and 20 mg of pyridoxol-HCl in 100 mL); pH 4.0-4.5, agar 2.5. This procedure was performed in triplicate. After bacterial growth, cell lysis was carried out placing the vials in an autoclave for 1 min at 15 pounds of pressure. From the resulting suspension, 5 mL were added to a tube where the whole sample was digested, in order to transform all the organic N into a mineral form (NH+3). The digestion was induced by adding 4 mL of concentrated sulfuric acid and 10 mL of 30 % hydrogen peroxide (V/V) to the tube containing the sample. The tubes were placed in the digestion equipment at an initial temperature of 180 °C for 120 min, and then, for 60 min at 375 °C. Subsequently, the tubes were removed from digester, and the content was gauged with distilled water into 50 mL. Samples were then recorded on the HACH DR/2000 spectrophotometer in order to quantify the total N (Nt) transformed into NH+3. The quantizing of Ntotal was completed in: 1 mL of the digestion product, plus 2 mL of the Nessler reagent, 1 drop of catalyst, and 1 drop of polyvinyl alcohol. Yellow colorations were obtained, which were compared with the standard calibration curve of the equipment. The calculation of fixed Nt was showed in µg mL-1.
Determination of the siderophore synthesis
From the cell suspension adjusted to an O.D.605nm=0.5 (from each isolation, as described above), 2.5 mL were taken and inoculated into 50 mL of Simon and Tesmann Broth (g L-1 broth: malic acid 10; NaCl 5.8; KCl 3.7; CaCl2·2H2O 0.15; Tris 12.1; magnesium chloride 0.1; ammonium chloride 1.1; Na2SO4 0.142; K2HPO4 0.272; pH 6.8). The bacterial cultures were incubated at 120 rpm at 32 °C during 5 d. Each isolate was inoculated in three replicates and an uninoculated control was set up under the same conditions. From the cultures, 10 mL of broth were taken and carried to centrifuge tubes in order to determine the siderophores produced. Centrifugation was performed at 4124 g for 10 min. Siderophores quantizing was completed in 1 mL of the supernatant, plus 1 mL of a ferric perchlorate and perchloric acid solution (5 mM and 0.14 M, respectively). After the reaction was obtained, a 525-nm absorbance was recorded in the spectrophotometer. The siderophore concentration was calculated using the linear regression equation of the calibration curve constructed with five concentrations (5, 20, 50, 80, 100 µg mL L-1) of a hydroxamate siderophore (deferoxamine mesylate, SIGMA) with the ferric perchlorate solution, and recorded with the same spectrophotometer.
Statistical analysis
An ANOVA was performed for each variable of plant growth promotion. The means of the treatments were compared with Tukey’s multiple comparison test (p≤0.05), using the IBM SPSS Statistics 19 software. For each isolate, the values obtained from the mean of three replicates of plant growth promoting features were compared with each other and with nine designed controls: 1) with the highest value in the indoleacetic acid production (Ctrl AIA); 2) with the highest value in the solubilization of phosphates (Ctrl SP); 3) with the highest value in siderophore synthesis (Ctrl Sider); and 4) with the highest value in biological nitrogen fixation (Ctrl FBN). In addition, three intermediate-level controls were designed for all plant growth promoting activities evaluated (Ctrl Medio_1, Ctrl Medio_2, Ctrl Medio_3), as well as two controls with the highest and lowest values obtained in all activities (Ctrl+ y Ctrl-). The similarities between these values were estimated based on the Euclidean Distance, were assembled using the means between groups comparison and were graphically represented in a dendrogram using the IBM SPSS Statistics 19 software (Hernández et al., 2004). This methodology enables the classification of the isolates according to their values in each plant growth promoting activities evaluated in order to select the means that are closer to the designed controls. The selected isolates continued with the molecular identification process.
Rhizobacteria molecular identification by the 16S rDNA gene
The selected Pseudomonas isolates were cultured in a Luria Bertani broth, at 150 rpm, at 30 °C, for 18 h and those of the Azotobacter genus were cultured in a DYGS broth, at 150 rpm for 30 h. DNA extraction was performed on 1 mL of the culture, using Bioline’s ISOLATE II Genomic DNA kit. The quality of the extracted DNA was verified using a horizontal electrophoresis system, in a 1 % (P/V) agarose gel at 120 V for 90 min, using TBE 0.5 X as the running buffer. The 16S rDNA gene was amplified in 50 µL of a reaction mixture composed of: 5 µL of buffer (1x); MgCl2 (2 mM); dNTP (200 µM); initiators 27F (5’ AGAGTTTGATCMTGGCTCAG 3’) (1 µM) and 1492R (5’ TACGGYTACCTTGTTACGACTT 3’) (1 µM) (Rohwer et al., 2002); 1.25 U of Taq DNA polymerase, and 1 µL of template DNA (isolation sample). The thermal cycler conditions were: an initial denaturing cycle (94 °C for 5 min), 30 denaturing cycles (94 °C for 30 s), annealing (57 °C for 45 s), an extension (72 °C for 90 s), and a final extension (72 °C for 7 min). Amplified fragments were observed by a 2 % agarose gel electrophoresis in TAE prepared with 0.5 µg mL-1 of ethidium bromide. The amplified product was sequenced at Corpogen. The result was analyzed using BLAST (Basic Local Alignment Search Tool) and at EMBL (European Molecular Biology Laboratory).
Results and Discussion
Isolation of Azotobacter and Pseudomonas genera rhizobacterium
Ten presumptive isolates of the Azotobacter genus and 32 of Pseudomonas genus were obtained from the rhizospheric soil samples -per their phenotypic characteristics. The Azotobacter colonies were selected because they presented viscous and abundant growth, with yellowish and brown pigmentation, and the presence of Gram-negative cells with cyst formation. This Azotobacter viscous growth has been associated with its production of exopolysaccharides (EPS) and polyhydroxybutyrate (PHB). An EPS is the alginate that participates in the formation of cysts in cells as a protection mechanism. The PHB is the main component of the carbon and energy stock of these bacterial cells, and is related to the biological nitrogen fixation, because it protects the nitrogenase against high oxygen concentration (Gauri et al., 2012).
The fluorescent isolates of the Pseudomonas genus sp. were selected based on the formation of a yellow-greenish pigment diffused on King B agar, and the presence of Gram-negative bacilli. Pigments are siderophore-type molecules with low relative molecular mass that act as Fe-chelating agents (considered very important for plant nutrition) (Aguado-Santacruz et al., 2012). Pseudomonas genus bacteria are found in the rice soils rhizosphere, and colonize the tissues of rice plants, which indicates their associative and endophytic behavior (Sivakamasundari and Usharani, 2012). From several tissues of rice plants, Phetcharat and Duangpaeng (2012) obtained 34 isolates identified as Pseudomonas sp. and a lower amount of rhizobacterium belonging to the Bacillus, Azotobacter, and Enterobacter genera. Habibi et al. (2014) reported that the Pseudomonas veronii, P. putida, P. monteilii, and P. mandelii species as rice endophytes. Souza et al. (2015) found isolates belonging to the Burkholderia, Enterobacter, and Pseudomonas genera associated with this culture. These results are related to those obtained in our study and show a strong occurrence of Pseudomonas in rice soils. However, Chennappa et al. (2014) and Sahoo et al. (2013) also report the isolation of Azotobacter species, especially in soils with pH closer to neutrality. Therefore, we can infer that there are several factors that influence the microbial diversity of these soils, and that the few Azotobacter genus isolates obtained can be related to the acidic nature of the soil where the samples were taken from -which are set apart by their strong (4.23) to moderate (5.60) acidity.
Description of plant growth promoting activities
The 42 bacteria isolates obtained showed all the plant growth promoting activities evaluated, although some of these features stand out more in some isolates than in others (Table 1).
Table 1 Characteristics related to the promotion of plant growth in isolated bacteria.
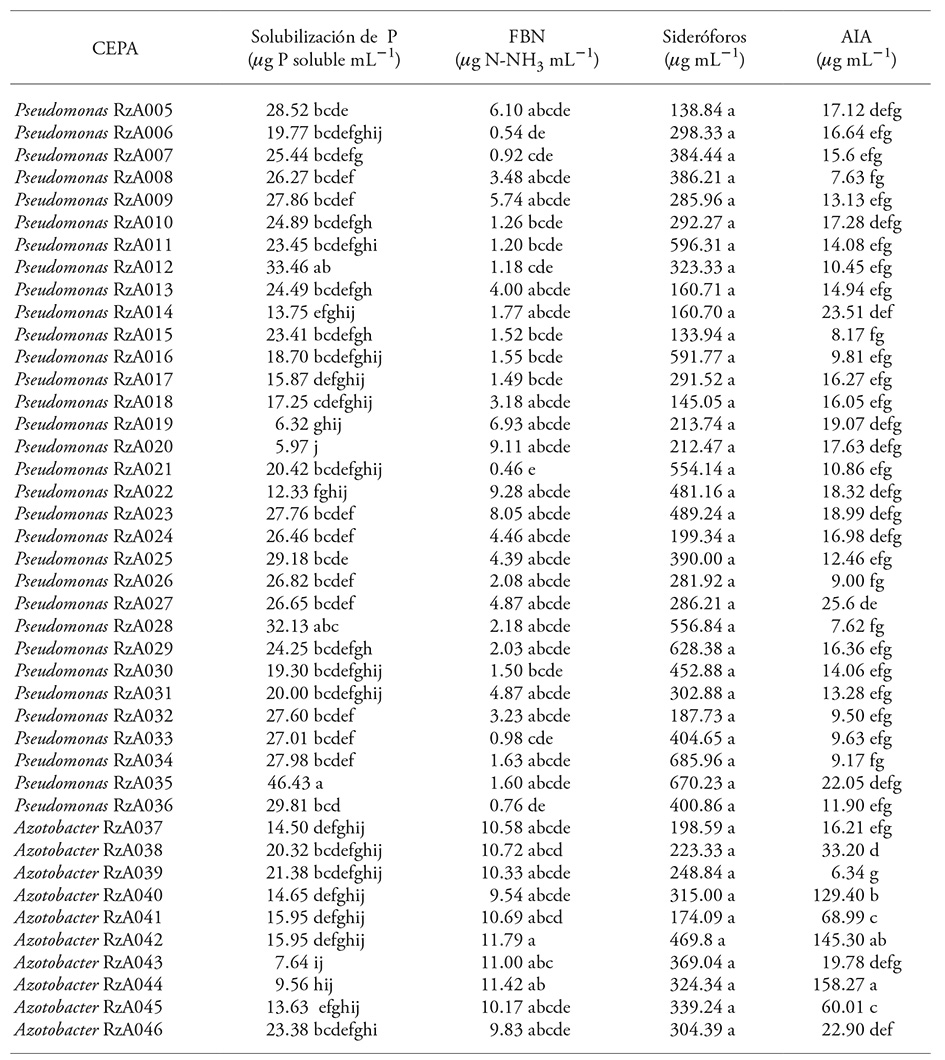
Mean of three replicates. Mean with different letters in a column are statistically different (Tukey; p≤005).
Production of indoleacetic acid (AIA)
Synthesis of AIA in Azotobacter isolates ranged from 6.34 to 158.27 µg mL-1. The values had great variability, but they showed this bacteria genus’ capacity to produce indolic compounds. The maximum value was obtained by the Azotobacter RzA044 isolate (158.27 µg mL-1) -which only showed similarity to the Azotobacter strain RzA042 (145.30 µg mL-1) -and was close to the maximum value recorded by Sahoo et al. (2013), who report a production close to 180 µg mL-1 -from A. vinelandii strain isolated from Indian rice soil. The other isolates were similar to each other, forming groups where the Azotobacter RzA040 and Azotobacter RzA041 isolates stood out. Hussein and Joo (2015) also reported -in Azotobacter strains isolated from Panax ginseng- a 32.29 and 46.68 µg mL-1 production, which are close to those obtained by Azotobacter RzA038 and RzA045 isolates. Barua et al. (2012) report a 23.16 µg mL-1 of indole compound production from an Azotobacter vinelandii strain (isolated from forest soils in India), which is similar to the values obtained by Azotobacter RzA043 and RzA046 strains.
The AIA production variability among same genus bacteria is also reported in other researches. Escobar et al. (2011) reported an 11.99 to 57.99 µg mL-1 range for indoleacetic acid production in Azotobacter strains isolated from vegetables roots and rhizospheric soil.
The AIA synthesis of Pseudomonas isolates ranged from 7.62 to 25.6 µg mL-1; RzA014, RzA027, and RzA035 strains stood out, but several isolates were not different (p>0.05). These values are close to those reported by Malik and Sindhu (2011) with a indoleacetic acid production range of 10.2 and 31.2 µg mL-1 in fluorescent Pseudomonas species isolated from chickpea crops. Lavakush et al. (2014) also report 10 to 18 µg mL-1 of AIA in Pseudomonas putida and Pseudomonas fluorescens strains isolated from rice soils. In other researches, the AIA production is higher than the one obtained in our study: Habibi et al. (2014) reported 37 µg AIA mL-1 in a P. mandelii isolate obtained from soil cultivated with rice, while Hussein and Joo, (2015) reported 35.9 to 47.54 µg AIA mL-1 of fluorescent Pseudomonas isolated from Panax ginseng.
The wide ranges of indole compounds production reported show the importance of quantifying this PGPR activity in the isolates obtained because their synthesis capacity may vary due to their genetic diversity and their environmental conditions (Karagöz et al., 2012).
Solubilization of phosphates
The isolates with the highest available P values in the SRS culture broth were those of the Pseudomonas genus, with 5.97 to 46.43 µg mL-1 values of available P; the RzA028 (33.13 µg mL-1), RzA012 (33.46 µg mL-1), and RzA035 (46.43 µg mL-1) isolates -which were statistically similar (Table 1)- stood out. The results recorded for all Pseudomonas isolates were similar to those reported for: P. putida (40 µg mL-1 available P) and P. pseudoalcaligens (45 µg mL-1 available P) isolated from marginal soils in India with scarce vegetation (Patel et al., 2012); Pseudomonas sp. strains isolated from cotton crops with less than 20 µg mL-1 of available P; P. otitidis isolated from olive (6.55 µg mL-1) and sesame plants (7.63 µg mL-1) (Saber et al., 2015). However, these values are lower than other rhizospheric Pseudomonas species’ such as: P. chlororaphis with 154.1 µg mL-1 (Yu et al., 2012) and P. putida with 140 and 150 µg mL-1 (Lavakush et al., 2014).
The Azotobacter genus isolates presented a 7.64 to 23.38 µg mL-1 soluble P production in the liquid culture medium. The values obtained by the Azotobacter isolates were similar to those recorded in the cultured medium with 13 of the 32 Pseudomonas genus fluorescent rhizobacteria isolates. They also matched the P availability in the culture medium recorded by the phosphatesolvent activity of A. chrococcum strains (18.63 µg mL-1 available P) (Abd El-Fattah et al., 2013), and were higher than those reported by Escobar et al. (2011) in Azotobacter strains isolated from tomato (0.46-2.46 µg mL-1 available P).
The Pseudomonas genus is set apart by its high phosphate-solubilizing capacity, although there are significant values in this biofertilization activity in Azotobacter (161.11 and 151.32 µg mL-1, Rojas-Tapias et al., 2012), and A. chrococcum isolates (41.3 and 93.72 µg mL-1, López-Ortega et al., 2013). Therefore, rhizobacterium are very important microorganisms for the plant, because they can directly increase P availability by solubilization, mineralization mechanisms, and, in alkaline soils, the reduction of pH, caused by the production of the following acids: lactic, acetic, oxalic, malonic, maleic, pyruvic, propionic, gluconic, citric, succinic, tartaric, fumaric, and trans-aconitic (Park et al., 2011; Marschner et al., 2011).
Biological nitrogen fixation
The isolates evaluated presented a variation in the amount of Ntotal from 0.46 to 11.79 µg mL-1 N-NH3. The isolate with the highest biological nitrogen fixation value was Azotobacter RzA042. Higher values were recorded for other Azotobacter isolates (9.54.-11.79 µg mL-1 N-NH3) regarding Pseudomonas (0.46-9.28 µg mL-1 N-NH3). Abdel-Aziez et al. (2014) reported a biological nitrogen fixation of 11.11-200 µg mL-1 N-NH3 by Azotobacter genus bacterium. The Pseudomonas genus also fixes nitrogen, especially the species P. putida PS9 (Ahemad and Khan, 2012), P. fluorescens and P. mendocina (Karagöz et al., 2012).
Azotobacter is the main group of heterotrophic free-living nitrogen-fixing bacterium associated with plants. The species of this genus can fix, at least, 10 mg N g-1 of carbohydrate, and N-fixing activity is higher in strains associated with rice than with other similar crops (Sahoo et al., 2013). Therefore, we can infer that this genus has a great biofertilizer potential in rice crops. In our study, although Ntotal values varied among the isolate, all showed N-fixing activity, which is one of the main characteristics of plant growth promoting rhizobacteria.
Siderophore production
There were no significant differences in siderophore production. The isolates with higher numerical production were Pseudomonas sp. RzA034 and RzA035 (685.96 and 670.23 µg mL-1 respectively), while the values of Azotobacter genus isolates were lower than 174.09 to 469.80 µg mL-1. Pseudomonas genus species produce hydroxamate-type siderophores (ferribactin, pseudobactin) and catechol-type siderophores (pioverdine) that sequester Fe III from their environment, when its conditions are deficient; in this way, they limit the phytopathogens’ growth, acting as a biocontrol mechanism (Aguado-Santacruz et al., 2012). Therefore, selecting isolates with this physiological feature is important in order to obtain an indirect benefit from plant growth promoting rhizobacteria. Abdel-Aziez et al. (2014) report a production of 11.3-59 µg mL-1 of siderophores per Azotobacter strain. Even though quantitative evaluations of siderophores synthesis by this genus are not frequent, the production of several biscatecholato-type siderophores were reported: azotoquelin (with a high affinity for Mo, V, Cu, Zn) (Bellenger et al.,) and protoquelin triscatecholato (with affinity for Fe III, Mn II, and Cu II) (Harrington et al., 2012). These authors indicate that the said siderophores are associated with the capture of Mo and V ions, which are required by the nitrogenase enzymatic complex in the atmospheric nitrogen fixation process.
Selection of rhizobacterium according to their plant growth promoting activities
The 42 isolates obtained were classified according to their PGPR activities comparing the nine controls designed with the values obtained. The dendrogram resulting from the grouping -according to the similarities of the medians- showed the development of two clusters (I and II) (Figure 1). Cluster I had a subcluster (IA) made up by two subclusters (IA1 and IA2). IA1 included two major subclusters: IA1a and IA1b, with 7 and 9 isolates, respectively. IA1b included phosphate solubilization controls (Ctrl SP), biological nitrogen fixation (Ctrl FBN), and negative control (Ctrl-), which indicates that the nine isolates linked with this subcluster had higher values only in the FBN activity or the solubilization of phosphates, but very low values in the production of AIA and siderophores (similar to Ctrl-).
In the IA2 subcluster, only the AIA production control (Ctrl AIA) was linked, because no isolate presented only a higher value in the AIA production synthesis activity. In the IB subcluster, three subclusters were formed (IB1, IB2 and IB3). In the IB1 subcluster, subclusters IB1a, IB1b and IB1c were formed. Nine isolates were included in the first two groups, and in the IB1c only the mean control 2 (CtrlMedio2) was linked. This mean control was designed with approximately 60 % of the highest available P values (27.86 µg mL-1), Ntotal (7.07 µg mL-1 N-NH3), indoleacetic acid (94.96 mg L-1), and siderophores (402.14 mg L-1). In subcluster IB2, the RzA040 isolation was associated with the Azotobacter genus, which recorded a value close to the mean control 2 (CtrlMedio2); therefore, it was selected as promising for the plant growth promotion. Subcluster IB3 linked 10 isolates and mean control 1, designed with approximately 50 % of the highest available P values (18.57 mg L-1), Ntotal (4.72 µg mL-1 N-NH3), indoleacetic acid (63.31 mg L-1), and siderophores (670.23 mg L-1). From this subcluster, the fluorescent Pseudomonas sp. RzA027 isolate was selected, because it presented similar values to mean control 1.
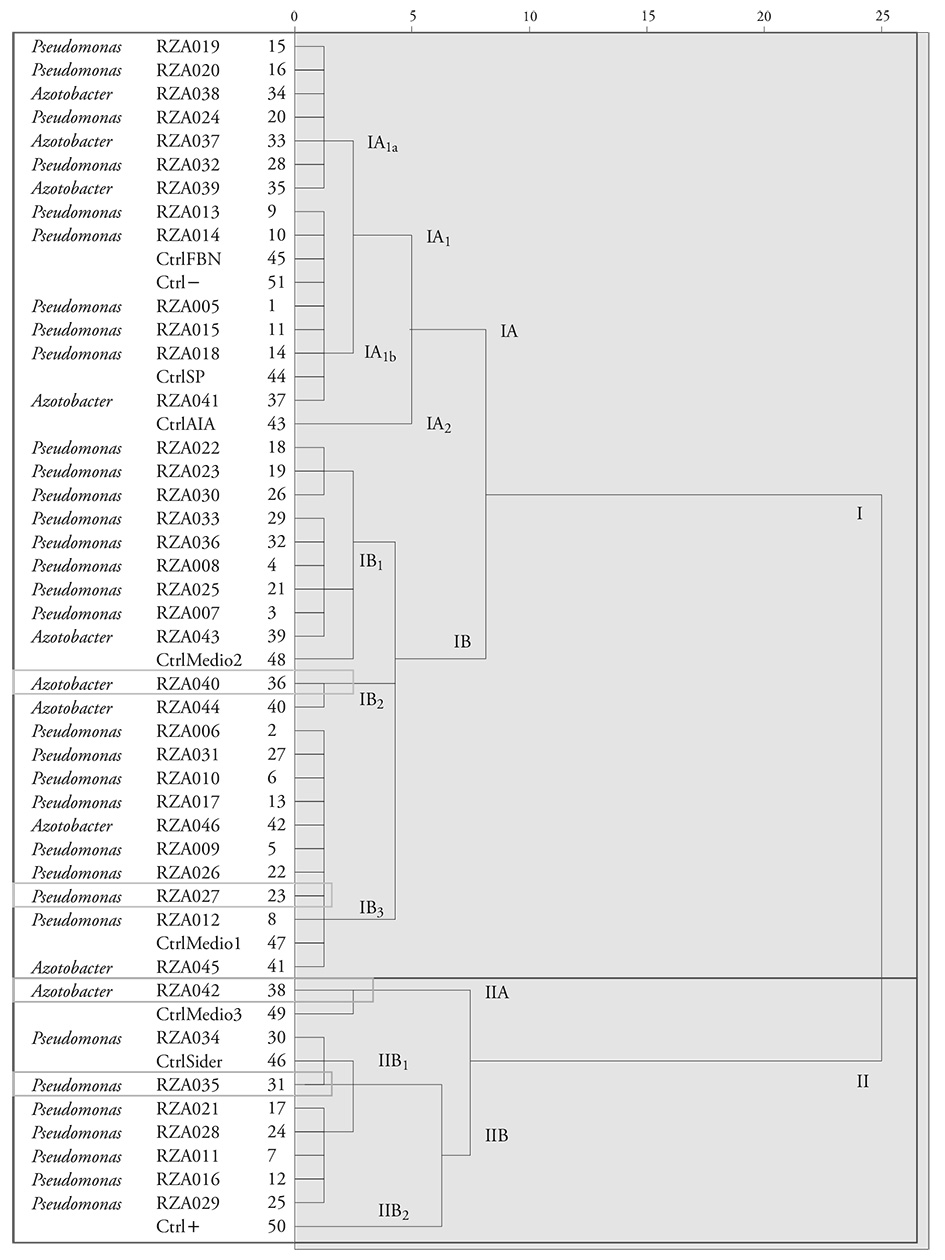
Figure 1 Similarity dendrogram in plant growth promoting activities by Azotobacter and Pseudomonas isolates.
Group II was made up by two subclusters (IIA and IIB). In subcluster IIA, the Azotobacter RzA042 isolate and the mean control 3 (CtrlMedio3) were classified. Mean control 3 was designed with approximately 80 % of the highest available P values (37.14 mg L-1), Ntotal (9.43 µg mL-1 N-NH3), indoleacetic acid (126.62 mg L-1), and siderophores (536.18 mg L-1). From this subcluster, the Azotobacter RzA042 isolate was selected, because it presented similar values to mean control 3.
In subcluster IIB, subclusters IIB1 and IIB2 were formed. In IIB1a, Pseudomonas RzA034 and RzA035 isolates were associated, as well as the siderophore production control (Ctrl Sider) -where the highest siderophore content is found (685.96 mg L-1). The Pseudomonas RzA035 isolate was selected from this group, because it represented the highest values of siderophore production, solubilization of phosphates, intermediates in biological nitrogen fixation, and AIA synthesis. In subcluster IIB2, only the positive control (Ctrl+) was linked, which indicates that no isolate recorded the highest values in the four activities evaluated.
The four isolates selected for molecular identification were the Pseudomonas RzA027 and RzA035, Azotobacter RzA040 and RzA042 strains, because they had representative values in the four features evaluated. They are considered to be promising for the continuation of the biofertilizers development process for rice cultivation in the region.
Molecular identification of selected Azotobacter and Pseudomonas isolates
The four strains showed a 1500 bp band in the 16S rDNA gene amplification (Figure 2). Analysis of the similarity between the nucleotide sequences obtained and those deposited in the GenBank database (using BLAST analysis) showed that the isolates belong to the Gamma Proteobacteria subclass of the Pseudomonadaceae family, and are related to the Azotobacter and Pseudomonas genera (Table 2).
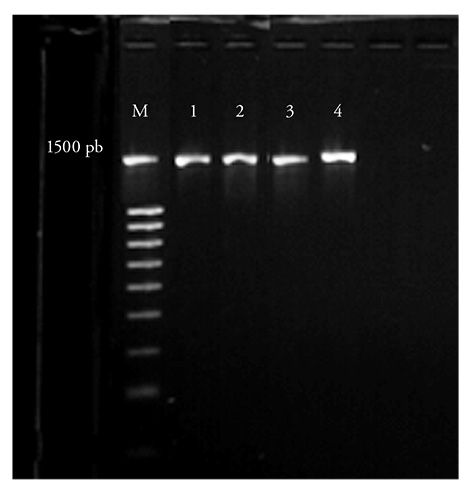
Figure 2 16S rDNA gene amplification. (M) 1500 bp DNA LADDER Biotium; (1) Pseudomonas RzA027; (2) Pseudomonas RzA035; (3) Azotobacter RzA040; (4) Azotobacter RzA042.
Conclusions
In the Zulia River irrigation district of Norte de Santander, Azotobacter and Pseudomonas genera rhizobacterium were isolated. These bacterium have plant growth promoting features that can be used in the development of biofertilizers to be applied in regional rice crops. The four isolates selected -because they have similar values to the designed controls- should be evaluated in rice cultivated fields, to determine their effect on plant growth and crop yield
Literatura Citada
Abd El-Fattah, D., W. Eweda, M. Zayed, and M. Hassanein . 2013. Effect of carrier materials, sterilization method, and storage temperature on survival and biological activities of Azotobacter chroococcum inoculant. Ann. Agric. Sci. 58: 111-118. [ Links ]
Abdel-Aziez, S., W. Eweda, M. G. Z. Girgis, and B. Abdel. 2014. Improving the productivity and quality of black cumin (Nigella sativa) by using Azotobacter as N2 biofertilizer. Ann. Agric. Sci . 59: 95-108. [ Links ]
Aguado-Santacruz G. A., Moreno-Gómez B., Jiménez-Francisco B., García-Moya E., y Preciado-Ortiz R. E. 2012. Impacto de los sideróforos microbianos y fitosideróforos en la asimilación de hierro por las plantas: una síntesis. Rev. Fitotec. Mex. 35: 9-21. [ Links ]
Ahemad, M., and M. S. Khan. 2012. Effect of fungicides on plant growth promoting activities of phosphate solubilizing Pseudomonas putida isolated from mustard (Brassica compestris) rhizosphere. Chemosphere 86: 945-950. [ Links ]
Andrade, L.F., G.L. Oliveira, D. de Souza, S. Nietsche, A. A. Xavier, M. R. Costa, A. M. Santos, M. C. Toledo, and D. F. Gomes. 2014. Analysis of the abilities of endophytic bacteria associated with banana tree roots to promote plant growth. J. Microbiol. 52: 27-34. [ Links ]
Aquilanti L., F. Favilli, and F. Clemeti. 2004. Comparison of different strategies for isolation and preliminary identification of Azotobacter from soil samples. Soil Biol. Biochem. 36: 1475-1483. [ Links ]
Arora, N. K., S. Tewari, and R. Singh. 2013. Multifaceted Plant-Associated Microbes and Their Mechanisms Diminish the Concept of Direct and Indirect PGPRs. Arora, N. K . (ed). Plant Microbe Symbiosis: Fundamentals and Advances. SPRINGER India. p.39. [ Links ]
Abdel-Aziez, S., S. W. Eweda, M. G. Z. Girgis, and B. Abdel. 2014. Improving the productivity and quality of black cumin (Nigella sativa) by using Azotobacter as N2 biofertilizer. Ann. Agric. Sci. 59: 95-108. [ Links ]
Barua, S., S.Tripathi, A.Chakraborty, S.Ghosh, and K.Chakrabarti. 2012. Characterization and crop production efficiency of diazotrophic bacterial isolates from coastal saline soils. Microbiol. Res. 167: 95-102. [ Links ]
Bellenger, J-P., F. Arnaud-Neu, Z. Asfari, S. C. B. Myneni, E. I. Stiefel, and A. M. L. Kraepiel . 2007. Complexation of oxoanions and cationic metals by the biscatecholate siderophore azotochelin. J. Biol. Inorg. Chem. 12: 367-376. [ Links ]
Cañón, R., V. Prato, M. A. Alterio, y D. Cárdenas. 2009. Efecto del uso del suelo sobre rizobacterias fosfatosolubilizadoras y diazotróficas en el distrito de riego del rio Zulia, Norte de Santander (Colombia). Revista Respuestas 14: 14-21. [ Links ]
Cárdenas, D., M. F. Garrido, R. R. Bonilla, y V. L. Baldani. 2010. Aislamiento e identificación de cepas de Azospirillum sp. en pasto guinea (Panicum maximum Jacq.) del Valle del Cesar. Pastos y Forrajes 33: 285-300. [ Links ]
Cárdenas, D.M., L. T.Ramírez, y L. Y.Moreno. 2013. Caracterización de Actividades Promotoras del Crecimiento Vegetal por Rizobacterias y su Efecto en Cultivo de Cilantro (Coriandrum sativum L.). 1a. ed. Ecoe Ediciones. Colombia. 138 p. [ Links ]
Chennappa, G., C. R. Adkar-Purushothama, U.Suraj, K.Tamilvendan, and M.Y.Sreenivasa . 2014. Pesticide tolerant Azotobacter isolates from paddy growing areas of northern Karnataka, India. World J. Microbiol. Biotechnol. 30: 1-7. [ Links ]
Cuevas, A. 2012. El clima y el cultivo de arroz en Norte de Santander. Revista Arroz 60: 4-8. [ Links ]
De Souza, R., A. Beneduzi, and A. Ambrosini. 2012. The effect of plant growth-promoting rhizobacteria on the growth of rice (Oryza sativa L.) cropped in southern Brazilian fields. Plant Soil 366: 585-603. [ Links ]
Escobar, C., Y. Horna, C. Carreño, y G. Mendoza. 2011. Caracterización de cepas nativas de Azotobacter spp. y su efecto en el desarrollo de Lycopersicon esculentum Mill. ‘tomate’ en Lambayeque. Scientia Agropec. 2: 39-49. [ Links ]
García de Salamone, I.E., J. M. Funes, L. Di Salvo, J.Escobar-Ortega , F.D’Auria , L.Ferrando L., and A. Fernandez-Escavino . 2012. Inoculation of paddy rice with Azospirillum brasilense and Pseudomonas fluorescens: Impact of plant genotypes on rhizosphere microbial communities and field crop production. Appl. Soil Ecol. 61: 196-204. [ Links ]
Gauri, S., S.Mandal, and B.Pati. 2012. Impact of Azotobacter exopolysaccharides on sustainable agriculture. Appl. Microbiol. Biotechnol. 95: 331-338. [ Links ]
Habibi, S., S. Djedidi, M. D. Firoz-Mortuza, N. Ohkama-Ohtsu, H. Sekimoto, and T. Yokoyoma. 2014. Physiological and genetic characterization of rice nitrogen fixer PGPR isolated from rhizosphere soils of different crops. Plant Soil 379: 51-66. [ Links ]
Halpern, M., A.Bar-Tal, M.Ofek, D. Minz, T. Muller, and U. Yermiyahu. 2014. The use of biostimulants for enchanching nutrient uptake. Adv. Agron. 130: 1-34. [ Links ]
Harrington, J. M., J. R. Bargar, A. A. Jarzecki, J. G. Roberts, L. A. Sombers, and O. W. Duckworth. 2012. Trace metal complexation by the triscatecholate siderophore protochelin: structure and stability. Biometals 25: 393-412. [ Links ]
Hernández, A., N. Rives, A. Caballero, A. Hernández, and M. Heydrich. 2004. Caracterización de rizobacterias asociadas al cultivo de maíz en la producción de metabolitos del tipo AIA, sideróforos y ácido salicílico. Rev. Colomb. Biotecnol. 6: 6-13. [ Links ]
Hussein, K. A., and J. H. Joo. 2015. Isolation and characterization of rhizomicrobial isolates for phosphate solubilization and indole acetic acid production. J. Kor. Soc. Appl. Biol. Chem. 23: 560-569. [ Links ]
IGAC (Instituto Geográfico Agustín Codazzi). 2006. Estudio General de Suelos y Zonificación de Tierras del Departamento Norte de Santander. Imprenta Nacional de Colombia. 359 p. [ Links ]
Karagöz, K., F.Ates, H.Karagöz, R. Kotan, and R. Çakmakç. 2012. Characterization of plant growth-promoting traits of bacteria isolated from the rhizosphere of grapevine grown in alkaline and acidic soils. Eur. J. Soil Biol. 50: 144-150. [ Links ]
King, E. O., M. K. Ward, and D. E Raney. 1954. Two simple media for the demonstration of pyocianin and fluorescin. J. Lab. Clin. Med. 44: 301-307. [ Links ]
Kuss A. V, V. V. Kuss, T. Lovato, e M. Lovato. 2007. Fixação de nitrogênio e produção de ácido indolacético in vitro por bactérias diazotróficas endofíticas. Pesq. Agropec. Bras. 42: 1459-1465. [ Links ]
Lavakush, J. Yadav, J.P.Verma, D.K.Jaiswal, and A.Kumar. 2014. Evaluation of PGPR and different concentration of phosphorus level on plant growth, yield and nutrient content of rice (Oryza sativa). Ecol. Eng. 62: 123-128. [ Links ]
López-Ortega, M., P. Criollo-Campos, R. Gomez-Vargas, M. Camelo-Rusinque, G. Estrada-Bonilla, M. F. Garrido-Rubiano, and R. R. Bonilla-Buitrago. 2013. Caracterización de bacterias diazotróficas solublizadoras de fosfato como promotoras de crecimiento en plantas de maíz. Rev. Colomb. Biotecnol. 2: 115-123. [ Links ]
Malik, D. K., and S. Sindhu. 2011. Production of indole acetic acid by Pseudomonas sp.: effect of coinoculation with Mesorhizobium sp. Cicer on nodulation and plant growth of chickpea (Cicer arietinum). Physiol, Mol. Biol. Plants 17: 25-32. [ Links ]
Marschner, P., D. Crowley, and Z. Rengel. 2011. Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis e model and research methods. Soil Biol. Biochem. 43: 883-894. [ Links ]
Park, J. H., N. Bolana, M. Megharaja, and R. Naidua. 2011. Isolation of phosphate solubilizing bacteria and their potential for lead immobilization in soil. J. Hazardous Mater. 185: 829-836. [ Links ]
Patel, D., C. Kumar, N. Tank, and M. Saraaf. 2012. Growth enhancement of chickpea in saline soils using plant growth-promoting rhizobacteria. J. Plant Growth Regul. 31: 53-62. [ Links ]
Phetcharat, P., and A. Duangpaeng. 2012. Screening of endophytic bacteria from organic rice tissue for indole acetic acid production. Procedia Eng. 32: 177-183. [ Links ]
Radwan, T. El-S. El-D., Z. K. Mohamed, and V. M. Reis. 2005. Aeração e adição de sais na produção de ácido indol acético por bactérias diazotróficas. Pesq. Agropec. Bras . 40: 997-1004. [ Links ]
Rohwer, F., V. Seguritan, F. Azam, and N. Knowlton. 2002. Diversity and distribution of coral-associated bacteria. Mar. Ecol. Prog. Ser. 243: 1-10. [ Links ]
Rojas-Tapias, D., A. Moreno-Galván A., S. Pardo-Díaz, M. Obando, D. Rivera, and R. Bonilla. 2012. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 61: 264-27. [ Links ]
Saber, F., A. Abdelhafez, E. Hassanand, and E. Ramadan. 2015. Characterization of fluorescent pseudomonads isolates and their efficiency on the growth promotion of tomato plant. Ann. Agric. Sci . 60: 131-140. [ Links ]
Sahoo, R., M. Ansari, T. Dangar, S. Mohanty, and N. Tuteja. 2013. Phenotypic and molecular characterisation of efficient nitrogen-fixing Azotobacter strains from rice fields for crop improvement. Protoplasma 251: 511-523. [ Links ]
Sarmiento, Y., A.H. Vergel, y D.M. Cárdenas. 2013. Evaluación de la estabilidad de Trichoderma sp. y Azotobacter sp. conservados por diferentes métodos. Rev. Colomb. Biotecnol . 15: 150-158. [ Links ]
Seck P. A., A.Diagne, S.Mohanty, and M.C.S. Wopereis . 2012. Crops that feed the world 7: Rice Food Security 4: 7-24. [ Links ]
Sivakamasundari, R., and G. Usharani. 2012. Studies on the Influence of Pseudomonas fluorescens and Chemicals on the Biocontrol Sheath Blight Incidence in Rice. Int. J. Pharmac. Biol. 4: 973-977. [ Links ]
Son, J. S., M. Sumayo, Y. J. Hwang, B. S. Kim, and S. Y. Ghim. 2014. Screening of plant growth-promoting rhizobacteria as elicitor of systemic resistance against gray leaf spot disease in pepper. Appl. Soil Ecol . 73: 1-8. [ Links ]
Souza, R., J. Meyer, R. Schoenfeld, P. Beschoren da Costa, and L. Passaglia. 2015. Characterization of plant growth-promoting bacteria associated with rice cropped in iron-stressed soils. Ann. Microbiol. 65: 951-964. [ Links ]
Sundara-Rao, W. V. B., and M. K. Sinha. 1963. Phosphate dissolving microorganisms in the soil and rhizosphere. Indian J. Agric. Sci. 33: 272-278. [ Links ]
Uribe-Vélez, D. 2011. El component microbiano del suelo como una herramienta para el desarrollo sostenible del cultivo del arroz. In: Uribe, D., y L. M. Melgarejo (eds). Ecología de Microorganismos Rizosféricos Asociados a Cultivos de Arroz de Tolima y Meta. Bogotá, D. C: Universidad Nacional de Colombia. pp: 19-21. [ Links ]
Vanegas, J., N. Florez-Zapata, y D. Uribe-Vélez. 2011. Bioprospección de microorganismos promotores de crecimiento vegetal para su aplicación en el cultivo de arroz. In: Uribe, D ., y L. M. Melgarejo (eds). Ecología de Microorganismos Rizosféricos Asociados a Cultivos de Arroz de Tolima y Meta . Bogotá, D. C: Universidad Nacional de Colombia . pp: 151-178. [ Links ]
Yu, X., X.Liu, T-H.Zhu, G-H. Liu, and C. Mao. 2012. Co-inoculation with phosphate-solubilzing and nitrogen-fixing bacteria on solubilization of rock phosphate and their effect on growth promotion and nutrient uptake by walnut. Eur. J. Soil Biol. 50: 112-117. [ Links ]
Received: February 2016; Accepted: January 2017











 texto em
texto em 



