Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista ALCONPAT
versão On-line ISSN 2007-6835
Rev. ALCONPAT vol.5 no.2 Mérida Mai./Ago. 2015
Articles
The potential of a method for the synthesis of ceramic-cementitious materials processed by an alternative route
1 Centro de Investigación y de Estudios Avanzados del IPN, Unidad Saltillo.
Formulations of thermochemically bonded ceramics based of silicoaluminate raw materials were characterized. The mixtures were prepared using low water:solid and these were pressed under up to 30MPa. The specimens were cured for 2 hours at 200°C and were further characterized. The flexural strength registered 6.9-15.7 MPa, which was higher than common cements conventionally processed. The microstructures were dense, suggesting a favorable response of the mixtures to the activation process. The flexural strength varied with the type and amount of mixed raw materials. X-ray diffraction indicated that the crystalline phases from the raw materials did not react; the formation of zeolites was not observed. The proposed processing is promising in order to obtain high strength in short curing times.
Keywords: geopolymers; ceramics chemically bound; activated clays; precast products
Se estudiaron formulaciones de materiales cerámicos ligados termoquímicamente empleando materias primas silicoaluminosas. Se prepararon mezclas con baja relación agua:sólidos y se procesaron mediante prensado hasta de 30 MPa. Las probetas se curaron a temperaturas de 200°C por 2 horas. Se evaluó la resistencia a la flexión después del tratamiento térmico; los valores registrados alcanzaron entre 6.9 y 15.7MPa, lo cual es superior a los cementos procesados por rutas convencionales. Las microestructuras obtenidas indicaron la formación de matrices densas, sugiriendo una respuesta favorable de las materias primas al proceso de activación. Las propiedades mecánicas variaron con la cantidad y tipo de materiales mezclados. La difracción de rayos X indicó que no hubo formación de fases zeolíticas y las fases cristalinas no reaccionaron durante el proceso de curado. El procesamiento propuesto es prometedor para obtener altas propiedades mecánicas en tiempos de curado cortos.
Palabras clave: Geopolímeros; cerámicos químicamente ligados; arcillas activadas; prefabricados
Estudaram-se o comportamento mecânico de cerâmicos-cimentício silicoaluminosos, ligados termo quimicamente. Empregou-se traços com baixa relação água/sólidos que foram prensados com até 30MPa, para obtenção dos corpos de prova. As amostras foram curadas a temperaturas de 200°C por 2h. Avaliou-se a resistência a flexão depois do tratamento térmico; os valores registrados alcançaram entre 6,8MPa e 15,7MPa, o qual é superior ao normalmente obtido com esses cimentos à temperatura ambiente. As microestruturas observadas indicaram a formação de matrizes densas, sugerindo uma resposta favorável das matérias primas ao processo de ativação. As propriedades mecânicas variaram com a quantidade e tipo de materiais utilizados. A difração de raios X indicou que não houve formação de fases zéoliticas e as fases cristalinas não reagiram durante o processo de cura. O processamento proposto é promissor para obter altas propriedades mecânicas em curtos períodos de cura.
Palavras-chave: Geopolímeros; cerâmicos termoquimicamente ligados; produtos prensados
1. INTRODUCTION
The tile production industry is an important consumer of water and energy due to the various stages of the processing and production, such as wet grinding, drying and firing. During the latter, the temperatures may reach above 1000°C by means of the use of fossil fuels. The mentioned operations contribute to the total cost of production, so savings in the number of operations is of importance. Moreover, in order to promote the sustainability of the production, the industry is interested in strategies leading to reductions in the consumption of fuels, which would in turn reduce CO2 emissions. This can be achieved by designing alternative routes of production, while preserving as much as possible the properties and quality demanded for the products, i.e. mechanical strength, wear resistance, etc.
A family of cementitious materials are those known as Chemically Bonded Ceramics (CMC), also known as geopolymers, which have been promoted by Davidovits since the decade of the 80, who registered several patents (Davidovits, 1982, Davidovits, 1991). The CMC are inorganic polymers, and some authors consider that the name of alkali activated cements is more adequate. In this paper the materials investigated will be referred as CMC.
The CMC can be of silicoaluminate nature, the structure of which is based on three dimensional arrangements of SiO4 and AlO4 tetrahedra bound by sharing oxygen atoms. These materials can be processed as cements and display properties of ceramic-like materials; the consolidation takes place via the dissolution of the raw materials under high pH conditions, and the co-polymerization of the referred species; the reaction temperatures commonly range from ambient to around 100°C.
1.1 Raw materials for the synthesis of CMC
The raw materials useful to prepare silicoaluminate CMC must comply with some specific requirements: (1) Chemical composition rich in SiO2 and Al2O3; (2) An abundant fraction of glassy phase, fundamental for the susceptibility to the alkaline attack; (3) Sufficiently small particle size in order to promote its reactivity.
Clays are silicoaluminate raw materials, and are among the most abundant minerals in the earth crust. Calcined clays have been used as raw materials in the production of CMC (Barbosa et al, 2000); most of the reports have been based on the use of metakaolin (Al2Si2O7) (Davidovits, 1982), which is obtained after firing kaolinite (2SiO2·Al2O3·2H2O). The latter is a clay of SiO2:Al2O3 configuration of the type 1:1 (Rowles y O´connor, 2003), constituted by one layer of Silicon atoms in tetrahedral coordination with Oxygen atoms and one layer of aluminum atoms in octahedral coordination with oxygen and OH- ions (also known as gibbsite layer). The chemical composition of kaolinite is (wt %) 46.54%SiO2; 39.50%Al2O3 39.50%; H2O 13.96%; it shows little reactivity towards alkali under normal conditions. However, after firing at 650-900ºC, the kaolinite losses the OH- groups and the aluminum layer collapses (Shvarzman et al, 2003) and the aluminum coordination changes to tetrahedral, while the layer of Silicon remains (Kakali et al, 2001). The resulting product is metakaolin, which is amorphous to X-ray diffraction. This conversion increases the reactivity towards alkaline environments even at room temperature, which is the key for the CMC materials. The kaolinitic minerals may contain impurities such as quartz and other clays, as well as the substitution of iron and/or titanium for aluminum. Regarding the purity, previous work in Cinvestav Saltillo (Arellano-Aguilar et al., 2014, Burciaga-Diaz et al., 2012, Burciaga, 2014) has shown that even low purity metakaolinitic minerals are useful to produce CMC.
1.2 Processing of CMC materials
Compared to the processing of conventional Portland cements, the CMC have lower environmental emissions and better technological properties; moreover, these can be prepared using widely available raw materials, processing fluid and moldable pastes. The CMC harden by virtue of chemical reactions that advance gradually with time, reaching properties of interest for a wide range of applications. The preparation of CMC depends on various factors, for which their effect has not been completely understood according to the literature, some of these are:
Type and amount of chemical activator
Thermal treatment
Chemical composition of the mineral
Glassy fraction of the mineral
Particle size
Amount of water
The chemical composition of the overall formulation plays a major role that defines the mechanical strength (Burciaga-Diaz et al, 2012); it is defined on the basis of molar ratios such as: SiO2/Al2O3, M2O/Al2O3 y M2O/H2O (Burciaga-Diaz and Escalante-García, 2004). The literature indicates variable compositional ranges for several raw materials; the ratios reported by the patents of Davidovits, do not always result in the best properties for any raw material. The chemical composition of the CMC can also be reported including the concentration of mineral raw materials and alkaline activators. The most common among the latter are the alkaline silicates of the type M2O:xSiO2, which promote high pH values (Palomo et al, 1999; Davidovits, 1984), where M can be Na or K.
The chemical reactions during the synthesis of CMC have been proposed to take place in three stages: (1) Destruction of the atomic structure of the mineral. The alkaline media offers chemical stimuli such as the variation of the ionic strength caused by the presence of alkaline metals that perform as electron-donors; this causes the breaking of the bonds Me-O, Si-O-Si, Al-O-Al y Al-O-Si, caused by the alteration of the electronic density around the Si and Al atoms. Some of the species formed include silicic acid (Si(OH)4), and anions such as Si-O-, Al(OH)4 -, Al(OH)5 2- y Al(OH)6 3-. (2) The mentioned species saturate the solution and a policondensation takes place, forming new chemical products, leading to the setting, and a reduction of the pH takes place due to the interaction of the hidrosilicates and hydroaluminates with the alkalis. (3) Precipitation of products as a result of the particles formed in the previous stage, which results in an enhancement of the mechanical strength; the reduction of pH leads also to de condensation of silica gel from the alkaline activator, which favors the strength.
The conventional synthesis of CMC involves mixing the mineral raw materials with the alkaline solutions of activators, as well as the molding and curing to attain solidification. The curing commonly takes place at temperatures from ambient to 120°C (Barbosa y McKenzie, 2003; de Vargas et al, 2011; Burciaga-Diaz et al, 2012). Curing at high temperature allows gains of high early strength, some authors have pointed that higher strengths are reached with higher temperatures, e.g. 60MPa after curing 24 h at 85°C (Palomo et al, 1999, Rowles y O´connor, 2003); however, it has also been reported that after curing at high temperature the strength gains are negligible (Arellano-Aguilar et al, 2014). Information about the use of curing temperatures above 100°C is scarce in the literature.
1.3 This investigation
There exist in the literature a considerable number of reports related to the synthesis of geopolymeric materials based on high purity clay minerals and industrial by products such as metallurgical slags, fly ash, muds, etc. Nonetheless, there exists a limited number on reports based on kaolinitic minerals of low grade (Burciaga-Diaz et al., 2012; Arellano-Aguilar et al., 2014). The latter are an abundant resource worldwide, so their potential is considerable and the opportunities must be explored (Davidovits, 2002), such is the case of this investigation. On the other hand, there are only a few papers on the synthesis of CMC materials that combine the CMC processing under the conditions proposed in this research (Asbridge et al., 2002, Zivica et al., 2011), i.e.: very low water/solid ratios and uniaxial compression.
This paper presents the results of an investigation to develop a strategy to synthesize CMC materials in the shape of small tiles, using those raw materials commonly consumed by the traditional ceramic industry of tile production, which are in turn of lower purity than those used in CMC synthesized as cementitious binders. In contrast to the traditional ceramic industry, this paper proposes a simpler route, with less processing steps, using less water, less energy and generating less CO2 emissions, but with the potential of preparing ceramic-like materials of properties similar to those currently produced by conventional routes but in a more sustainable way.
2. EXPERIMENTAL PROCEDURE
2.1 Materias Primas
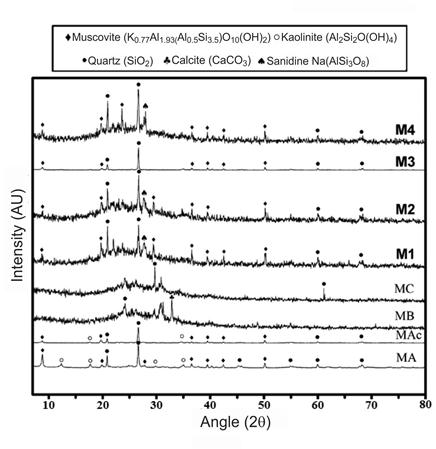
Some experimental details are omitted to protect authorship rights and industrial secrets. Three silicoaluminate minerals were employed, labelled as MA, MB y MC; the MA was fired at 700°C and was labelled as MAc. Table 1 shows the chemical composition obtained by X-ray fluorescence spectroscopy (FRX), from which the differences in chemical composition among the silicoaluminate minerals were evident; MA shows high content of iron. The mineralogy of the raw materials was characterized by X-ray diffraction, the results are shown with the results in Figure 4 for reasons of space and for a better comparison and understanding of the results. The difference among MA and MAc was the disappearance of the reflections of kaolinite, although the other crystalline phases persisted after the firing at 700°C; on the other hand, MB and MC showed an amorphous hump in the 2theta position typical for silicoaluminates.
2.2 Processing of materials
After a series of preliminary tests carried out to determine a set of experimental parameters, 4 mixtures were prepared using the raw materials from Table 1; here the mixtures are made of MAc and any of the other 3 raw minerals. Table 2 lists the formulations prepared. The alkaline activators were chemical compounds of Na2O·SiO2·H2O with weight ratios SiO2/Na2O lower than 2. The amounts of Na2O used were similar to those commonly reported for CMC from the literature using calcined clays. The amount of water used was the minimum necessary to provide the mixtures a consistency useful for the conformation of green bodies ready for the subsequent processing stage.
Table 2 Systems investigated.
| Mixture | Component 1 | Component 2 | Ratio Component/Component2 |
|---|---|---|---|
| M1 | MAc | MB | High 2/1 |
| M2 | MAc | MB | Low 3/1 |
| M3 | MAc | MA | Low 3/1 |
| M4 | MAc | MC | Low 3/1 |
The powdered minerals and activator solutions were mixed using a high speed mixer. The semi-dry mixtures were placed in the cavities of molds of dimensions of 4x16cm, the thickness of the resulting specimens was of 0.8-1.0cm. The mixtures were subjected to uniaxial compression using pressures up to 30.4 MPa. The tile specimens were demolded and thermally treated at 200° for 120 minutes and then left to cool to room temperature before characterization.
2.3 Characterization of materials
The specimens were tested by flexural strength using an hydraulic machine following the ISO 10545-4; due to difficulties associated to the processing of the specimens, only the flexural strength was measured, which is more important than the compressive strength for tile samples. The density was measured by the Archimedes method using liquid mercury. After the mechanical testing, the sample relics were used for further characterization. A fraction of the samples was ground in a planetary mill with agate media in order to pass the 105(m mesh, before characterization by DRX (Phillips PW3040) under the following operating conditions: CuKα radiation (1.542 Å), range 10-70° 2θ, step of 0,03° 2θ and incidence time of 3s per step. Solid fragments of specimens were mounted in resin for characterization by scanning electron microscopy (MEB Philips XL30ESEM coupled with energy dispersive spectroscopy, EDS) at an acceleration voltage of 20 ekV. The specimens were polished and coated with carbon. EDS spot analysis were performed to determine semi-quantitatively the chemical composition of specific areas in the samples, the measuring time was of 30 seconds for each analysis.
3. RESULTS AND DISCUSSION
Table 3 presents the results of compressive strength and density of the formulations investigated, which showed notable variations in flexural strength. The highest strength was for the mixture M1 (MAc-MB with low content of MB), this was close to the requirements for conventional ceramic tiles and 35% higher than the strength of the mixture M4 (MAc-MC with high content of MC). Higher contents of MB among mixtures M1 and M2 resulted in a reduction of 43% of the strength. The density values did not correlate to those of strength, i.e. M3 was denser and also weaker than mixtures M1 y M4; this could be due to differences in the intrinsic mechanical properties of the reaction products formed during the thermochemical activation.
Table 3 Mechanical properties and density of the mixtures investigated.
| Mixture | Flexural Strength (MPa) | Density (g/cm3) |
|---|---|---|
| M1 | 15.7 | 2.01 |
| M2 | 8.9 | 1.76 |
| M3 | 6.9 | 2.21 |
| M4 | 11.6 | 1.88 |
It is noteworthy that the flexural strength values registered were superior to those reported for CMC processed in a conventional way, which are commonly much lower than 10 MPa after 28 days; this indicates that the proposed processing method is quite efficient to attain high strength in considerably short curing times. Considering that for concretes, the flexural strength is approximately 10% of the compressive strength of the specimen, the mixtures investigated would be expected to potentially show compressive strengths of 69 to 157 MPa after only 2 h of preparation. The obtained flexural strength results could be considered similar to those reported previously using uniaxial compression and low water/solid ratios (Zivica et al., 2011); they reported compressive strengths of up to 146 MPa, which would correspond to an estimate of approximately 15 MPa of flexural strength. Nonetheless, the minerals used in this investigation are of far lower grade that those of the referred report, which makes our route more economical.
Figure 1 presents the microstructure of the mixture M1, obtained by backscattered electron imaging. The matrix of reaction products was dense, in agreement with the good flexural strength. Some particles of MB showed internal porosity. Some particles of MAc showed bright zones, which occasionally showed an elongated morphology; the brightness of such zones is attributed to a higher emission of backscattered electrons due to the presence of compounds of higher average atomic number, such zones could be unreacted particles that have not incorporated water, as happens in the reaction products that appear of a darker gray tone.
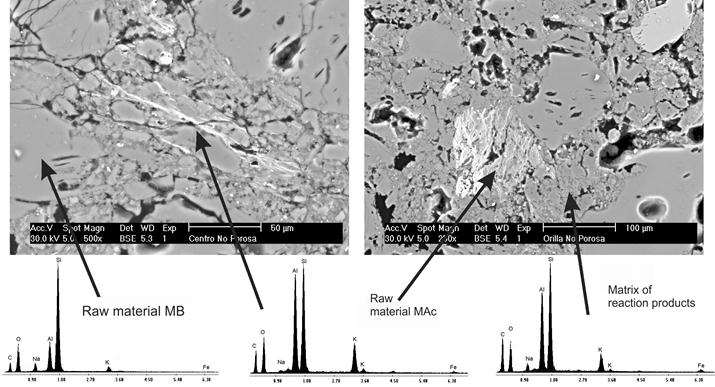
Figure 1 Microstructure obtained by scanning electron microscopy and backscattered electrons of mixture M1.
Figure 2 shows the microstructure of mixture M3 (the lowest flexural strength). The densification of the matrix of reaction products was similar to that of mixture M1, so the reduced strength is attributed to the formation of reaction products of lower intrinsic strength. The microstructure shows some bright zones, which were rich in Fe y Ti, in agreement with the chemical composition of the MAc.
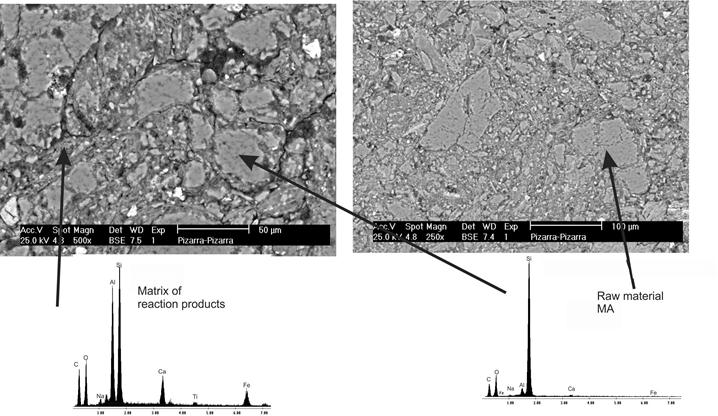
Figure 2 Microstructure obtained by scanning electron microscopy and backscattered electrons of mixture M3.
Figure 3 shows the micrographs from the microstructure of mixture M4, which displayed a similar densification as mixtures M1 and M3. The bright zones showed high contents of Fe and Ti (high atomic number) in the matrix corresponding to MA, while the matrix of MC showed a chemical composition consistent with a silicoaluminate in most of the particles (similar to MB).
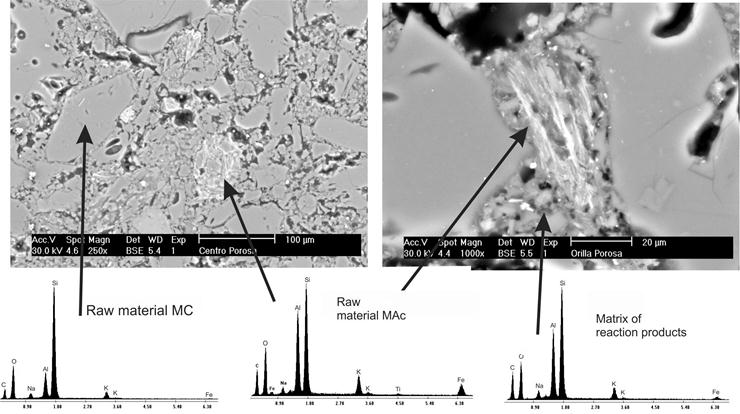
Figure 3 Microstructure obtained by scanning electron microscopy and backscattered electrons of mixture M4.
The results show that the type and amount of Component 2 (see Table 2) influences directly on the resulting properties. The differentiation among reaction products and unreacted raw materials is complicated in the micrographs, as both are silicoaluminates, so the differentiation by means of the gray tone is not as easy as in the case of hydrated conventional Portland cement. However, due to the high flexural strength obtained, it can be inferred that the reaction cementitious products are intimately intermixed with the unreacted raw materials.
Figure 4 presents the XRD patterns of the investigated mixtures, the patterns of the unreacted raw materials are included for reference purposes. In general terms, it was noted that the crystalline fractions remained unreacted after the solidification, indicating that these are inert during the curing process at 200°C for 2h. The formation of zeolites was not observed as reported for some CMC materials. On the other hand, the reaction products of cementitious CMC are characterized by an amorphous nature. For the mixtures M1, M2 y M4 the amorphous hump persisted, although it was noted that such humps were widened and shifted slightly to the left, indicating the formation of new reaction products of amorphous nature but different to those of the starting raw materials. In contrast, for the mixture M3 the amorphous hump was not clear, although the consolidation and mechanical strength indicated the formation of reaction products, so it is possible that the high crystallinity of the specimens overlaps and overshadows the presence of amorphous products.
4. CONCLUSIONS
The uniaxial pressing favors the densification of the matrices of the chemically bonded binders, which resulted in high flexural strengths of 6.9-15.7 MPa
The thermal processing accelerates the reaction processes, promoting the formation of the reaction products of cementitious nature, which are intimately intermixed with the unreacted raw materials in dense microstructures.
The chemical activation process was reinforced by the uniaxial pressing and thermal activation, using Na2O concentrations similar to those reported for common chemically activated binders
The combination of chemical activation and thermal treatment, could be called thermochemical activation and has the potential to produce ceramic-like materials of similar properties as those processed at more than 1000°C, but with savings in the energy and water demands.
More research is required to optimize the processing and for a better understanding and characterization of the structure of the mixtures, in order to understand the mechanisms of reaction.
Referencias
Arellano-Aguilar R., Burciaga-Díaz O., Gorokhovsky A., Escalante-Garcia J.I. (2014), Geopolymer mortars based on a low grade metakaolin: Effects of the chemical composition, temperature and aggregate:binder ratio, Construction and Building Materials,V.50, pp. 642-648. [ Links ]
Asbridge A.H., Page C.L., Page M.M. (2002), "Effects of metakaolin, water/binder ratio and interfacial transition zones on the microhardness of cement mortars", Cem Conc Res, V.32, pp. 1365-1369. [ Links ]
Barbosa F.F., MacKenzie J.D., Thaumaturgo C. (2000), Synthesis and characterization of materials based on inorganic polymers of alumina and silica: sodium polysialate polymers, International Journal of Inorganic Materials, V. 2, pp. 309-317. [ Links ]
Barbosa V.F., MacKenzie K.J. (2003), Thermal behavior of inorganic geopolymers and composites derived from sodium polysialate, Mater Res Bull, V. 38, pp. 319-31. [ Links ]
Burciaga Díaz O. (2004), Investigación inicial del uso de caolín del estado de Zacatecas en la preparación de cerámicos a base de polímeros inorgánicos, Tesis de licenciatura, Instituto Tecnológico de Zacatecas/Cinvestav Saltillo. [ Links ]
Burciaga Díaz O., Escalante García J.I. (2004), Efecto de parámetros químicos de soluciones alcalinas sobre las propiedades mecánicas de polímeros inorgánicos base metacaolín, Memorias del 26 congreso internacional en metalurgia y materiales, Saltillo, Coahuila (MEX), articulo 16. [ Links ]
Burciaga-Diaz O., Escalante-Garcia J I, Gorokhovsky A. (2012), Geopolymers based on a coarse low-purity kaolin mineral: Mechanical strength as a function of the chemical composition and temperature, Cement & Concrete Composites, V. 34, pp. 18-24. [ Links ]
Davidovits J., Boutterin C. (1982), Procédé de fabrication de revêtements de sols ou de murs par polycondensation de géopolymères, FR Brevet 82 10864. [ Links ]
Davidovits J. (1984), Synthetic mineral polymer compound of the silicoaluminates family and preparation process, US Patent 4,472,199. [ Links ]
Davidovits J. (1991), Geopolymers: Inorganic Polymeric New Materials, J Thermal Analysis, Vol 37, pp. 1633-1656. [ Links ]
Davidovits J. (2002), 30 years of successes and failures in geopolymers applications. Market trends and potential breakthroughsGeopolymer 2002 conference, October 28-29, Melbourne Australia, pp 1-16. [ Links ]
de Vargas A.S., Dal Molin CC, Antônio CF, da Silva FJ, Pavão B, Veit H (2011),The effects of Na2 O/SiO2 molar ratio, curing temperature and age on compressive strength, morphology and microstructure of alkali-activated fly ash-based geopolymers. CemConcrComp, V. 33, pp. 635-60. [ Links ]
Kakali G., Perraki T., Tsivilis. S. 2001Thermal treatment of kaolin: the effect of mineralogy on the pozzolanic activity. Applied Clay Science, V. 20, pp. 73-80. [ Links ]
Palomo A., Grutzeck M.W., Blanco M.T. (1999), Alkali-activated fly ashes A cement for the future, Cem Concr Res, V. 29,pp. 1323-1329. [ Links ]
Rowles M, O´Connor B (2003), Chemical optimization of the compressive strength of aluminate geopolymerssynthetised by sodium silicate activation of metakaolinite, J of Materials Chemistry, V. 13, pp. 1161-1165. [ Links ]
Shvarzman A., Kovler K., Grader G.S. (2003),The effect of dehydroxylation/amorphization degree on pozzolanic activity of kaolinite, Cement and Concrete Research, V. 33, pp. 405- 416. [ Links ]
Zivica V., Balkovic S., Drabik M. (2011), "Properties of metakaolin geopolymer hardened paste prepared by high-pressure compaction", Con and Bui Mat, V. 25, pp. 2206-2213. [ Links ]











 texto em
texto em