Introduction
BC and traditional models of study
Among females, BC is the most commonly diagnosed cancer and the leading cause of cancer death. More than 2.1 milliaxon new cases are diagnosed every year worldwide, with an estimated incidence of 24.2% and a mortality rate of 15% in females.1 The most used methods of study for BC include 2D monolayer cells, patient-derived xenografts (PDXs), and genetically engineered mouse models (GEMMs). 2D monolayer cells have some advantages as easy management, feasible establishment, and are the most economic model for BC research2. Although, it is a model that does not bear similarity to the original tumor as it becomes immortalized; it does not reflect the interaction with the microenvironment; can acquire mutations that do not originate in the original tumor due to the 2D culture and do not acquire a constitution like an organ, therefore, the cellular hierarchy is lost. On the contrary, PDXs and GEMM models are more complex and suitable to outmatch some problems inherent to cell lines. The PDXs consist of tumor tissue or tumor cells from a patient, which are implanted into an immune compromised or humanized mouse. PDXs are models that can be used to evaluate drugs for treatments, preserve a certain degree of tumor hierarchy, heterogeneity, and functions from the original tissue. Beckhove et al., 2003, developed the first PDX using human primary BC transplants and DeRose et al., 2011, established clinically defined BC subtypes PDX models3,4. In the case of GEMMs, they are useful to study genetic pathways, therapeutic approaches, cancer progression, and metastasis. The first GEMM to model Human Epidermal Growth Factor Receptor 2 (HER2+) BC was obtained by Muller et al., 1988, and a GEMM developed to model BRCA1 BC was obtained by Behbod et al., 1999, to study the involvement of specific genes in oncogenesis5,6. Although these advantages, there are caveats that prevent its translation into clinics. Among them, the complete heterogeneity of the tumor is not preserved, its maintenance is more expensive and technically more difficult, and in the case of GEMMs, the establishment takes longer to perform. Both models are hampered by the interspecies difference, the microenvironment is not fully recapitulated and mouse stroma can interfere with therapeutic response, for instance. They show poor clinical predictability and reproducibility, specific therapies cannot be tested, and high-throughput screening cannot be performed7-9. Hence, there is still a gap in research that requires other models10.
Organoids definition
Organoids are three-dimensional (3D) structures that can be derived from pluripotent stem cells (PSCs), adult cellular tissue (stem or differentiated cells), embryonic progenitors, tissue segments, and whole organ explants11. The concept was first used in 1946 concerning a tumor case study12. Its meaning evolved to commonly refer to tissues or structures that resemble an organ; however, until the development of organoids in 2009, this concept was used specifically for self-organizing in vitro structures13. They are a useful tool to investigate organogenesis, repair, homeostasis, and disease modeling, including single-gene disorders and more complex maladies, such as cancer14,15. The advent of the “organoid era” began with the establishment and development of organoids from the intestine, until today the best characterized system13. Since then, organoids from several other tissues have been established and modified genetically for disease modeling or have been obtained from tumor tissues, leading to the development of the so-called tumor or cancer organoids16.
Strengths as models of study
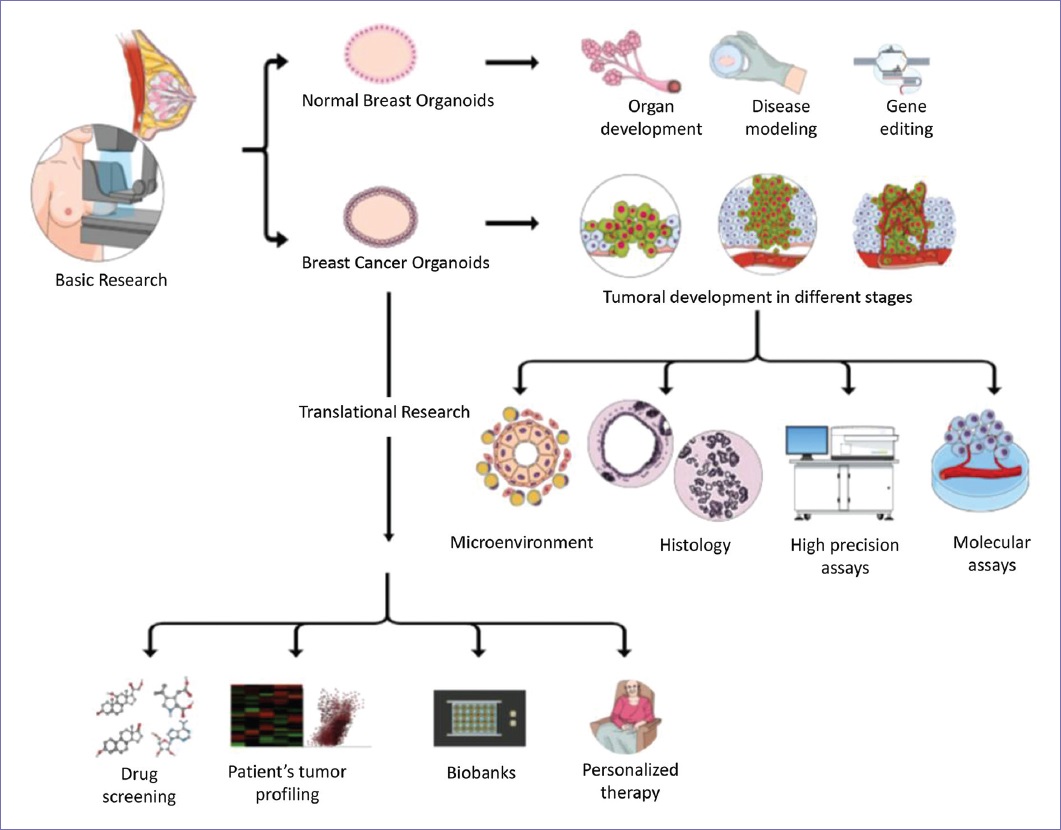
Breast cancer organoids (BCOs) have become an option for cancer study because they offer advantages and alternatives over the traditional methods. BCO can be established from the tumor tissue in culture, even from minor sources. Other benefits include the lower generation time for a stable organoid model, the efficiency of passage, and overall, the cost is affordable in comparison with animal models17. BCOs also provide a solid and reproducible platform to perform high-precision assays, which is limited and not always reproducible in the other models. This advantage can be applied in drug screening for novel treatments, single-cell profiling for transcriptome and epigenome analysis, and whole-exome and whole-genome sequencing, among other approaches18. Furthermore, BCOs preserve the 3D structure, all the cells and interactions involved in the tumor which are lost in the 2D cultures, together with the tumor heterogeneity including immune components and intercellular interactions between tumorigenic cells, the matrix, and the tumor niche19. Furthermore, because its technology does not depend on an animal, there are no interspecies interferences20. Moreover, BCOs are amenable to translational research including creating self-personalized models of a patient tumor, which can be molecularly profiled and tested for multiple drugs to find better therapeutic and individualized options. They can be preserved in long collections known as biobanks for further investigation. In addition, their application in clinical trials with comparison purposes is increasing, as it seems to be a more accurate model for personalized medicine (Fig. 1).
Disadvantages
The use of BCO has been largely discussed, as they still have limitations. It has been demonstrated that BCOs resemble the primary tumor at genomic, transcriptomic, and proteomic level. Nonetheless, it is debated until which point these signals remain identical to the tumor of origin. If the culture conditions interfere and, for example, the length and number of passages can also alter the expression levels of the BCO, leading to a loss of intratumor heterogeneity21. They initially provide a platform to understand the niche, but as the culture condition is extended, specific cell types could be enriched. In particular, a report has examined that culture favors the growth of a percentage of benign cells that in the tumor would act as supporters of growth, and in consequence, the natural evolution of the tumor is masked in vitro22. When the BCOs are not “contaminated” by such epithelial supporters cells, are possible to study the effect of particular environmental factors and/or perform cocultures with stromal cells to comprehend these interactions, but as such there are authors that comment that these systems are no longer per se the original tumor23. Other concerns include that each study of BCO has differences in the culture media, time for passaging, and/or strategies for generating the organoids; as consequence, there is a strong need of international standardization in the pipelines for isolation, enrichment, and characterization techniques employed. A collective effort should be made to establish clear guidelines and ways to assess quality and validity in organoid models24. For instance, some authors use or not extracellular matrix components25 and synthetic scaffold designs, among others. For example, a technical caveat was related with the effect of frozening BCO. In a recent study, BCOs from frozen tissues matched viability and drug response from BCO from fresh tissues with an optimized slow freezing technique in dimethyl sulfoxide26, this shows how improvements in the protocols are crucial for BCO standardization. One of the arguments against them is their relative high cost in comparison with 2D monolayer cells. It is correct that this technology is more complex, so therefore, it depends on more technological and human-trained staff for its management. Although, in comparison with the PDXs and GEMMs, they are cheaper27. Nonetheless, it has been seen that due to their advantages and more accessible management, its use has increased both in laboratories and in clinical trials. There is an ongoing collective effort to standardize and/or improve protocols, which could translate into better results that are worth the investment24. In addition, recent findings using transmission electron microscopy studying ultrastructural characteristics of BCO have concluded that these models recapitulate mammary gland morphology and display specific structural features that could be used to classify and identify BC subtypes28. A 3D bioprinting platform was developed to control the 3D formation of mammary organoids, which is adjustable to diverse culturing protocols and potentially to BCO, adding efficiency and scalability29. In addition, new methods with a high success rate are being proposed for the generation of BCO from surgical and biopsy samples30. These denote that as more technology is used for the characterization/generation of BCO, current technological difficulties will be overcome.
Applications of BCO
Organoids from extremely rare BC types
BCOs allow exploration of rare forms of BC that lacked solid models due to its infrequency, which lead to limited therapeutic options. Nowadays, this technology is used to characterize rare forms of BC. Pan et al., 2020, established a BCO from a 65-year-old woman with Paget disease, which resembled the original tumor and was useful for genomic characterization finding novel copy number alterations, mutational signatures, and somatic mutations, proving its value for clinicopathological research31. Another BCO system was described for giant papillary carcinoma, an infrequent form of duct lobular BC. Furthermore, it was applied for drug sensitivity tests that included endocrine and targeted therapies and resulted quite sensitive to fulvestrant, which has important implications for personalized medicine32. Thinking about its potential, it would be interesting the future development of an organoid system from male BC, which still lacks a reliable model of study.
Exploration of BC microenvironment
BCOs offer the possibility to study the microenvironment along with its specific interactions. It has been seen that the cocultures of normal breast organoids with fibrospheres are useful for understanding epithelial-stromal interactions33. BCO cocultures with fibroblasts have been performed, proposing novel tools such as optical coherence tomography, to assess stromal-epithelial interactions to study premalignancy34 and reveal how stromal cells promote cancer invasion through regulation of basal gene expression35. In addition, novel scaffold-free platforms are being used for studying normal breast and BCO to analyze diverse stimuli from the microenvironment, as well as neoplastic progression allowing the analysis of multi-phenotypic and multi-morphologic states36.
Study of BC hallmarks
BCOs are allowing in-depth study of challenging cancer hallmarks such as angiogenesis, invasion, metastasis, inflammation, and deregulation of cellular metabolism, among others. For instance, it has been demonstrated the influence of a stromal cell line that secretes vascular endothelial growth factor (VEGF) in angiogenesis and proliferation of BCO models from MCF-737. Furthermore, a potential relation between vessel formation and metastasis was observed in cocultures of a mouse BCO with a tissue-engineered 3D microvessel model, where tumor organoids integrated into the endothelial cell lining and facilitated intravasation of circulating tumor cells38. It was observed that human cancer cells competently invaded organoids with a microvessel network of human endothelial cells coupled to the mouse circulatory system allowing extravasation of tumor cells39. To study cancer invasion, another interesting approach used organoids derived from non-tumor MCF10A cells cocultured with tumor MDA-MB 231 which allowed monitoring invasion through epithelium and basement membrane40. Using organoid studies, it was detected a subpopulation of HER2+ early cancer cells which can intravasate, activate an epithelial-mesenchymal transition dependent on Wnt and ultimately metastasize41. Regarding inflammation, a very recent publication found a link between it and metastasis in triple-negative breast cancer (TNBC), where the axis A20/TNFAIP3-CDC20-CASP1 was strongly associated with poor prognosis and survival. Besides, TNBCO treated with inflammation and necroptotic inhibitors blocked this axis-mediated metastasis42. Deregulating energetics is of particular interest in the search for specific tumor characteristics amenable to treatment. A relation between cellular energetics and invasion was reported by Zhang et al., 2019, using BCO, where invasive cancer cells rearrange into the leader and follower positions. Leader cells exhibited higher glucose uptake than follower cells and together with other energetic features, point toward metabolic regulation in different tumor cells43. Another study using organoid model demonstrated that tumor recurrence is caused by residual cells that survive therapeutic regimens by acquiring metabolic shifts different from normal and primary tumors including altered lipid metabolism and elevated ROS44. Indeed, more studies about cellular energetics and metabolic reprogramming are needed. Xiao et al., 2022, performed a metabolic study in TNBC and using patient-derived BCO, a potential target: sphingosine-1-phosphate (S1P) was identified for luminal androgen receptor (LAR) BC subtype, proving the potential of these studies for personalized medicine45. Furthermore, it would be interesting to explore emerging cancer hallmarks such as epigenetics, phenotypic plasticity, and the role of senescent cells in BC.
BCO biobanks
A biobank is a collection that gathers and stores biological material and data associated, to perform molecular/genetic studies, to compare among specimens from the same disease or against normal specimens, etc. For the field of oncology, they are a benefit that can be used for drug design/development, treatment response analysis, and personalized medicine46. Its use has become so important that currently, there are “onco-biobanks” derived from different cancers such as gastrointestinal47, colorectal48, and glioblastoma49, among others. Sachs et al., 2018, developed a biobank of BCO, providing a protocol where primary and metastatic BCOs were obtained, recapitulating multiple distinct subtypes of BC and were used to perform drug screening concomitant with results obtained from in vivo models and patients’ response to ER inhibitor tamoxifen50. In a very complete approach, Dekkers et al., 2021, published protocols for the long-term culture and culturing conditions of 45 biobanked samples including BCO from different BC subtypes, as well as the methodology for genetic manipulation and orthotopic organoid transplantation in mice for tumor growth visualization and cancer cell behavior studies51. Another biobank of TNBC was developed by Bhatia et al., 2022, characterizing different cell types, candidate genes, and survival pathways related to BC progression52.
Personalized medicine
BCO in clinical trials
We performed a search about organoids in clinical trials (www.clinicaltrials.gov). Using the word “organoid,” we found 142 studies registered until June 2022. By adding “breast cancer” to our search, we found 22 clinical trials, representing 15.5% of all the trials employing organoids. Many of them were proposed as a platform for personalized medicine, allowing comparison of BCO against PDXs to corroborate results (e.g., NCT02732860 and NCT04703244), evaluation with one (e.g., NCT03544047), or several drugs (e.g., NCT03925233 and NCT03896958) for BC treatment based on organ-like culture. Some were applied in specific forms of BC, such as tumors with positive estrogen receptor (e.g., NCT04727632), negative HER2 (e.g., NCT04450706), positive HER2 (e.g., NCT04281641 and NCT05429684), TNBC (e.g., NCT05134779 and NCT05404321), advanced/metastatic disease (e.g., NCT04655573 and NCT04526587), and including patients with a germline pathogenic variant with a moderate to high lifetime risk of BC (e.g., NCT04531696). Additionally, clinical trials are using BCO to study, predict, prevent, and treat the metastatic recurrence of TNBC (NCT05464082). It was noted that before 2015, there were very few clinical trials including BCO (2/22) and the majority of reported clinical trials are quite recent. Thus, they have updates but no results reported. Only one of them was withdrawn (NCT04281355). Nonetheless, BCO use has increased in the last years and clinical trials now are including them primarily focused on oncology precision. The summarized characteristics of each study are found in Table S1.
Drug screening (DS) and personalized therapy
With the development of new BCO models and techniques as next-generation sequencing (NGS), the future is set toward personalized medicine. In a larger BCO platform obtained in China, Pan et al., 2021, performed DS looking for novel treatment options, evaluating tamoxifen, fulvestrant, paclitaxel, palbociclib, and carboplatin on neoadjuvant BCO with diverse degree of sensitivity to these drugs which demonstrate the value of organoids in DS and individualized treatment53. Chew et al., 2021, analyzed both BCO and PDX models of triple-negative BC (TNBC) identifying aberrantly activated protein kinases, specifically FGFR4 (fibroblast growth factor receptor 4) that could be targeted with tyrosine kinase inhibitors54. Furthermore, in TNBC, Ge et al., 2021, identified a microtubule-associated complex containing tektin4 and histone deacetylase 6 (HDAC6). BCO and PDXs, which have lost tektin4, were sensitive to ACY1216, a HDAC6 inhibitor, proposed as a new therapeutic strategy55. In an important percentage of BC cases, despite the treatment, tumors tend to develop resistance. As well, there is also the undesired possibility of relapse. In both cases, the combination of novel drugs with known chemotherapy regimens is explored. This type of evaluation can be performed in BCO, as done by Whittle et al., 2020, for testing a combination of inhibitors of CDK4/6, BCL2 together with fulvestrant (for estrogen receptor-positive BC). When this triple therapy was assessed in BCO derived from patients, tumor responsiveness augmented significantly56. Shao et al., 2020, employed whole-genome-wide RNA interference screening and a drug pressure model in BCO. The mechanism associated with cisplatin resistance was identified. DS allowed identification of an important number of drugs that were useless to cisplatin-resistant models and that cotreatment with bortezomib overcame such resistance57. Li et al., 2021, worked over HER2-positive BC resistant to anti-HER2 tyrosine kinase inhibitors. Their study included this subtype of BCO and found that by combining inhibition of CDK12 and anti-HER2 drugs sensitize/resensitize tumors to treatment58. Novel methodologies that could be applied to BCO and DS are under development. Such is the case of a method established by Mukundan et al., 2022, employing cytometry assays where calcein AM and propidium iodide staining were used to analyze the dose-dependent effect of drugs in tumor spheroid models59. Another interesting study was published by Walsh et al., 2014, where optical metabolic imaging of BCO was performed to measure antitumor drug responses to select optimal drug combinations60. Up until now, there was a lack of models that could recapitulate characteristics of advanced BC, either metastatic or refractory. Thus, little information could be obtained about patient stratification or prediction of cancer treatment outcomes. Despite this, BCOs are offering options to these patients and are valuable for studying specific subtypes of cancer patients and intrinsic and/or acquired resistance pathways61,62. As an example, organoids derived from advanced BC with malignant pleural effusion were used to DS yielding sensitivity to everolimus and capecitabine, the latter was consistent with the patient’s clinical response63. Even a combination of models as PDX-derived organoids has been used for exploration of metastatic BC with either Food and Drug Administration-approved and experimental DS against recurrent tumors, where treatment was reoriented in the clinic and the patient’s metastases showed remission for 5 months64. Nikulin et al., 2021, developed an organoid model from metastatic BC and tested 3,3’-diindolylmethane, a suppressor of mir-21-5p, overcoming drug resistance by enhancing response to the combination of cyclophosphamide and methotrexate65. Another study applied to refractory BC was performed by Chen et al., 2021, where a platform of patient BCO was developed for testing microtubule-targeting DS. Also, patients who received at least one drug predicted to be sensitive by BCO achieved partial response, stable disease, or long disease-free survival61. In addition, other techniques such as tumor-on-a-chip platform and 3D scaffolds are being applied to BCO to rapidly assess drug sensitivity to tailor drug therapies66,67. In other approach, for prevention, breast organoids derived from BRCA1 mutated tissue detected that inhibition of RANKL signaling with denosumab reduced proliferation, which is a form of preventive medicine targeted specifically to BRCA1 mutation carriers68.
Conclusions
Naturally, organoids for the study of BC have hurdles, although we have presented that the benefits outweigh the limitations of other traditional models. Therefore, we consider that BCOs are of vital importance to understand advanced processes of oncogenesis, the interaction with the microenvironment, the elucidation of survival pathways used in the neoplastic transformation, and metastasis. Furthermore, its multiple applications include drug discovery and screening, exploration of novel treatment strategies, the establishment of biobanks, and improvement of personalized medicine, which demonstrate that this model of study is important to find the missing pieces of BC research.
Supplementary data
Supplementary data are available at Mexican Journal of Oncology online (doi: 10.24875/j.gamo.22000110). These data are provided by the corresponding author and published online for the benefit of the reader. The contents of supplementary data are the sole responsibility of the authors.











 nova página do texto(beta)
nova página do texto(beta)