Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.50 no.3 Texcoco Abr./Mai. 2016
Plant protection
Susceptibility of Diaphorina citri Kuwayama (Hemiptera: liviidae) to insecticides, in Veracruz, Mexico
1Entomología y Acarología. Colegio de Postgraduados. 56230. Montecillo, Estado de México,
2Agroecosistemas Tropicales. Colegio de Postgraduados. Campus Veracruz. 91690. Predio Tepetates, Municipio de Manlio E Altamirano, Veracruz, México..
The management of the citrus bacterial disease Huanglongbing (HLB) is more effective when focused on the control of the insect vector in Regional Control Areas (RCA). To support the regional management strategy of insecticides, susceptibility of two Diaphorina citri populations to the insecticides endosulfán, dimethoate, imidacloprid, malathion, methomyl, abamectin and lambda-cyhalothrin was determined. Imidacloprid formulated as a commercial product was evaluated by systemic absorption in orange seedlings (Citrus sinensis L.) cv. Valencia. The other technical grade formulated products were evaluated by topical application in dilutions with acetone. Mortality was registered 24 һ after applying the insecticides. For each product the LD50 or LC50 was obtained through Probit analysis and the resistance factor was calculated. Cazones colony was susceptible to the seven insecticides evaluated, thus these values are proposed as a baseline for monitoring resistance. Martinez population also showed susceptibility to abamectin, endosulfán, lambda-cyhalothrin, imidacloprid and malathion, and resistance to dimethoate (87.52 X) and methomyl (83.58 X).
Key words: Diaphorina citri; citrus; chemical control; HLB; Asian citrus psyllid; resistance
El manejo de la enfermedad bacteriana de los cítricos Huanglongbing (HLB) es más efectivo si se enfoca al control del insecto vector en Áreas Regionales de Control (ARCO). Para apoyar la estrategia de manejo regional de insecticidas se determinó la susceptibilidad de dos poblaciones de Diaphorina citri a los insecticidas endosulfán, dimetoato, imidacloprid, malatión, metomilo, abamectina y lambda-cialotrina. El imidacloprid formulado como producto comercial se evaluó por absorción sistémica en plántulas de naranja (Citrus sinensis L.) cv. Valencia. Los demás productos formulados en grado técnico se evaluaron por aplicación tópica en diluciones con acetona. La mortalidad se registró 24 h después de aplicar los insecticidas. Para cada producto se obtuvo la DL50 o CL50 mediante análisis Probit y se calculó el factor de resistencia. La colonia Cazones fue susceptible a los siete insecticidas evaluados, por lo que estos valores se proponen como base para la vigilancia de la resistencia. La población de Martínez también mostró susceptibilidad a abamectina, endosulfán, lambda-cialotrina, imidacloprid y malatión, y resistencia a dimetoato (87.52 X) y metomilo (83.58 X).
Palabras clave: Diaphorina citri; cítricos; control químico; HLB; Psílido Asiático de los Cítricos; resistencia
Introduction
Huanglongbing (HLB) is considered the most destructive citrus disease. Its capacity of dissemination and pathogenicity represents a high risk for citrus production in Mexico and other parts of the world (SENASICA, 2014). Diaphorina citri Kuwayama (Hemiptera: Liviidae), also known as the Asian Citrus Psyllid (ACP), is the primary vector of HLB, thus its control is vital for the adequate management of the disease (Halbert and Manjunath, 2004; SENASICA, 2014). The association of ACP and HLB has left defenseless more than 67 000 citrus producers in Mexico, who concentrate a production of more than 7 million Mg, with an approximate value of MX$10.2 billion (SENASICA, 2014). To halt the expansion of the disease, the Mexican government, as in other countries, has initiated a control program based on three strategies: chemical control of the vector (ACP) in Regional Control Areas (RCA), the production and use of certified plants and the elimination of infected trees at the moment of their detection (SENASICA, 2014; Lewis-Rosenblum et al, 2015). However, the common practice among the producers is the indiscriminate application of various active insecticide materials each season (Boina et al, 2009). In the present situation, it is foreseen that the development of resistance to different active ingredients will increase considerably in the coming years. Tiwari et al. (2012) documented that ACP can survive the application of various insecticides; mainly those that share at least one resistance mechanism and that were previously used intensively to control other citrus pests. This meant that to control D. citri in orchards of Florida, U.S., the initial dose of imidacloprid would be increased 35 times and 18 that of chlorpyrifos (Tiwari et al, 2011).
In integrated pest management it is important to detect early changes in susceptibility to insecticides in use in field populations and thus avoid unnecessary applications to prolong the useful lifespan of the available insecticides (Georghiou and Taylor, 1986; Diaz-Zorilla etal., 2011 ). This early detection facilitates the application of optional control measures and is the basis of the chemical control programs of a determined pest or vector. The objective of the present study was to determine the susceptibility of a population of D. citri, from Martínez de la Torre, to different insecticide materials of common use in the citrus regions of Veracruz, Mexico. The susceptibility of this population was compared with that of a laboratory population to determine the relative response of activity and to infer the development of resistance.
Materials and Methods
The study was carried out from May of 2012 to April of 2013 in the Vectors Laboratory of the Graduate Program of Plant Health-Entomology and Acarology, Colegio de Postgraduados, Campus Montecillo, Estado de Mexico, and in the Local Board of Plant Health of the ejido La Palma in Martínez de la Torre, Veracruz.
Populations of Diaphorina citri
Two populations of Diaphorina citri were obtained for the study; these were named Cazones and Martinez colonies and their identity was verified using keys for Psylloidea proposed by Yang (1984) and Burckhardt (2007). Cazones colony was collected from orange jasmin (Murraya panicuL·ta (L.), Jack; Rutaceae) plants and in orange orchards (Citrus sinensis L. cv. Valencia, Rutaceae), in both cases without regular applications of insecticides, in the community of Cazones de Herrera, Veracruz. This colony was maintained isolated in a greenhouse and free of selection pressure from insecticides since the year 2009. Adults from this colony were transferred to plants of cv. Valencia of 4 months age, maintained in a substrate of vermicompost, leaf soil and agrolite (3:2:1), in plastic bags of 30 x 30 cm placed in entomological cages (60 x 40 x 60 cm) covered with fine mesh. Adults remained in the shoots of the plants during one week to allow egg laying, then were removed with an aspirator. Infested plants were maintained in the cages in the greenhouse (25 ± 5 °С) and 12:12 h light:darkness) until the emergence of new adults, organisms used in the bioassays.
Martinez colony was collected from commercial lime (Citrus Խւքօսս (Tan) cv. Persa) orchards in the ejido La Palma, Martínez de la Torre, Veracruz. Farmers of this ejido indicated the lack of effectiveness of some applications of insecticides made against D. citri in the field. Trees with shoots infested by nymphs of the 5th instar were located during February, March and April of 2013, and were anticipatedly covered with fine mesh to capture the adults. Adults emerged between 24 to 48 h (a total of approximately 10 thousand insects), were confined in the aspirators and in groups of 20 individuals per aspirator: then were transferred in a cooler to the laboratory where they were evaluated.
Insecticides
Insecticides most used by the growers for ACP control were selected; six in analytical grade and a commercial formulation; they represented different toxicological groups according to the classification of Lagunes-Tejeda and Villanueva-Jiménez (1994). Common names, active ingredient concentration, manufacturer and toxicological group of these insecticides are as follows: endosulfán, 96 %, Syngenta, organochlorine-cyclodiene (ОС-Cd); dimethoate, 98 %, Sigma Aldrich, aliphatic organophosphate dimethyl (FA-SM); malathion, 96 %, ChemService, carboxylated organophosphorus (F-Cx); methomyl, 90 %, Sinochem Hebei Corp, monomethyl carbamate (CA-MM); abamectin, 95 %, Sigma-Aldrich, microbial insecticide (I-MICR); lambda-cyhalothrin, 92.1 %, Syngenta Agro, pyrethroid (PIRT-I); and imidacloprid, commercial formulation used at 30.20 %, Helmfidor of Mexico, neonicotinoid (NIC). Imidacloprid dilutions in the bioassays were made with distilled water; six other insecticides were diluted with analytical grade acetone (Reasol®).
Bioassays
Bioassays with contact insecticides in technical grade were made with the topical method described by Tiwari et al. (2011). To evaluate each dose of insecticide, 20 adults of 1 to 3 dof emergence and after 2 h of fasting they were used for each replicate. Adults were anesthetized with CO2 for 2 min to facilitate treatments application (Mann et al, 2012). Then, with an entomological brush (000), adults were placed on the underside of an orange (C. sinensis cv. Valencia) leaf disc that was introduced in a Petri dish (Ø 4.0 cm), containing 3 mm of a 1 % agar-agar (Merck®) substrate; the Petri dish was covered by a nylon mesh for ventilation. Insecticides (0.2 μL) were applied with a chromatographic syringe (Hamilton®) of 10 μL, coupled to repeated dispenser (Hamilton® model PB-600-1), on the pronotum of each insect. Petri dishes were maintained at 27± 1 °С, 50 % RH and photoperiod of 14:10 h light:darkness. Mortality was registered 24 h after treatment application.
Imidacloprid bioassay was performed using the plant systemic method proposed by Boina et al. (2009). The roots of Valencia citrus seedling of 120 d of age, were inmersed 48 h in a known concentration of the insecticide diluted in distilled water. Then, the seedling was transferred to a recipient with distilled water. A circular (Ø 4.0 cm) entomological clip cage was held on a leaf. Twenty adults of 1 to 3 d from emergence, previously subjected to a fasting period of 2 h, were introduced to the cage through a lateral orifice. Mortality was registered 24 h after application and the treatments were maintained in the greenhouse under the previously described conditions.
Initially, six logarithmic doses between 0.00001 and 1 % were used to detect the dose that caused percentages of insect mortality in the 10 to 90 % were used interval. At the end, eight logarithmic doses were selected in the interval to carry out the full bioassay. For each dose, five replicates were used of 20 individuals of 1 to 3 d of age, performed on different days, and 800 insects treated with each insecticide were obtained. Each replicate included a control, where acetone was applied in the topical bioassay or distilled water in the systemic bioassay.
The percentage of individual mortality was registered 24 h after each application; an individual was considered dead when mobility was not observed after the abdomen was pressed with a dissection needle. The maximum level of acceptable mortality in the control was ≤12 %. Mortality in the treatments was corrected with the equation of Abbott (1925). The experimental design was completely randomized with five replicates.
Statistical analysis
Results were analyzed through the Probit procedure in SAS® v. 9.2 (SAS, 2009). Values obtained were the LD50 or LC50 (lethal dose or concentration that kills 50 % of the exposed population, expressed in ng AI insect-1, or mg mL-1, respectively), the confidence limits (CL) at 95 % and the log dose-mortality response line. The resistance factor (RF) was obtained by dividing the LD50 or LC50 of the Martinez population by the LD50 or LC50 of the Cazones population. The LD50 or LC50 of the compared populations were not considered significantly different from each other when their LC overlapped (Finney, 1971; Robertson and Priesler, 1992).
Results and Discussion
The adults of D. citri exhibited different susceptibility to the seven insecticides evaluated. The Cazones colony was more susceptible than the Martinez colony; the former was maintained isolated from the selection pressure of insecticides for more than five years, which allowed it to be considered as the reference colony in this study. Resistance of pests to insecticides is one of the most serious problems that prevents the advancement of vector management programs. Switching insecticides when the one previously used is no longer effective is not a viable solution (Ortega et al, 1998). Knowing the susceptibility of field populations to the insecticides can help to reduce the use of non-effective products, which increase production costs and contaminate the environment.
Lambda-cyhalothrin showed highest toxicity in the Cazones colony, according to the LD50; in decreasing order, it was followed by abamectin, dimethoate, methomyl, malathion, imidacloprid and endosulfán. The group of pyrethroids (PIRT) presented the highest toxicity, followed by the microbials (I-MICR), aliphatic phosphates (FA-SM), carbamates (CA-MM) and carboxylated phosphates (F-Cx). The group of organochlorine cyclodienes (ОС-Cd) was the least toxic (Table 1). Slopes registered for all of the insecticides were higher than 0.84±0.13, which indicated certain uniformity of the colony for responding to the application of the products evaluated.
Table 1 Toxicity of insecticides in adults of D. citri, from Cazones de Herrera (Cazones colony) and Martínez de la Torre (Martínez colony), Veracruz, Mexico.
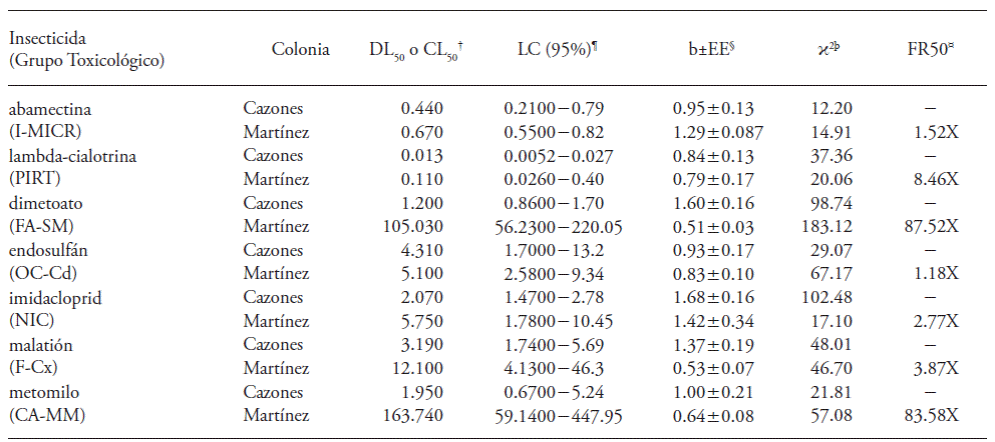
†LD or LC50: Lethal dose or concentration 50 in ng of active ingredient per insect (ng AI insect-1); ¶LC: Confidence limits at 95 %; §b±EE: slope with standard error; ϸϰ2: estimation of the straight line in the regression; ¤FR50: resistance factor 50 (LD50or LC50 of Martinez/ LD50 or LC50 of Cazones). The value of imidacloprid is expressed in mg mL-1 to represent a Lethal Concentration 50 (LC50).
Lambda-cyhalothrin was also the most toxic product for the Martinez population, according to the DL . Methomyl showed the lowest toxicity. Between these two products in order of effectiveness are abamectin, endosulfán, malathion and dimethoate. The PIRT group was also effective, in contrast to the Cazones colony, the CA-MM were least toxic. Registered slopes varied from 0.51 ± 0.03 (dimethoate) to 1.29±0.087 (abamectin), which indicated that population response was uniform to selection with abamectin, lambda-cyhalothrin and endosulfán, and heterogeneous for malathion, dimethoate and methomyl. The latter two presented a high resistance factor (RF50) (Table 1).
The RF50 of each insecticide evaluated in the Martinez colony (Table 1 ), with respect to the Cazones colony, indicated susceptibility to the insecticides abamectin, endosulfán, lambda-cyhalothrin, imidacloprid and malathion, considering that values were similar to those of the reference population and to the overlap of the confidence limits. Estimated methomyl and dimethoate RF50 values indicated that genes that supplying resistance to these insecticides were expressed in adults of D. citri from Martinez de la Torre. Separation of the confidence limits of log dose-Probit lines allowed the confirmation of the lack of susceptibility of the Martinez colony according to the LD50 of the evaluated toxic. This showed the capacity of field population adults to survive, independently of the presence of these products (Table 1).
Cazones colony has been maintained free of the pathogen for more than five years and from the application of pesticides. Adults of this colony were susceptible to the seven insecticides evaluated, thus the log dose-Probit lines of each insecticide can be used as reference for managing the resistance of the psyllid in Veracruz, Mexico (Prabhaker et al, 2006). According to Tiwari et al. (2010), the use of insects free of HLB gives certainty to the bioassays, since the susceptibility of insects to insecticides is reduced in populations of ACP infected with Candidatus Liberibacter asiaticus.
Martinez colony contains resistance genes in detectable frequency for the insecticides methomyl and dimethoate. In Mexico and in particular in the Persa lime producing region of Martínez de la Torre, insecticides of the principal chemical groups (organochlorines, organophosphates, carbamates, neonicotinoids and pyrethroids, among others) have been used against D. citri, since they first were sold for this purpose (Córtez-Mondaca et al, 2010; Díaz-Zorrilla et al, 2011). It is probable that the resistance levels registered in our study are related to the previous history of exposure. This would concur with the observations of some farmers of the region, with respect to the lack of control of D. citri with applications of conventional insecticides, and with Tiwari et al. (2011); they indicated that the resistance of D. citri to organochlorines and neonicotinoids insecticides was higher in areas of Florida, where they were used with more frequency.
In contrast, the relative susceptibility of the Martinez colony to abamectin, lambda-cyhalothrin, endosulfán, malathion and imidacloprid could be explained by the scant selection of the resistance mechanisms associated to these molecules. Therefore, the frequency of resistance genes is not yet found at detectable levels. High levels of resistance sometimes have shown opposite effects. In populations of D. citri of Florida exposed to sub-lethal doses of imidacloprid, increments of detoxifying enzymes (glutathione transferase (GTS) and cytochrome P450 oxidases) were detected, allowing them to survive the exposure to insecticides (Tiwari etal, 2011; 2012). The resistance to chlorpyrifos and fenpropathrin continues to increase in Florida, and these products are used intensively for the control of this pest (Tiwari et al, 2013). Imidacloprid is a costly product, but it is effective against D. citri (Hernández-Fuentes et al., 2012). The product exerts an agonist action in the postsynaptic acetylcholine receptors (IRAC, 2014), which is the specific site of the insects that confers them low toxicity to mammals (Liu etal, 2005; Phua et al, 2009). Furthermore, it requires an adequate and opportune application to be translocated and to take down the populations (Tomizawa and Casida, 2003).
Dimethoate is a product used in Mexico for the control of sucking insects for various decades (Hernández-Fuentes et al, 2012). It inhibits the action of the acetylcholinesterase (IRAC, 2014), and it is used in the control of D. citri in orchards with a low infestation rate. Dimethoate is widely accepted by the growers because apparently it is effective against diverse pests. This product is recommended in technical brochures distributed by the Federal Government (Córtez-Mondaca et al, 2010; Díaz-Zorrilla et al, 2011; Robles-González etal, 2011). However, in those texts it is emphasized that the product should only be applied once a year and at the recommended dose to avoid the selection of resistant insects (Córtez-Mondaca et al,2010). Dimethoate can be degraded by more than four types of detoxifying enzymes, thus resistance crossed with various insecticides that share at least one resistance mechanism could be created (Lagunes-Tejeda and Villaueva-Jiménez, 1994). This phenomenon is common among organophosphate compounds and carbamates (Tiwari et al, 2012) and it can explain partially the tolerance of the field population to methomyl (83-58 X) and the separation of the response lines between the colonies studied. Acetylcholinesterase (АСҺЕ) and GTS (Zhu and Gao, 1999; Gao and Zhu, 2000; Tiwari et al, 2012) are the enzymes present in common mechanisms that were identified in the tolerance of various insects to dimethoate and methomyl. Methomyl is not authorized for the control of D. citri in Mexico and apparently was not used in recent years to combat ACP in Martínez de la Torre. This confirms the importance of having the susceptibility reference for most of the possible toxicological groups, even products that could be authorized soon for the control of ACP, and prevent control problems (Prabhaker et al, 2006). The continuous and anarchic application of insecticides in the citrus regions of Veracruz will lead to reducing the useful life of the toxicological groups. This may be the case of dimethoate with chlorpyrifos, which share resistance mechanisms by action of GTS and cytochrome P450 oxidases (Ortega, 1998; Ortega et al, 1998; Tiwari et al, 2011). The use of dimethoate and methomyl should be limited in Martínez de la Torre, due to the resistance levels found in D. citri, in order not to continue incrementing resistance in these and other products. According to Georghiou and Taylor (1986), the capacity of controlling a species could be lost with presence of 1 % of resistant individuals in one to seven generations.
The development and establishment of resistance is possible in scenarios with high selection pressure, without adequate rotation of insecticides or with the use of the same toxicological group (Ortega et al, 1998; Lagunes-Tejeda et al, 2009). Resistance is a dynamic process (Georghiou and Mellon, 1983), thus it appears necessary to carry out a constant monitoring in each agricultural area. The base lines obtained in this study are a reference for monitoring resistance to these insecticides applied against D. citri. According to Tiwari et al. (2011), in 1998 the presence of D. citri was reported in citrus orchards of Florida; after that, the regional management of the pest has been carried out continuously, based on the rational and selective use of insecticides. Management of D. citri is feasible based on toxicological groups; therefore, the frequency of application is reduced, the use of mixtures and of insecticides of prolonged environmental persistence is avoided, the most opportune moments are defined for carrying out chemical control actions per region and methods of lower environmental impact are incorporated.
Conclusions
The Cazones colony of D. citri originally from Cazones de Herrera, Veracruz, was susceptible to the insecticides endosulfán, dimethoate, imidacloprid, malathion, methomyl, abamectin and lambdacyhalothrin, The Martinez colony, Martínez de la Torre, Veracruz, showed susceptibility to endosulfán, imidacloprid, abamectin, malathion and lambda-cyhalothrin and resistance to dimethoate and methomyl.
The log dose-Probit lines of each insecticide of the Cazones colony can be a reference for decision making in the management of resistance of D. citri.
Literatura Citada
Abbott, W. S. 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18: 265-267. [ Links ]
Boina, D. R., E. O. Onagbola, M. Salyani, and L. L. Stelinski. 2009. Antifeedant and sublethal effects of imidacloprid on Asian citrus psyllid, Diaphorina citri. Pest Manage. Sci. 65: 870-877. [ Links ]
Burckhardt, D. 2007. Order Sternorrhyncha, superfamily Psylloidea. Arthropod Fauna UAE 1: 159-169. [ Links ]
Córtez-Mondaca, E., J. I. López-Arroyo, L. M. Hernández Fuentes, A. Fu-Castillo, y J. Loera-Gallardo. 2010. Control químico de Diaphorina citri Kuwayama en cítricos dulces, en México: Selección de insecticidas y épocas de aplicación. Folleto Técnico No. 35. INIFAP, México. 22 p. [ Links ]
Díaz-Zorrilla, U., H. Cabrera-Mireles, J. A. Villanueva-Jiménez, F. D. Murillo-Cuevas, y J. I. López-Arroyo. 2011. Selección de insecticidas y épocas de aplicación para el control del psílido asiático en limón Persa en Veracruz. Folleto Técnico No. 6. INIFAP, México 16 p. [ Links ]
Finney, D. J. 1971. Statistical Method in Biological Assay. Griffing, London. 2nd Ed. 668 p. [ Links ]
Gao, J. R., and K. Y. Zhu. 2000. Comparative toxicity of selected organophosphate insecticides against resistant and susceptible clones of the greenbug, Schizaphis graminum (Homoptera: Aphididae). J. Agric. Food Chem. 48: 4717-4722. [ Links ]
Georghiou, G. P., and R. B. Mellon. 1983. Pesticide resistance in time and space. In: Georghiou, G. P. , and T. Saito (eds). Pest Resistance to Pesticides. Plenum Press, New York. pp: 1-46. [ Links ]
Georghiou, G. P. , and C. E. Taylor. 1986. Factors influencing the evolution of resistance. In: Committee on Strategies for the Management of Pesticide Resistant Pest Populations, National Research Council (eds). Pesticide Resistance: Strategies and Tactics for Management. National Academic Press, Washington, D.C. pp: 157-169. [ Links ]
Halbert, S. E., and K. L. Manjunath. 2004. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Fla. Entomol. 87: 330-353. [ Links ]
Hernández-Fuentes, L. M., M. A. Urias-López, J. I. López-Arroyo, R. Gómez-Jaimes, y N. Bautista-Martínez. 2012. Control químico de Diaphorina citri Kuwayama (Hemiptera: Psyllidae) en lima Persa Citrus latifolia Tanaka. Rev. Mex. Cienc. Agric. 3: 427-439. [ Links ]
IRAC. Insecticide Resistance Action Committee. 2014. Arthropod Pesticide Resistance Database. Michigan State University. http://www.irac-online.org/content/uploads/modo_de_accion_Oct11.pdf . (Consulta: agosto 2014). [ Links ]
Lagunes-Tejeda, A., J. C. Rodríguez-Maciel, y J. C. de Loera Barocio. 2009. Susceptibilidad a insecticidas en poblaciones de artrópodos de México. Agrociencia 43: 173-196. [ Links ]
Lagunes-Tejeda, A. , y J. A. Villanueva-Jiménez. 1994. Toxicología y Manejo de Insecticidas. Colegio de Postgraduados en Ciencias Agrícolas. Montecillo. Texcoco, México. 264 p. [ Links ]
Lewis-Rosenblum, H., X. Martini, S. Tiwari, and L. L. Stelinski. 2015. Seasonal movement patterns and long-range dispersal of Asian citrus psyllid in Florida citrus. J. Econ. Entomol . 108: 1-8. [ Links ]
Liu, Z., M. S. Williamson, S. J. Lansdell, I. Denholm, Z. Han, and N. S. Millar. 2005. A nicotinic acetylcholine receptor mutation conferring target-site resistance to imidacloprid in Nilaparvata lugens (brown planthopper). PNAS 102: 8420-8425. [ Links ]
Mann, S. R, S. Tiwari, J. M. Smoot, L. R. Rouseff, and L. L. Stelinski. 2012. Repellency and toxicity of plant-based essential oils and their constituents against Diaphorina citri Kuwayama (Hemiptera: Psyllidae). J. Appl. Entomol. 136: 1-10. [ Links ]
Ortega A., L. D. 1998. Resistencia de Bemisia argentifolii a insecticidas: Implicaciones y estrategias de manejo en México. Manejo Integrado de Plagas (Costa Rica) 49: 10-25. [ Links ]
Ortega A., L. D., A. Lagunes T, J. C. Rodríguez M., C. Rodríguez H., R. Alatorre R, y N. M. Bárcenas O. 1998. Susceptibilidad a insecticidas en adultos de mosquita blanca Trialeurodes vaporariorum (West.) (Homoptera: Aleyrodidae) de Tepoztlán, Morelos, México. Agrociencia 32: 249-254. [ Links ]
Prabhaker, N., S. Castle., F. Byrne, J. Henneberry, and N. Toscano. 2006. Establishment of baseline susceptibility data to various insecticides for Homalodisca coagulata (Homoptera: Cicadellidae) by comparative bioassay techniques. J. Econ. Entomol . 99: 141-154. [ Links ]
Phua, D. H., C. C. Lin, M. L. Wu, J. F. Deng, and C. C. Yang. 2009. Neonicotinoids insecticides: an emerging cause of acute pesticide poisoning. Clinical Toxicol. 47: 336-341. [ Links ]
Robertson, J. L, and H. K. Preisler. 1992. Pesticide Bioassays with Arthropods. CRC Press, Boca Raton, Florida. 127 p. [ Links ]
Robles-González, M. M., J. J. Velázquez-Monreal, M. A. Manzanilla-Ramírez, M. Orozco-Santos, R. Flores-Virgen R, y J. I. López-Arroyo. 2011. Control químico de Diaphorina citri en limón Mexicano. Insecticidas convencionales, productos alternativos y épocas de aplicación. Folleto Técnico 1. INIFAP. Campo Experimental Tecomán. Tecomán, Colima, México. 36 p. [ Links ]
Salcedo, D., R. Hinojosa, G. Mora, I. Covarrubias, F. De Paolis, C. Cíntora y S. Mora. 2010. Evaluación del impacto económico de Huanglongbing (HLB) en la cadena citrícola mexicana. IICA. Oficina del IICA en México. México, D.F. En línea: http://www.iica.int/Esp/regiones/norte/mexico/Publicaciones%20de%20la%20Oficina/B2009e.pdf (Consulta: febrero 2014). [ Links ]
SAS. Statistical Analysis System. 2009. SAS/STAT® 9.2 User’s Guide. Second Edition. SAS Institute, Cary, NC, USA. 7886 p. [ Links ]
SENASICA. Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria. 2014. Situación Actual y Perspectivas del Huanglongbing y el Psílido Asiático de los Cítricos en México. http://www.senasica.gob.mx (Consulta: febrero 2014). [ Links ]
Tiwari, S., K. Pelz-Stelinski, and L. Stelinski. 2010. Effect of Candidatus Liberibacter asiaticus infection on susceptibility of Asian citrus psyllid, Diaphorina citri, to selected insecticides. Pest Manage. Sci . 67: 94-99. [ Links ]
Tiwari, S. , R. S. Mann, M. E. Rogers, and L. L. Stelinski. 2011. Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manage. Sci . 67: 1258-1268. [ Links ]
Tiwari, S. , L. L. Stelinski, and M. E. Rogers. 2012. Biochemical basis of organophosphate and carbamate resistance in Asian citrus psyllid. J. Econ. Entomol . 105: 540-548. [ Links ]
Tiwari, S. , N. Killiny and L. L. Stelinski. 2013. Dynamic insecticide susceptibility changes in Florida populations of Diaphorina citri (Hemiptera: Psyllidae). J. Econ. Entomol . 106: 393-399. [ Links ]
Tomizawa, M., and J. E. Casida. 2003. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu. Rev. Entomol. 48: 339-364. [ Links ]
Yang, C. T. 1984. Psyllidae of Taiwan. Taiwan Museum Special Public. Series 3: 1-305. [ Links ]
Zhu, K. Y., and J. R. Gao. 1999. Increased activity associated with reduced sensitivity of acetylcholinesterase in organophosphate resistance greenbug, Schizaphis graminum (Homoptera: Aphididae). Pestic. Biochem. Physiol. 68: 138-147. [ Links ]
Received: May 01, 2015; Accepted: November 01, 2015











 texto em
texto em 


